Abstract
Proton-coupled electron transfer (PCET) reactions and thermochemistry of 5,6-isopropylidene ascorbate (iAscH−) have been examined in acetonitrile solvent.iAscH− is oxidized by 2,4,6-tBu3C6H2O• and by excess TEMPO• to give the corresponding 5,6-isopropylidene ascorbyl radical anion (iAsc•−), which persists for hours at 298 K in dry MeCN solution. The stability of iAsc•− is surprising in light of the transience of the ascorbyl radical in aqueous solutions, and is due to the lack of the protons needed for radical disproportionation. A concerted proton-electron transfer (CPET) mechanism is indicated for the reactions of iAscH−. Redox potential, pKa and equilibrium measurements define the thermochemical landscape for 5,6-isopropylidene ascorbic acid and its derivatives in MeCN. These measurements give an O–H bond dissociation free energy (BDFE) for iAscH−of 65.4 ± 1.5 kcal mol−1 in MeCN. Similar studies on underivatized ascorbate indicate a BDFE of 67.8 ± 1.2 kcal mol−1. These values are much lower than the aqueous BDFE for ascorbate of 74.0 ± 1.5 kcal mol−1 derived from reported data.
Ascorbic acid (vitamin C) is involved in a wide range of biochemical processes.1 It is primarily a redox cofactor, being oxidized to the ascorbyl radical and then to dehydroascorbate. Studies of the ascorbyl radical in aqueous media are complicated by its transient nature.1c Here we report that in ‘dry’ acetonitrile, the soluble 5,6-isopropylidene ascorbyl radical (iAsc• −, eq 1) is surprisingly long-lived, as is the parent ascorbyl radical.2 These long lifetimes have facilitated detailed reactivity and thermo-chemical studies. While ascorbate reactivity is often described as electron transfer,1 at pH 7 one-electron oxidation to the ascorbyl radical occurs with concomitant loss of a proton (a proton-coupled electron transfer (PCET) process).3 Ascorbate often reacts by concerted transfer of e− and H+, including reactions with cytochrome b561 nitrosobenzenes, quinones and the reduction of tocopheroxyl radicals to tocopherols (vitamin E).4,5 These were historically called hydrogen atom transfer reactions, but they are probably better termed PCET or (our preference) concerted proton-electron transfer (CPET), because the ascorbate proton is in the molecular plane while the electron is removed from a π-orbital.6 Described here are a number of CPET reactions of ascorbates in MeCN, which reveal an unusual solvent dependence of the O–H bond dissociation free energy.
5,6-Isopropylidene semidehydroascorbyl radical (iAsc•−) is conveniently generated by reaction of 5,6-isopropylidene ascorbate (iAscH−) with the 2,4,6-tri-tert-butyl phenoxyl radical (tBu3PhO•)7 in MeCN (eq 1). Rapid and quantitative reduction of tBu3PhO• to
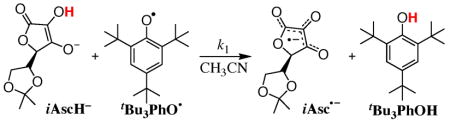 |
(1) |
tBu3PhOH is observed by UV-Vis and1H NMR spectroscopies. 5,6-Isopropylidene ascorbic acid (iAscH2) is an organic soluble ascorbate analog,8 available from Sigma-Aldrich. iAscH− can be isolated as nBu4N+iAscH−, a low melting solid, or generated in situ by addition of one equivalent of the strong base DBU to iAscH2 in MeCN (DBU = l,8-diazabicyclo[5.4.0]undec-7-ene).9
The ascorbyl radical product of reaction 1 (iAsc•−) has been characterized by its UV-visible, EPR, and ESI-mass spectra. By stopped flow spectrophotometry, the disappearance of tBu3PhO• (λmax = 382, 401, 634 nm) is accompanied by growth of a band at 377 nm (Figure 1a). The λmax and ε377 (3900 ± 200 M−1 cm−1) of this species are similar to those reported for the ascorbyl radical anion in water (360 nm, 3300 M−1 cm−1).1b,10 ESI-mass spectra of iAscH− + tBu3PhO• reaction mixtures9 show a negative ion at m/z = 214, as well as major peaks at m/z = 213 and 215 due to iAscH− and dehydroascorbate (iAsc)11 (from loss of H+ from the 4-position12). The m/z = 214 ion is the ascorbyl radical anion.
Figure 1.
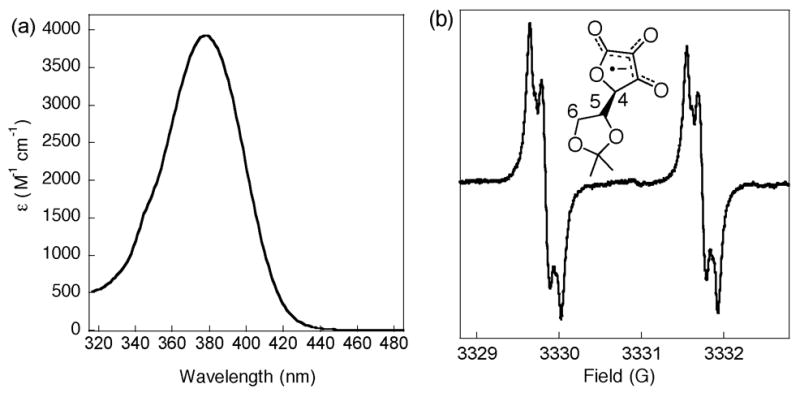
(a) UV-Visible spectrum and (b) X-band EPR spectrum of iAsc’“ in CH3CN at 298K, generated via reaction (1)
The room temperature X-band EPR spectrum of iAsc•− in MeCN (Fig. 1b), prepared by mixing a slight excess of iAscH− with tBu3PhO•, is centered at gMeCN = 2.0059 with hyperfine couplings of 1.91 [H4], 0.05 [H5], and 0.12, 0.16 G [H6].9 These are close to the values reported for iAsc•− in DMSO and in water (e.g., gdmso =2.00563, gH2O=2.00520).13
iAsc•− generated via reaction 1, at ca. 0.1 mM concentrations, decays over about two hours at room temperature. This stability is surprising given that the underivatized radical anion disproportionates rapidly in aqueous solutions at pH 7 (k ~ 3 × 106 M−1 s−1; t1/2 ~3 ms at 0.1 mM).14 The decay of iAsc•− in MeCN forms iAscH− and iAsc by1H NMR and ESI-MS (eq 2), analogous to the
| (2) |
decay of the ascorbyl radical in H2O.14 The decay of iAsc•− in MeCN appears to be highly dependent on the proton activity: drying the MeCN with activated alumina extends the lifetime, and addition of 56 mM H2O causes complete decay within 30 min. The addition of 0.1 mM CF3CO2H results in immediate loss of the absorbance at 377 nm. This indicates that the stability of iAsc•− arises from effectively “starving” the reaction of protons, since, unlike the decay of most organic radicals, disproportionation stoichiometrically requires H+ (eq 2). Analogous preparations of iAsc•− in “dry” CH2Cl2 and DMSO give similarly stable solutions.
Stopped-flow kinetic studies of reaction 1 give k1, = (3.4±0.5) × 106 M−1 s−1 and kH/kD 3.2 ± 0.6 at 289 K.15 The analogous reaction of 2,6-di-tert-butyl-4-methoxy-phenoxyl forms iAsc•− and the corresponding phenol, about six times slower: k = (5.3 ± 0.5) × 105 M−1 s−1; kH/kd = 3.5 ± 0.6 The reaction of iAscH− and the nitroxyl radical 2,2,6,6-tetramethyl-piperidin-1-oxyl (TEMPO) in MeCN results in production of iAscH•− and TEMPOH (eq 3) as observed by UV-Vis and 1H NMR spectroscopies. Aqueous ascorbic acid has long been known to reduce nitroxyl radicals.16 Kinetic measurements in MeCN using excess TEMPO yielded k3 = 1720 ± 150 M−1 s−1 at 298 K, and ΔH3‡ = 3.4±0.5 kcal mol−1,
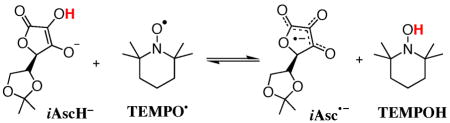 |
(3) |
ΔS3‡ = −32 ± 2 cal K1 mol−1. Spectrophotometric titration of iAscH− with limited TEMPO gives the equilibrium constant for eq 3, k3 = 1.2 ± 0.2 (ΔG°3 = −0.15 ± 0.10 kcal mol−1).9
The stability of iAsc•− allows the ready determination of the thermochemistry of electron, proton, and hydrogen atom transfers in this system (Scheme 1; all data in MeCN).9 Titrations of iAscH2 with N-methyl morpholine (pka = 15.59 in MeCN),17 and iAscH− with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (pka = 26.03),18 give pka(iAscH2) = 18.3 ± 0.3 and pka(iAscH−) = 28.8 ± 0.5. Cyclic voltammetry of iAscH− and iAsc2−19 show quasi-reversible waves (ΔEP ~ 0.2 V) at −0.410 ± 0.015 and −1.30 ± 0.02 V vs. Cp2Fe+/0. The pka(AscH•) is calculated from these E1/2 and pka values, using the square in Scheme 1 as a thermochemical cycle: 1.37[pka(iAscH−)−pka(iAscH•)] − 23.1[E(iAscH−)− E(iAsc2−)] = 0.
Scheme 1.
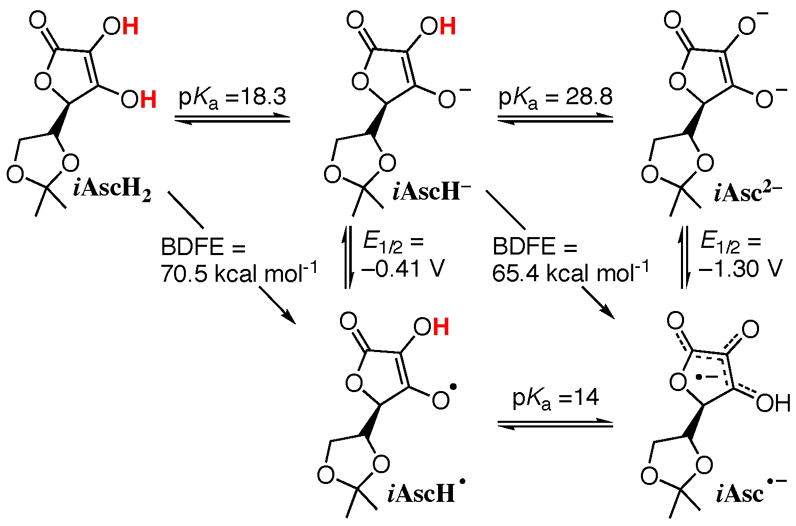
Thermochemistry of the AscH2 system in MeCN
The pka and E1/2 values indicate, using eq 4, that the O-H bond dissociation free energies (BDFEs) for iAscH2 and iAscH− in MeCN are 70.5 ± 2.0 and 64.4 ± 2.0 kcal mol−1, respectively (CG = 54.9 kcal mol−1 in MeCN versus Fc0/+).20 The equilibrium in eq 3
| (4) |
(K3 = 1.2) and the known21 BDFE(TEMPO-H) of 66.5 ± 1 kcal mol−1 give an independent measure of BDFE(iAscH−), 66.4 ±1.0 kcal mol−1. This value is in good agreement with that obtained from E1/2 and Pka data, and indicates a consensus BDFE for iAsc− of 65.4 ±1.5 kcal mol−1 in MeCN (Scheme 1). This corresponds to a bond dissociation enthalpy of roughly 70 ± 1 kcal mol−1.20b
With underivatized ascorbate, the tetrabutylammonium salt is sufficiently soluble in MeCN to examine its similar reactivity. Reaction with TEMPO forms the parent ascorbyl radical, based on its characteristic optical spectrum (λmax = 377 nm), which then decays slowly over ca. 1 hour. This reaction has keq = 0.11 ± 0.07 which implies BDFE(ascorbate) = 67.8 ± 1.2 kcal mol−1 in MeCN.9 Aqueous thermochemical data for ascorbate22 yield an aqueous O–H BDFE of 74.0 ± 1.5 kcal mol−1 (eq 4 with CG = 57.5 kcal mol−1 in H2O versus NHE20).9 The BDFE is 6.2 ± 1.8 kcal mol−1 higher in H2O than in MeCN. Preliminary studies of the aqueous ascorbate + TEMPO reaction (pH 7 phosphate buffer) seem consistent with these conclusions. The difference in BDFE of 6.2 ± 1.8 kcal mol−1 is a very large given that homolytic bond strengths are typically not very sensitive to the solvent.23
The mechanism of the reaction of iAscH− and TEMPO in MeCN is indicated to be concerted H+/e− transfer following the analysis used by Njus to implicate this mechanism for ascorbate + tocopheroxyl radicals.4b Alternative pathways of (i) initial proton transfer (PT) followed by electron transfer (ET) or (ii) initial ET followed by PT are not possible because of the high free energies of the initial steps: ΔG°PT ≅ 40 kcal mol−1 and ΔG°ET ≅ 35 kcal mol−1 - from the data in Scheme 1 and the known thermochemical values for TEMPO(H).24 These ground state free energy changes are the minimum activation barriers for these pathways (ΔG3‡ ≥ ΔG°) and both are much larger than the observed Eyring barrier, ΔG3‡ = 13.0 ± 0.1 kcal mol−1. The same treatment indicates that iAscH− + tBu3PhO• (eq 1) also likely proceeds via CPET.9
In conclusion, the 5,6-isopropylidene ascorbyl radical (iAsc•−) is readily generated from the corresponding ascorbate by phenoxyl and nitroxyl radicals. Remarkably, this radical is stable for hours in ‘dry’ acetonitrile, likely because protons are required for radical disproportionation (eq 2). Mechanistic and thermochemical data indicate that these reactions proceed by concerted H+/e− transfer, rather than a stepwise path involving separate electron and proton transfers. The O–H bond dissociation free energy (BDFE) of iAscH− in MeCN is determined to be 65.4 + 1.5 kcal mol−1 from two different approaches, and the BDFE of underivatized ascorbate in MeCN is 67.8 +1.2 kcal mol−1. These values are significantly lower than the BDFE of ascorbate, 74.0 + 1.5 kcal mol−1, derived from aqueous thermochemical measurements. Future studies will explore the origin of this large variation in ascorbate O–H BDFEs, which could reflect an unusual sensitivity to local solvation effects and could be important for enzymatic reactions of ascorbate.
Supplementary Material
Supporting Information Available: Experimental details for kinetic and thermochemical studies. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank Ms. K. Whitaker for assistance with the EPR spectra, and the U.S. National Institutes of Health (GM50422) and the University of Washington for support.
References
- 1.(a) Packer L, Fuchs J, editors. Vitamin C in Health and Disease. Vol. 5 Marcel Dekker; New York, NY: 1997. [Google Scholar]; (b) Davies MB, Austin J, Partridge DA. Vitamin C Its Chemistry and Biochemistry. The Royal Society of Chemistry; Cambridge: 1991. [Google Scholar]; (c) Bielski BHJ. Ascorbic Acid: Chemistry, Metabolism, and Uses. In: Seib PA, Tolbert BM, editors. Adv Chem Series. Vol. 200. ACS; Washington D.C: 1982. pp. 81–100. [Google Scholar]
- 2.All reactions were performed in Burdick and Jackson low water brand acetonitrile ([H2O] ~ 3 × 10−4 M), unless otherwise noted.
- 3.(a) Huynh MHV, Meyer TJ. Chem Rev. 2007;107:5004–5064. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mayer JM. Ann Rev Phys Chem. 2004;55:363–390. doi: 10.1146/annurev.physchem.55.091602.094446. [DOI] [PubMed] [Google Scholar]
- 4.(a) Njus D, Wigle M, Kelley PM, Kipp BH, Schlegel HB. Biochemistry. 2001;40:11905–11911. doi: 10.1021/bi010403r. [DOI] [PubMed] [Google Scholar]; (b) Vuina D, Pilepiæ V, Ljubas D, Sankoviæ K, Sajenko I, Uršic S. Tet Lett. 2007;48:3633–7. [Google Scholar]; (c) Biondi C, Galeazzi R, Littarru G, Greci L. Free Rod Res. 2002;36:399–404. doi: 10.1080/10715760290021243. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bisby RH, Parker AW. Arch Biochem Biophys. 1995;317:170–178. doi: 10.1006/abbi.1995.1150. [DOI] [PubMed] [Google Scholar]; (b) Njus D, Kelley PM. FEBS Lett. 1991;284:147–151. doi: 10.1016/0014-5793(91)80672-p. [DOI] [PubMed] [Google Scholar]
- 6.(a) Mayer JM, Hrovat DA, Thomas JL, Borden WT. J Am Chem Soc. 2002;124:11142–11147. doi: 10.1021/ja012732c. [DOI] [PubMed] [Google Scholar]; (c) Litwinienko G, Ingold KU. Ace Chem Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]; (a) Costentin C, Evans DH, Robert M, Saveant J-M, Singh PS. J Am Chem Soc. 2005;127:12490–12491. doi: 10.1021/ja053911n. [DOI] [PubMed] [Google Scholar]
- 7.Manner VW, Markle TF, Freudenthal J, Roth JP, Mayer JM. Chem Comm. 2008:256–258. doi: 10.1039/b712872j. [DOI] [PubMed] [Google Scholar]
- 8.Cf. Wittine K, Gazivoda T, Markuš M, Mrvoš-Sermek D, Hergol-Brundiæ A, Celine M, Ziher D, Gabelica V, Mintas M, Raiæ5-Maliæ5 S. J Mol Struc. 2004;687:101–106.Olabisi AO, Wimalasena K. J Org Chem. 2004;69:7026–7032. doi: 10.1021/jo049319i.Zhang L, Lay PA. J Am Chem Soc. 1996;118:12624–12637.Warren JJ, Mayer JM. J Am Chem Soc. 2008;130:2774–2776. doi: 10.1021/ja711057t.
- 9.Full details given in the Supporting Information.
- 10.Schuler RH. Radiat Res. 1977;69:417–433. [PubMed] [Google Scholar]
- 11.Ohmori M, Takagi M. Agric Biol Chem. 1978;42:173–174. [Google Scholar]
- 12.Cioffi N, Losito I, Terzano R, Zambonin CG. Analyst. 2000;125:2244–2248. doi: 10.1039/b007299k. [DOI] [PubMed] [Google Scholar]
- 13.Kirino Y. Chem Lett. 1974;3:153–158. [Google Scholar]
- 14.Bielski BHJ, Allen AO, Schwarz HA. J Am Chem Soc. 1981;103:3516–3518. [Google Scholar]
- 15.k’s from global analysis of the spectral data using SPECFTT™ software.9
- 16.Paleos CM, Dais P. JCS Chem Comm. 1977:345–346. [Google Scholar]
- 17.Isutzu K. Acid-Base Dissociation Constants in Dipolar Aprotic Solvents. Blackwell Scientific; Oxford: 1990. [Google Scholar]
- 18.Kaljurand I, Kütt A, Soovälti L, Rodima T, Mäemets V, Leito I, Koppel IA. J Org Chem. 2005;70:1019–1028. doi: 10.1021/jo048252w. [DOI] [PubMed] [Google Scholar]
- 19.Asc2− generated from nBu4NOH (1M in MeOH) + AscH2 in acetonitrile.
- 20.(a) Tilset M. In: Electron Transfer in Chemistry. Balzani V, editor. Vol. 2. Wiley-VCH; Weinheim: 2001. pp. 677–713. [Google Scholar]; (b) Mader EA, Davidson ER, Mayer JM. J Am Chem Soc. 2007;129:5153–5166. doi: 10.1021/ja0686918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mader EA. PhD Thesis. University of Washington; Seattle, WA: 2007. Mader EA, Manner VW, Wu A, Mayer JM. manuscript in preparation. (c) See Supporting Information.
- 22.(a) Williams NH, Yandell JK. Aust J Chem. 1982;35:1133–1144. [Google Scholar]; (b) Creutz C. Inorg Chem. 1981;20:4449–4452. [Google Scholar]
- 23.Mulder P, Korth HG, Pratt DA, DiLabio GA, Valgimigli L, Pedulli GF, Ingold KU. J Phys Chem A. 2005;109:2647–2655. doi: 10.1021/jp047148f. [DOI] [PubMed] [Google Scholar]
- 24.pka(TEMPOH+) ~ −3, and E1/2(TEMPO0/−) = −1.91 V vs. Fc+/0.9
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental details for kinetic and thermochemical studies. This material is available free of charge via the Internet at http://pubs.acs.org.


