Abstract
Twist1 is a basic helix-loop-helix (bHLH) factor that plays an important role in limb development. Haploinsufficiency of Twist1 results in polydactyly via the inability of Twist1 to antagonistically regulate the related factor Hand2. The mechanism modulating Twist1-Hand2 antagonism is via phosphoregulation of conserved threonine and serine residues in helix I of the bHLH domain. Phosphoregulation alters the dimerization affinities for both proteins. Here we show that the expression of Twist1 and Twist1 phosphoregulation mutants result in distinct limb phenotypes in mice. In addition to dimer regulation, Twist1 phosphoregulation affects the DNA-binding affinities of Twist1 in a partner dependent and cis-element dependent manner. In order to gain a better understanding of the specific Twist1 transcriptional complexes that function during limb morphogensis, we employ a series of Twist1-tethered dimers that include the known Twist1 partners, E12 and Hand2, as well as a tethered Twist1 homodimer. We show that these dimers behave in a manner similar to monomerically expressed bHLH factors and result in distinct limb phenotypes that correlate well with those observed from the limb expression of Twist1 and Twist1 phosphoregulation mutants. Taken together, this study shows that the Twist1 dimer affinity for a given partner can modulate the DNA binding affinity and that Twist1 dimer choice determines phenotypic outcome during limb development.
Keywords: bHLH factors, Twist1, Hand2, limb development, transcription, and dimerization
Members of the Twist family of bHLH proteins are evolutionarily conserved transcription factors that play fundamental roles in the normal development of a number of tissues including extraembryonic membranes, teeth, jaw, heart, and limbs (1–3). Although bHLH/HLH proteins can be organized into 5 groups, bHLH factors can be generally classified into 2 groups; the ubiquitously expressed E-proteins (Class A) and the tissue-specific/restricted bHLH factors (class B) (for review (4)). The classic model for bHLH function is that a heterodimer containing a member from class A and a member from class B interacts via the amphipathic α-helices. This interaction juxtaposes the basic domains of both proteins such that a DNA binding domain is formed which recognizes a DNA cis-element termed an E-box. (CANNTG) (4). The requirement for class A/class B heterodimerization for many bHLH factors is well established and in the case of the skeletal myogenic bHLH factors (MyoD1, Myogenin, Myf5 and Mrf4) heterodimerization is critical for proper biological function (4,5). Other class B factors such as members of the Twist family of bHLH proteins not only function as heterodimers with E-proteins, but can also form homodimers and heterodimers with other class B bHLH proteins (1–3,6). It is thought that the control of bHLH dimerization is a mechanism that could control transcriptional response.
Recently, we have shown that members of the Twist family of bHLH proteins contain an evolutionarily conserved threonine and serine within helix 1 that can be phosphoregulated by the actions of protein kinase A (PKA), protein kinase C (PKC), and B56δ-containing protein phosphatase 2A (PP2A) (2,3). The consequence of altering the charge on these conserved residues affects the dimerization affinities that Twist family members have for their potential bHLH partners. In the case of Twist1, these partners include itself, E12/E47 and Hand2 (3,6). Our working hypothesis is that the phosphoregulation of Twist-family members alters the dimerization affinities allowing for new bHLH complexes to form within a cell thus driving or changing the cells identity program (2,3). The mechanism for this model involves dimer choice, and access and/or ability of the dimer complex to bind DNA and thus regulate transcription. Support for this hypothesis can be seen in the autosomal dominant disease Saethre-Chotzen Syndrome (SCS). There are over 70 documented mutations within human TWIST1 that are associated with the causation of SCS (7). We recently showed that a sub-population of these TWIST1 mutations disrupts the evolutionarily conserved consensus PKA site and consequently these mutations are poorly phosphorylated. Moreover, phosphorylation mutants of Twist1 affect the dimerization characteristics of Twist1 with itself, E-proteins, and the related co-expressed Hand2 (3). Through both gain-of-function and loss-of-function analysis, we show that during limb development Twist1 and Hand2 function antagonistically; moreover, we show that a hypophosphorylation mutation in Twist1 that correlates to the phosphorylation state of Twist1 in patients with SCS cannot antagonize the actions of Hand2 supporting our idea that the bHLH dimer choice is a critical regulatory step in driving the phenotype of a cell (for review (8)).
The functional role of Twist1 in the developing limb is twofold. First, Twist1 maintains an Fgf8-Fgf10-FgfR2 autoregulatory signaling loop, which promotes the development of the apical ectodermal ridge (AER) facilitating limb outgrowth (9). In addition, Twist1 also regulates the expression of Gli2 and Gli3, which are key positive and negative regulators of Sonic Hedgehog (Shh) activity. Shh activity defines anterior-posterior limb patterning via the zone of polarizing activity (ZPA). Twist likely activates or maintains a graded pattern of Gli3 activity, which then suppresses Shh signaling within the anterior mesenchyme (9).
When the physiological ratio of Hand2:Twist1 expression is perturbed by either Twist1-haploinsufficiency or by Hand2 overexpression, shh expression is expanded. This results in polydactyly via the formation of an additional/expanded ZPA in the anterior of the forming limb (3,9–11,12). Hand2 induced-expression of shh is also associated with an direct antagonistic relationship with the shh-repressor Gli3 (13), suggesting that a precise transcriptional balance of both Twist1 and Hand2 are essential for both normal limb outgrowth and patterning. Also involved but not currently understood is the role that E-protein expression has on modulating Twist1 and Hand2 regulation of limb formation.
In this study, we set out to investigate the consequences of Twist1 gain-of-function analysis using wild type and phosphoregulation mutants of Twist1 in the developing limb and explore the affects of Twist1 phosphoregulation on DNA binding affinities to a representative set of E-box cis-elements. We show that the expression of wild type Twist1, hypophosporylated Twist1 (Twist1T125;S127A), and phosphorylation-mimic Twist1 (Twist1T125;S127D) indeed results in distinct phenotypes within the developing mouse limb and in addition to changes in dimerization affinity, altering the charge of helix I can affect DNA binding in a cis-element dependent manner. Furthermore, the expression of tethered Twist1 dimers (Twist1-Twist1, Twist1-E12 and Twist1-Hand2), where the partner choice is dictated by expressing the dimer pair as a single polypeptide, show specific phenotypic effects on limb morphogenesis that correlate to those observed when expressing the monomeric wild type and phosphorylation mutant Twist1 proteins.
EXPERIMENTAL PROCEDURES
Plasmids
Expression plasmids for Twist1, Twist1T125;S127A, and Twist1T125;S127D were described previously (2,3). Tethered dimer constructs were generated by cloning cDNAs for wild type Twist1, Hand2, and E12 into pcDNA3 (Invitrogen) modified to include a glycine-rich linker G(SGGG)3SGG that joins the two cDNAs (6). The 5prime; cDNAs were PCR amplified as HindIII-BamHI fragments with the stop codon removed to allow for translation of both cDNAs as a single protein. The 3prime; cDNAs were PCR amplified as KpnI-XbaI fragments. The glycine linker had HindIII and BamHI sites at the 5prime; end and KpnI and XbaI sites at the 3prime; end for cloning purposes. CATCTG E-box and CGTCTG D-box reporter constructs were previously described (14). The CATATG E-box reporter was made similarly by inserting 3-copies head-to tail into the SacI and BglII sites of the luciferase vector pGL3P (Promega). Prx transgenic constructs were generated as follows: For all clones containing a Hand2 cDNA, an internal BglII site within the coding region was first mutated to destroy the site while maintaining amino acid sequence (5prime;-GG AAG AAA GAG CTG AAT GAA ATC TTG AAA AGC ACA GTG AGC-3prime;) using the Quickchange Mutagenesis Kit (Stratagene) following the manufacturer’s instructions. BglII sites were then added to the 5′ end of the clones and 3′ of the poly A signal via PCR. Sequences were verified to be free of PCR generated mutations by sequence analysis of both strands. Verified inserts were then cloned into the BglII cloning site of the Prx promoter-containing construct. Orientation was determined by sequence analysis. Prx promoter sequence plus cDNA monomer or tethered inserts were liberated by SalI digestion and given to the IU transgenic core for gel purification and F0 production.
Electrophoretic Mobility Shift Assays
The various bHLH and tethered bHLH proteins were in vitro transcribed and translated using Promega’s TNT system as described in the manufacturer’s protocol. 3 probes were used for EMSA: a Hand E-box (5′-gga ttc cat tgc atc tgg att cca gag-3′), a degenerate E-box sequence shown to bind Hand1 and Hand2 termed a D-box (5′-cat tgc att ggc gtc tgg cat tgc att -3′) (14), and an E-box that was shown to bind Twist1 (5′-gat ccc tcg cat atg ttg aa -3′) (6). Single stranded sense probes were 5′ end labeled and then annealed to cold antisense to generate double stranded DNA. EMSA assays were performed as described using a binding buffer containing 2mM MgCl2, 2mM DTT, 25mM Tris pH 7.5, 25mM NaCl, 12% Glycerol, and 0.1% NP40 (15). Competitors used in the assay are the unlabeled oligos used for probes as specific competitors and oligos with the sequences in italics substituted for the binding target sequences (5′-gga ttc cat tgt gct acg att cca gag-3′); (5′-cat tgc att ggg cag agg cat tgc att -3′); (5′-gat ccc tcg tga ttg ttg aa -3′) as non-specific competitors. EMSA experiments were repeated 5 times using new programmed lysates, in which protein expression was verified via immunoblotting prior to EMSA analysis. Individual experiments employed a single batch of programmed lysates for each probe and the EMSA reactions and gels were run on the same day.
Immunoblotting
In vitro programmed reticulocyte lysates were collected, and 5 μL of lysates were run through 10% or 12% SDS PAGE gels, electroblotted and incubated with the indicated antibody as described (2). Blots were visualized using the Super Signal Luminescent detection protocol (Pierce).
Cell culture and transfections
HEK293 cells were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, glutamine, and antibiotics. Cells were plated and transfected as described (16) with the indicated constructs using a CaPO4 procedure.
Luciferase assays
Luciferase assays were preformed using the Dual Luciferase Assay kit (Promega) following the manufactures protocol. Cell lysates were read using a 96 well microtitre plate luminometer (Forma). Data represents 6-independent experiments and error bars denote standard error.
Generation and analysis of transgenic animals
Transgenic DNA was purified using resin columns (Qiagen), digested, and given to the IU transgenic facility for gel purification and microinjection into mice. Southern blotting of yolk sac DNA identified E17.5 F0 animals carrying the transgenes of interest. Skeletal preps were preformed exactly as described (17).
RESULTS
DNA-binding is altered in Twist1 helix I phosphoregulation mutants
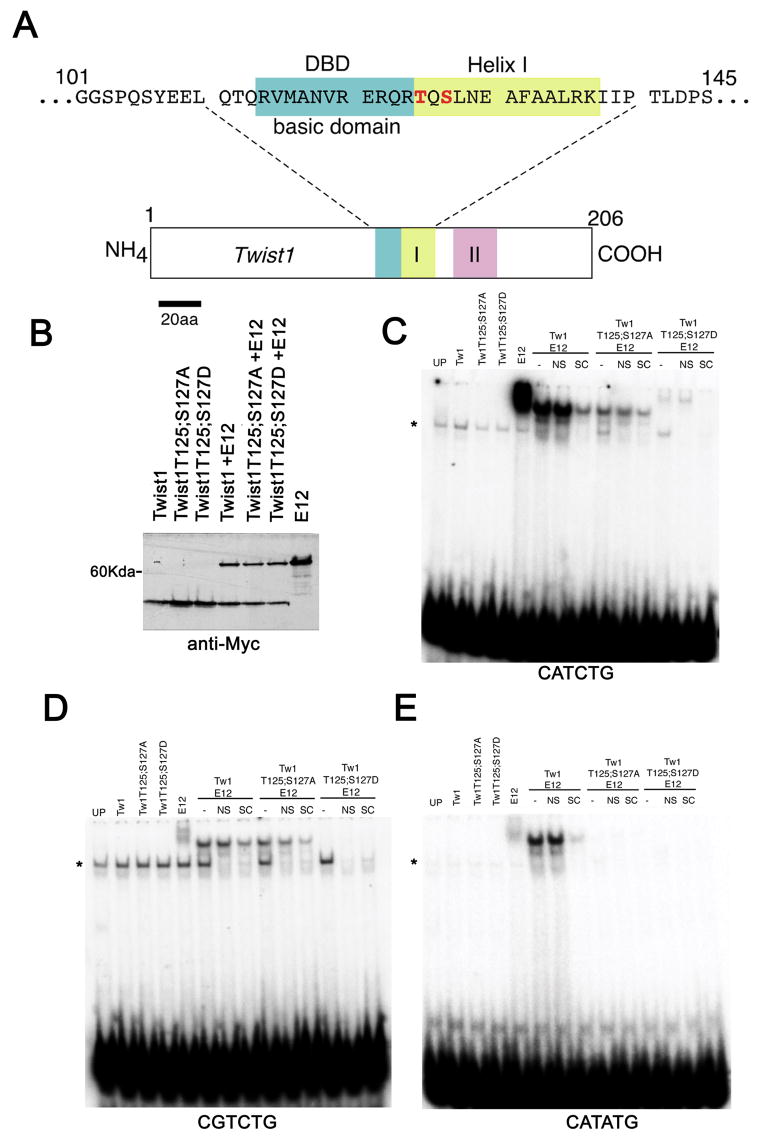
As is shown in figure 1A, T125 and S127 are positioned at the boundary between the basic DNA-binding domain (DBD) and helix I of the bHLH. Our earlier findings show that the alteration of charge at T125 and S127 results in changes of the dimerization affinities between Twist1 and its bHLH partners, but in no case have we shown that these alterations result in the complete loss of Twist1 dimerization with another bHLH partner (3). Considering this, we sought to determine if DNA-binding was also affected in Twist1 phosphoregulation mutants. We thus performed Electrophoretic Mobility Shift Assays (EMSA) using in vitro transcribed and translated Twist1, Twist1T125;S127A, and Twist1T125;S127D alone and co-translated with E12 from CMV-driven expression vectors containing a SP6 promoter sequence for in vitro transcription and translation. To control for input protein, all proteins were tagged with the myc-epitope and were in vitro transcribed and translated. Immunoblot analysis of the input protein shows that equivalent amounts of the protein were added to all binding reactions using each DNA probe, thus differences in the intensity of the shifted probe reflect differences in the DNA-binding affinity (Fig 1B). We first looked at DNA binding to an CATCTG E-box core, which binds the related Hand factors (18,19). No DNA-binding is observed from the reticulocyte lysates programmed to express Twist1, Twist1T125;S127A, or Twist1T125;S127D indicating that the homodimers are not binding at detectable levels (Fig. 1C). Twist1 homodimer formation has been demonstrated previously (3) and is discussed below. In contrast, lysates programmed to express E12 show a strong probe shift indicating that E12 homodimers can bind the Hand E-box. Lysates programmed to express both Twist1 and E12 also show a smaller DNA mobility shift than that of the E12 homodimer, which is not competed away by the addition of excess non-specific (NS) competitor but is competed by excess unlabeled competitor (SC) (Fig. 1C). When Twist1 is replaced with Twist1T125;S127A and is co-translated with E12 a similar complex is able to form; however, the amount of shifted probe is significantly less indicating that DNA binding affinity is diminished. Of note, we do not observe any E12 homodimer shifted probe based on the migration pattern of the mobility shifts indicating that the majority of the stable dimer complexes formed are indeed Twist1T125;S127A-E12 heterodimers. Conversely, lysates programmed with Twist1T125;S127D and E12 fail to shift this Hand E-box at any detectable level under our experimental conditions (Fig. 1C). What we do observe is a higher mobility shift that based on its migration pattern corresponds to an E12 homodimer and is not competed by NS but is competed by SC. Given this observation and previous FRET analysis (3), we feel that both homodimer and heterodimer complexes are able to form under these conditions and that the E12 homodimer has a stronger DNA binding affinity and/or is more stable and therefore competes out the binding of Twist1T125;S127D and E12 for the Hand E-box (Fig. 1C).
Figure 1.
(A) Schematic of murine Twist1 showing the amino acid sequence of the basic DNA binding domain (DBD) and helix I. T125 and S127 are denoted in red. (B) Western blot showing expression of myc-tagged proteins within programmed rabbit reticulocyte lysates. Proteins expressed are denoted over each lane and represent the same amount of protein used in each EMSA assay. (C) EMSA using the CATCTG or Hand E-box. Unprogrammed lysate (UP) shows the presence of non-specific protein-DNA complexes (*). Specific DNA-protein complexes are not competed by non-specific competitor (NS) and are masked by the addition of a molar excess of unlabeled probe (SC). (D) EMSA analysis using a CGTCTG core D-box. (E) EMSA analysis using a CATATG core Twist E-box.
The next probe that we employed in our analysis was a degenerate E-box that has been termed a D-box and contains a CG in place of the CA of the CANNTG motif, which can be transactivated by Hand1 in trophoblasts (14) (Fig. 1D). EMSA analysis shows no homodimer DNA binding of singly translated Twist1 or helix I mutant proteins; however, we were surprised to observe that E12 homodimers shift this degenerate sequence weakly (Fig. 1D). Lysates programmed with both Twist1 wild type and E12 proteins also shift the D-box sequence resulting in a protein-DNA complex that migrates at a slightly faster mobility than the E12 homodimer (Fig. 1D). The observation that Twist1 can bind this degenerate sequence known to bind Hand factors is reasonable given the evolutionary conservation within the Twist-family of factors. We were surprised to observe that lysates programmed with both Twist1T125;S127A and E12 were able to shift the D-box probe as efficiently as that of Twist1 wild type + E12. This suggests that hypophosphorylation of Twist1 does not effect DNA binding to this specific cis-element. Similar to what we observed using the Hand E-box probe, lysates programmed with Twist1T125;S127D and E12 fail to show any significant DNA-binding to the D-box probe.
Finally, we tested an CATATG E-box sequence that was previously identified as binding drosophila Twist both as a homo and a heterodimer (6). Although drosophila Twist has been shown to bind this sequence as a homodimer (6), lysates programmed for mouse Twist1 and Twist1 helix I mutants fail to show any observable homodimer DNA/protein mobility shifts using this single cis-element as an EMSA probe (Fig. 1E). Similar to what we observed using the D-box, lysates programmed with E12 shift this E-box weakly. Once again, lysates programmed with both Twist1 and E12 shift the probe strongly (Fig. 1E); in contrast, neither the hypophosphorylation or phosphorylation mimic of Twist1 shifts this probe when co-translated with E12. Of note, translated Hand2 as well as Hand2 co-translated Twist1 failed to shift any of the employed cis-elements; however, Hand2 co-translated with E12 shifted all 3 cis-elements (data not shown; see Fig. 2)). Together these data show that in addition to alterations in dimerization affinities, Twist1 phosphoregulation can influence DNA binding in a cis-element dependent manner.
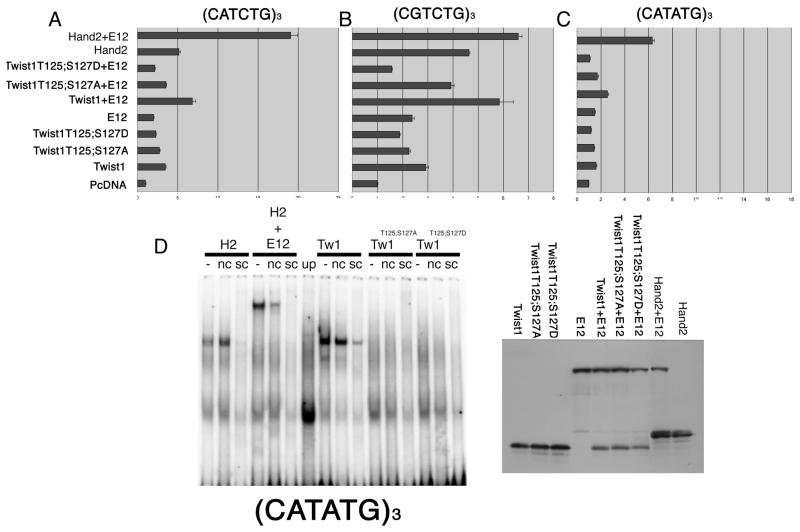
Figure 2.
Luciferase analysis of Twist1 monomers on the CATCTG Hand E-box. (A) Transactivation of an SV40 minimal promoter driven by 3-CATCTG cis-elements (Hand E-box). Relative light units (RLU) are shown and reflect the ratio of firefly: renillia luciferase activity. (B) Transactivation of an SV40 minimal promoter driven by 3-CGTCTG cis-elements (D-box). (C) Transactivation of an SV40 minimal promoter driven by 3-CATATG cis-elements (Twist E-box). All experiments were preformed at least 6 times and error bars denote standard error. (E) EMSA using a (CATATG)3 probe showing Hand2 and Twist1 homodimer binding. Protein loading is shown by immunoblot.
In addition to DNA-binding, we employed luciferase analysis using a luciferase reporter driven by 3-copies of the above-mentioned cis-elements to determine if transcriptional activity in vitro is affected (Fig. 2A). Transactivation of the CATCTG E-box shows that Twist1 increases luciferase activity approximately 3-fold, whereas, Twist1 T125;S127A and D show diminished transactivation. Transactivation of the CATCTG E-box with E12 alone shows a 2.5 fold increase in luciferase activity (Fig. 2A). Consistent with EMSA results, co-expression of Twist1 + E12 results in a 6 to 7-fold increase in luciferase activity. Twist1T125;S127A, which can bind to this sequence when co-expressed with E12 in EMSA (albeit with less affinity than wild type Twist1) transactivates less efficiently than wild type (Fig 2A). Co-expression of Twist1T125;S127D + E12 shows levels of transactivation that are nearly identical to that observed when Twist1T125;S127D is expressed alone. (Fig 1C & 2). Expression of Hand2 alone and when coexpressed with E12 also shows activation of the multimerized cis-element-driven reporter.
Transactivation of the CGTCTG D-box shows Twist1 increases luciferase activity 2.5-fold. Although the alanine point mutant shows similar transactivation, the aspartic acid mutant exhibits less transcriptional activity (Fig 2B). When coexpressed with E12, there is no difference in transcriptional activity between Twist1 + E12 and Twist1T125;S127A + E12 consistent with the equivalent DNA binding affinities shown previously (Fig 1D & 2B). Co-expression of Twist1T125;S127D + E12 does not enhance the transactivation of the D-box, reflecting the observed poor DNA-binding in the EMSA analysis (Fig 1D &2B). Although Hand2 DNA-binding was not detectable on the single D-box cis-element, significant activity is observed when Hand2 is expressed with the multimerized D-box reporter. This transcriptional activation is further increased when Hand2 is coexpressed with E12 correlating with its ability to bind the single D-box probe in EMSA (data not shown).
Transactivation of the drosophila Twist CATATG E-box was the weakest of the tested cis-elements; nevertheless, the luciferase activity correlates with the observed DNA binding (Fig. 2C). Expression of the Twist1 and the Twist1 phosphorylation mutants resulted in 1.5 fold or less transactivation, which is not considered significant. Similar findings are observed when expressing Hand2. Although Twist1 + E12 showed a modest 2.5-fold increase in luciferase activity, neither of the Twist1 phosphorylation mutants resulted in any significant transactivation, directly correlating with the observed DNA binding with this cis-element in our EMSA analysis (Fig 1E & 2C). Hand2 expressed with E12 exhibits the strongest transactivation of this reporter construct (Fig. 2) Taken together, these data show that alterations in the charge on helix I can affect both dimerization affinities (3) and DNA-binding affinities for Twist1 in a cis-element dependent manner. These differences are reflected by the transcriptional activation of these cis-elements in tissue culture.
Given that drosophila Twist is shown to bind as a homodimer (6) and our observed transcriptional activity shown above with Twist1 (Fig. 2), we were puzzled as to why the homodimers were not binding in our EMSA analysis. It was pointed out in review that the transcriptional assays were performed on multimerized cis-elements; where as, the EMSA analysis employed a single copy of the cis-element. Therefore, we repeated our EMSA analysis employing the same multimerized cis-element sequences used in the transcriptional assays. To our surprise, the EMSA analysis performed using the multimerized E-boxes increased DNA-binding affinities revealing homodimer binding (Fig. 2D). The use of the (CATATG)3 probe shows that both Hand2 and Twist1 can each specifically bind as homodimer complexes that are faster in mobility then Hand2 + E12 which was used as a control. Interestingly, neither of the Twist1 helix I point mutants show a detectable shift of this E-box (Fig. 2D). As Twist1 and Twist1 phosphorylation mutants are shown to form homodimers (3), we conclude that decreased DNA-binding affinity of T125;S127A and D is observed. Western blot shows that equal protein levels were employed. Similar results are observed for the (CATCTG)3 and (CATCTG)3 probes (data not shown). These results support previous findings from drosophila (6) and suggest that DNA binding is enhanced by multiple cis-elements within a localized region. These data also confirm that the transcriptional activation of the employed reporter clones is likely the result of the protein dimers directly binding the DNA within the cells. Therefore, to determine the affects (if any) of changing protein dimerization choice and DNA binding affinities of Twist1 in vivo, we made use of the Prx-limb promoter (20) to ectopically express Twist1 wild type and phosphoregulation mutants in F0 transgenic mice.
In vivo expression of Twist1, hypophosporylated Twist1T125;S127A and phosphorylation-mimic Twist1T125;S127D helix 1 mutants alter normal limb development in distinct ways
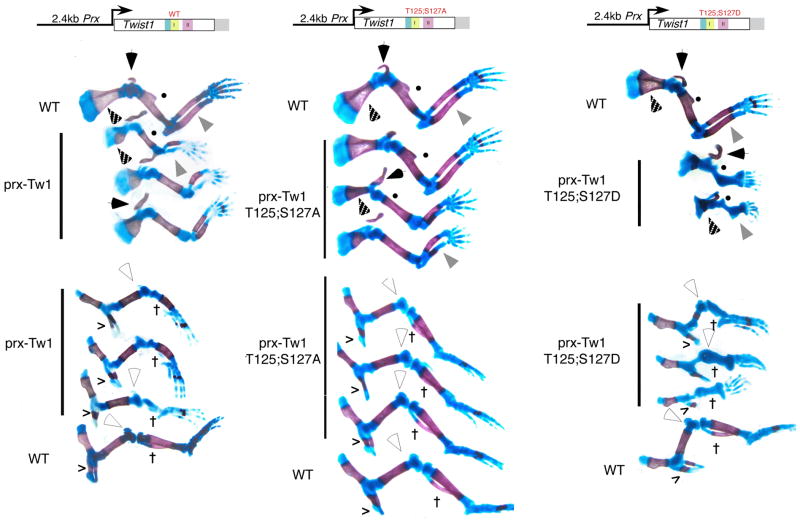
To look at the effects of modulating Twist1 phosphoregulation on limb patterning, we made use of the Prx limb promoter to express various forms of Twist1 in the developing limb (Fig. 1) (20). The Prx limb enhancer is ideal for the ectopic expression of factors in the developing limbs as this enhancer shows robust Prx-lacZ limb expression throughout both fore and hind limbs at E10.5, well prior to any observable limb patterning and matches well with the observed Twist1 expression (3,20). F0 embryos were collected at E17.5 as limb development is largely completed by this stage. Results show that Twist1 wildtype, T125;S127A, and T125;S127D expression produces distinct limb phenotypes (Fig. 3). Ectopic expression of wild type Twist1 causes an overall reduction in fore limb size, but has no effect on digit number. The clavicles of these mice (black arrowhead) are misshapen. Both the radius and ulna are reduced in size and the ulna in particular shows a reduction in ossification. It also appears that the size of the ulna is disproportionate to the radius as bending of the radius in 2 out of 3 F0 embryos suggests growth rates are not matched. The humerus of Prx-Twist1 wildtype transgenics is smaller compared to wild type littermates and lacks the deltoid tuberosity (Fig 3. closed circle). Ossification within the scapula is reduced in Prx-Twist1 transgenics and the superior border of the scapula is absent/reduced (Fig. 3 dotted arrowhead).
Figure 3.
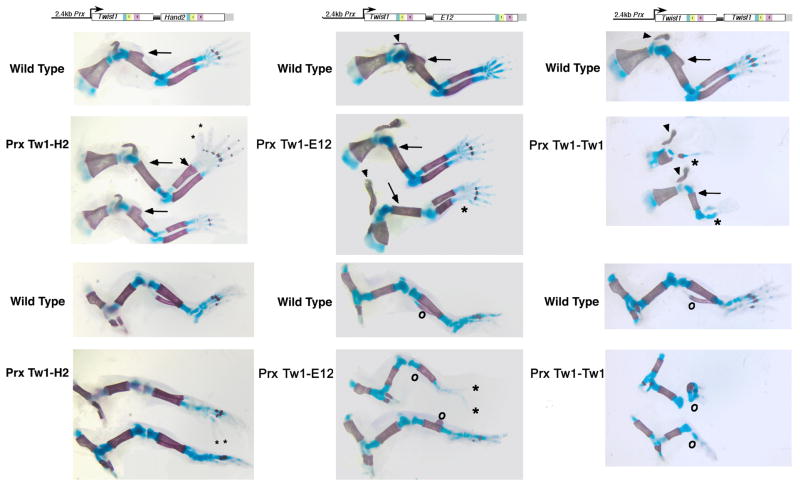
Expression of Twist1 and phosphoregulatory mutants in mouse limbs. Monomer-Prx constructs were used to generate E17.5 F0 embryos. Embryos were stained with Alcian blue and Aiizarine S as described (17) from 1 wild type and 3-transgenic positive littermates. Limbs were dissected away for detailed analysis. Filled arrowhead, clavicle; filled circle, deltoid tuberosity; gray arrowhead, ulna; dotted arrowhead, scapula; open arrowhead, patella; cross, fibula; v, iliac bone.
Hind limb defects are also observed in Prx-Twist1 mice. Consistent with the fore limb phenotypes, hind limb digit numbers are normal; however, a loss of the fibula (Fig. 3 †) and a reduction in both the ossification and size of the tibia are evident (Fig. 3). Defects in the cartilage of the knee joint results in a semicircular bending of the hind limb and the size of the patella is greatly reduced (Fig. 3 open arrowhead). Femurs although shorter, appear phenotypically normal as do iliac (hip) bones, which display normal amounts of ossification (Fig. 3). Thus ectopic Twist1 expression does not affect digit number or distal limb development but does alter more medial regions of the limb in contrast to the phenotypes observed with Prx-Hand2 expression (21).
We previously identified a number of Twist1 SCS mutations that compromise T125;S127 phosphoregulation and that hypophosporylated Twist1 fails to antagonize Hand2 function resulting in polydactyly (3). To look at the effect of ectopically expressing this haploinsufficient SCS allele, Prx-Twist1T125;S127A transgenic E17.5 F0 embryos were generated and compared to wild type and Prx-Twist1 mutant mice (Fig. 3). Prx-Twist1T125;S127A phenotypes are similar to those observed in Prx-Twist1 forelimbs; however, they are clearly less severe. Prx-Twist1T125;S127A clavicles (black arrowhead) are misshapen in all three E17.5 day embryos; however, the sizes of the radius and ulna are closer to that of wild type mice. Similar to Prx-Twist1 forelimbs, 2 out of 3 Prx-Twist1T125;S127A ulnas show a reduction in ossification (Fig. 3). The humerus of the Prx-Twist1T125;S127A embryos is also closer in size to that of the non-transgenic embryo; however, the loss of the deltoid tuberosity (Fig 3. closed circle) in 2 of the 3 forelimbs is evident. Less severe hind limb defects are also observed in Prx-Twist1T125;S127A embryos as seen by a reduced limb size and loss of fibula in 2 of 3 examples (Fig. 3). These findings suggest that in addition to the inability of the Twist1 helix I mutation to antagonize Hand2 in limb development (3), Twist1 helix I hypophosphorylation F0 embryos exhibit a reduced gain-of-function phenotype when compared to the expression of Twist1 wild type F0 transgenic embryos further supporting the notion that hypophosporylated helix I functionally mimics Twist1 SCS alleles.
As the Prx-Twist1 transgenic embryos had more pronounced limb defects than a Twist1 SCS associated mutation, we were curious as to the effects of ectopically expressing a Twist1 helix I phosphorylation mimic on limb phenotype. Consistent with our predictions, Prx-Twist1T125;S127D F0 embryos exhibit the most severe limb defects (Fig. 3). Fore limbs are greatly reduced in size and show global reductions in ossification, but in all cases fore and hind limb digit numbers are normal. The F0 embryos exhibit a loss of the ulna (or fusion of the ulna with the radius) and the Prx-Twist1T125;S127D humerus is small and lacks sites of ossification. Interestingly, the deltoid tuberosity is visible on each humerus in contrast to the deltoid tuberosity of the Prx-Twist1T125;S127A and Prx-Twist1 F0 embryos (Fig 3. closed circle). In addition, the clavicles of Prx-Twist1T125;S127D embryos appear to be less severely effected than those of either the Prx-Twist or Prx-TwistT125;S127A F0 embryos (Fig. 3).
Considering the possible variation in expression from each F0 line generated, the resultant phenotypes are extremely consistent within each group of embryos. Moreover, the finding that hypophosporylated Twist1 results in the least severe limb defects correlates well with its association with the haploinsufficient human disease SCS as a TWIST1 null allele. Taken together, these results suggest that the differences in DNA-binding and transcriptional activity observed for Twist1, Twist1T125;S127A and D are in fact reflected by observable differences in limb phenotypes in vivo. What is still unclear is which Twist1 bHLH dimer partners are being employed to generate the different observed phenotypes? In order to gain a better insight into this question, we employed a tethered-dimer approach.
Twist1 tethered dimer complexes exhibit DNA binding and transcriptional activity
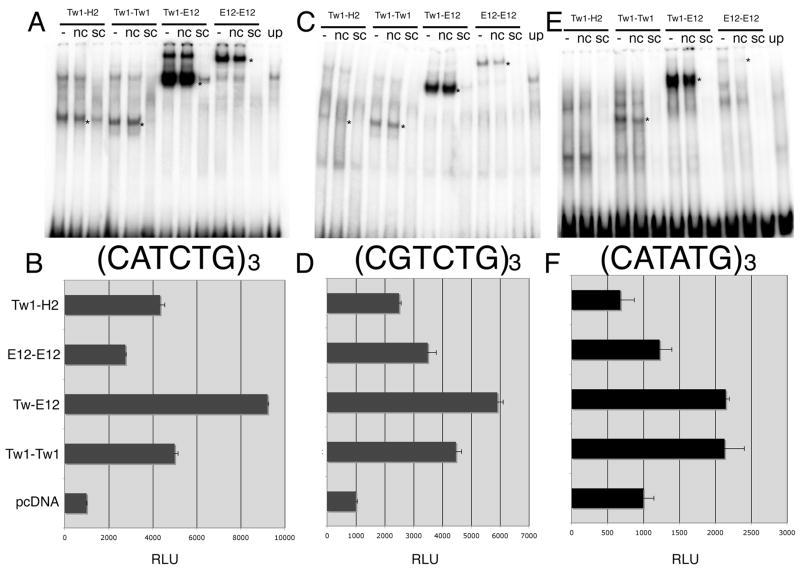
Our first goal in testing the Twist1 tethered molecules was to determine the DNA binding affinities for the previously employed cis-elements. EMSA analysis was performed using tethered Twist1-Twist1 homodimers, Twist1-E12 heterodimers, E12-E12 homodimers, and Twist1-Hand2 heterodimers using the multimerized cis-element probes (Fig. 4A–F). In addition to the DNA binding analysis, the transcriptional activity of the Twist tethered proteins was assessed using the same E-box and D-box luciferase constructs that we employed to test the Twist1 and Twist1 phosphorylation mutant monomers (Fig. 4D–F). Examining the DNA binding and transcriptional activity on the employed cis-elements, we find that Twist1-Hand2 tethered heterodimers, which did not bind to the monomeric cis-element sequence (data not shown), can bind to the (CATCTG)3 and (CGTCTG)3 probe, but not to the (CATATG)3 probe (Fig. 4A, C, E). Transcriptional activity in HEK293 cells correlates with the EMSA findings where Twist1-Hand2 transactivates the CATCTG and CGTCTG sequences but not the CATATG cis-element (Fig. 4A–F). Also consistent with data obtained from singly expressed proteins both, Twist1-Twist1 and Twist1-E12 tethered complexes bind to each cis-element in EMSA analysis (Fig. 4A–F). Transactivation of the cis-elements show that the Twist1-E12 tether exhibits the highest level of transactivation directly correlating with the levels of activity observed when the proteins were individually expressed (Fig. 2, 4D–F). Together this data shows that Twist1 homodimer, Twist1-E12 and Twist1-Hand2 heterodimer complexes both bind DNA and transactivate the employed cis-element targets, we conclude that these artificial transcriptional complexes are functional and we next set out to test these tethered clones in vivo.
Figure 4.
EMSA and luciferase analysis of Twist1 tethered dimers on the (CATCTG)3 E-box. (A) EMSA showing specific DNA-protein interactions of Twist1-Hand2 heterodimers (Tw1-H2), Twist1 homodimers (Tw1-Tw1), Twist1-E12 heterodimers (Tw1-E12), and E12-E12 homodimers. Unprogrammed lysate (UP). Specific complexes are indicated by *. (NS) non-specific competitor; (SC) molar excess of unlabeled probe. (B) Transactivation of an SV40 minimal promoter driven by 3-CATCTG cis-elements. RLU are shown and reflect the ratio of firefly: renillia luciferase activity. (C) EMSA of Twist1 tethered dimers on the (CGTCTG)3 D-box. (D) Transactivation of an SV40 minimal promoter driven by 3-CGTCTG cis-elements. (E) EMSA of Twist1 tethered dimers on the (CATATG)3 E-box. (F) Transactivation of an SV40 minimal promoter driven by 3-CATATG cis-elements. Error bars denote standard error.
Expression of Twist1 tethered dimers in the developing limb reflects unique roles for different Twist complexes
To gain a better understanding of how specific Twist1 dimers function in vivo, we generated Prx-Twist1-Hand2, Prx-Twist1-E12, and Prx-Twist1-Twist1 F0 E17.5 transgenic embryos to correlate with the phenotypes observed with the Prx-expressing Twist1 and Twist1 phosphoregulation mutant F0 transgenic embryos. When E17.5 Prx-Twist1-Hand2 F0 transgenic embryos were examined, we observed either no phenotype or preaxial polydactyly (Fig. 5 left). One of the transgenic embryos exhibits 2 extra digits on both fore and hind limbs. Interestingly, a thickening of the distal end of the radius is evident on the fore limbs (Fig. 5). The deltoid tuberosity is also absent from these fore limbs (Fig. 5 arrow). The loss of the deltoid tuberosity phenotype is not reported with Prx-Hand2 ectopic expression (21); however, it is observed with Prx-Twist1 expression (Fig. 3). Other than the polydactyly (a Hand2 overexpression phenotype), no other malformations were observed in the Prx-Twist1-Hand2 hind limbs. As polydactyly and loss of the deltoid tuberosity is observed, it suggests that the Twist1-Hand2 transcriptional complex is actively modulating transcription in vivo.
Figure 5.
Analysis of limb phenotypes resulting from Twist1 tethered dimer expression. The indicated tethered-dimers were used to generate E17.5 F0 embryos. 2-transgenic positive fore and hind limbs are shown for Tw1-H2 (left), Tw1-E12 (center), and Tw1-Tw1 (right). One wild type representative is shown followed by two transgenic embryos stained with Alcian blue and Aiizarine S. Black arrowheads denote clavicle; black arrow, deltoid tuberosity; small asterisk, polydactyly; large asterisk, ulna; open circle, fibula.
Analysis of the Prx-Twist1-E12 F0 transgenic embryos at E17.5 shows a similar phenotype to that observed with the expression of wildtype Twist1 and the milder phenotype observed using Twist1T125;S127A (Fig. 5 center). F0 E17.5 transgenic embryos show the loss of the deltoid tuberosity (Fig. 5 arrow) as well as a misshapen clavicle (Fig. 5 arrow head). In one of the two Prx-Twist1-E12 fore limbs, we also observe a shortening of the ulna similar to that observed with wild type Prx-Twist1 and the Prx-expression of the hypophosporylated Twist1 mutant shown in Figure 2. Hind limb phenotypes include the loss of the fibula (open circle) and the reduced ossification of the tibia. These phenotypes are most consistent with what we observed with the Prx-Twist1T125;S127A F0 embryos. Phenotypes distinct to the Prx-Twist-E12 expressing transgenic embryos are the presence of an ectopic outgrowth on the tibia that may be the remnant of the fibula (open circle) and a reduced number of digits (2 marked by *) in the hind limbs of one of the Prx-Twist-E12 transgenic embryos. Of note, this is the first defect in autopod development that we have observed using Prx-Twist expression constructs that does not involve the expression of Hand2.
The final dimer pair tested is the Prx-Twist1-Twist1 homodimer. Phenotypes observed with the E17.5 transgenic F0 embryos effect the distal limb outgrowth (Fig. 5 right). The fore limbs display normal scapula and clavicle development (arrowhead); however, the more extreme example (upper) exhibits a missing humorous and a single radius or ulna that extends to an autopod with a single digit and the ossification is greatly reduced. In a less extreme example (lower), the humerus is present but reduced in size and lacks a deltoid tuberosity (arrow). The radius and ulna lack ossification and the autopods are misshapen with oddly placed digits (Fig 5 right). Hind limb phenotypes include a severe reduction/absence of fibula and tibia along with misshapen autopods (Fig. 5 right). The iliac and femur appear relatively normal. These phenotypes correlate most closely to those observed with the Prx-Twist1T125;S127D expression (Fig. 3 & 5). Taken together, these data suggest that Twist1 phosphoregulation not only influences dimer choice but can also impact the DNA binding affinity to specific cis-elements thereby differentially affecting developmental transcriptional programs. Furthermore, by locking Twist1 into specific dimer complexes, the phenotypic outcomes resulting from the gain-of-function analysis are as distinct as those observed with the monomeric expression of Twist1 phosphoregulation mutants, suggesting that different Twist1 dimers play specific roles in limb development.
DISCUSSION
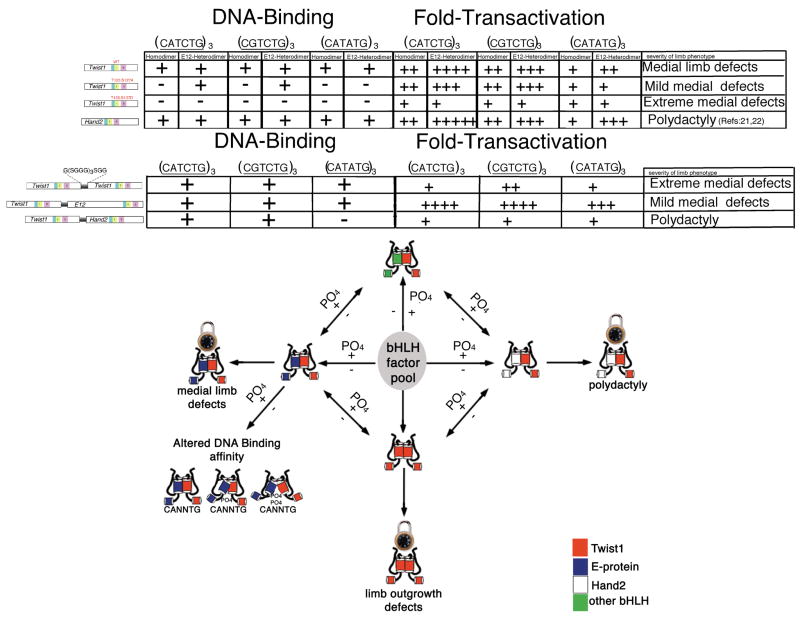
Helix I phosphoregulation of the Twist-family of bHLH proteins is a mechanism by which these factors can modulate their dimer partner choice (2,3). Here we show that in addition to dimer control, phosphoregulation also impacts DNA-binding preferences to E-box sequences and the impact appears to be cis-element dependent (Fig. 1& 2). Furthermore, ectopic limb expression of Twist1 and Twist1 phosphoregulation mutants produce unique phenotypes confirming that helix I phosphoregulation can define Twist1 function in vivo (Fig. 3). As the evolutionarily conserved phosphoregulated residues reside at the boundary between the basic domain and helix I, this finding suggests that protein-protein contacts within helix I are strengthened or relaxed given the phosphorylation state and bHLH partner choice, which adds or subtracts flexibility (breathability) to the juxtaposition of the DNA binding basic domain (Fig. 6). This flexibility then mediates changes in the ability of the dimer to bind DNA in a cis-element specific manner and has the potential to change the affinity of a Twist1 dimer complex for one gene program to another or alternatively, to keep a functional Twist1 complex in an inactive state poised to mediate transcription upon the change in the phosphorylation of T125 and/or S127. Interestingly, we show that the employment of multimerized cis-elements as EMSA probes increases the sensitivity of the DNA binding which is necessary in order to visualize Twist1 and Hand2 homodimers bound to specific DNA sequences as well as Twist1-Hand2 tethered heterodimer. Although Hand2 homodimers and Twist1-Hand2 heterodimers were shown to form (3) this is the first demonstration of DNA-binding for these complexes. The observed discrepancy in the monomer vs. multimer probe binding may reflect that multiple cis-elements facilitate cooperative DNA binding, explaining why multimerized cis-elements are proven more efficient in transactivation assays then promoters driven by a monomeric enhancer sequence.
Figure 6.
Data summary and proposed model for Twist-family phosphoregulation. Earlier studies show that Twist-family phosphoregulation of the evolutionarily conserved threonine and serine within helix I modulates changes in dimerization with various bHLH partners from the bHLH factor pool (2,3). In this study, EMSA data from Twist wildtype and mutant monomers coexpressed with E12 suggests that in addition to dimer control helix I phosphoregulation modulates DNA binding by perhaps regulating flexibility at the junction of helix I and the basic DNA-binding domain. Considering if variations in one or both helix I sites are phosphorylated in the case of Twist-family homo and heterodimers the “breathability“ of this junction will regulate protein-DNA interactions and switch protein-DNA affinity to different cis-element sequences. By locking Twist1 into specific dimer complexes via the use of a flexible linker, the ability to affect the bHLH factor pool is diminished and gain-of-function phenotypes result from direct transcriptional regulation and not from the titration of other bHLH factors. Distinct phenotypic outcomes suggest that different Twist1 dimers control specific aspects of limb morphogenesis.
Given the complexity of the Twist-family regulatory mechanism where both the levels of expression and the phosphorylation state of helix I effect dimer choice and DNA-binding, it is difficult to assess the functions for specific Twist1 transcriptional complexes; therefore, we employed a tethered dimer approach similar to that used for Twist and daughterless in drosophila (6). In the drosophila study Twist-Twist and Twist-daughterless tethered dimers were assessed in vivo showing that Twist homodimers specified mesoderm whereas Twist-Daughterless transcriptional complexes repressed gene expression required for somatic myogenesis (6). By forcing the dimer choice expressed in the developing limb via the use of tethered Twist1 transcriptional complexes, we observe distinct phenotypes that correlate well with the expression of wildtype and mutant Twist1 monomers (Fig. 6). Twist-E12 tethered dimers produce phenotypes similar to those observed with Twist1T125;S127A expression and Twist1-Twist1 phenotypes correlate to those observed with the expression of Twist1T125;S127D. Interestingly, Twist1-Hand2 tethered dimers in part phenocopy Prx-Hand2 expression (21) by inducing polydactyly suggesting that the mechanism for Hand2/Twist antagonism could be competition for 3rd party bHLH proteins as well as forming a biologically active heterodimer.
Twist1-Twist1, Twist1-E12, and Twist1-Hand2 tethered dimers express a single fused protein that binds DNA in a manner similar to co-expressed monomer proteins; moreover, tethered dimers transactivate E-box driven reporters in a manner similar to the monomerically expressed proteins in tissue culture (Fig. 6).
One could argue that when over expressing monomeric Twist1 (or any bHLH factor), we will affect the dimerization choices of the endogenous bHLH factors as well as affect the direct regulation of transcription via interaction (either directly or indirectly) with various gene promoters. Indeed, we believe that both of these mechanisms hold true when expressing monomeric Twist1 and Twist1 mutant proteins and that these mechanisms are reflected in SCS (3). Until this study, we were unsure if a Twist1-Hand2 dimer exhibited any direct biological function other than serving as a mechanism of mutual inhibition via sequestration from E-proteins. Our observation of direct DNA binding as well as preaxial polydactyly in Prx-Twist1-Hand2 tethered dimer mice suggests that indeed there is a direct transcriptional function for the Twist1-Hand2 dimer. Given that the tethered proteins will not disrupt the dimerization of endogenous bHLH proteins, the phenotypes that we observed here reflect only the direct alteration of gene expression by Twist1 tethered dimers. We feel that the phenotypic differences between the tethered Twist1 and monomeric Twist1 experiments reflect this important distinction in mechanisms.
When tethered Twist1-complexes are expressed ectopically in the limb via the Prx promoter, distinct phenotypes are observed that reflect the functional differences that unique Twist1 dimers exert on limb morphogenesis. As stated previously, Twist1-Hand2 expression results in polydactyly, a phenotype previously associated with ectopic Hand2 expression. (21,22). Ectopic Hand2 expression in the early limb induces the ectopic expression of Shh, which forms a secondary zone of polarizing activity (ZPA) resulting in extra digits (21,22). Twist1 limb expression (via the regulation of the repressor Gli3) represses Shh expression in the anterior of the limb bud (9) restricting ZPA expansion. Hand2 overexpression overrides this repression and results in Twist1 haploinsufficiency (which results in polydactyly) inadvertently allowing for an increased ratio of Hand2 to Twist1; moreover, Hand2 haploinsufficiency rescues the polydactyl phenotype seen with Twist1 haploinsufficiency (3,8). The finding that the Twist1-Hand2 tethered dimer can result in polydactyly suggests that the induction of Shh by Hand2 is dominant to that of the repression of Shh by Twist1 perhaps by Twist1-Hand2 directly regulating Shh and/or by reducing expression of the Shh repressor Gli3. Additionally, the loss of the deltoid tuberosity (a phenotype not observed in Hand2 ectopic expression (21)) suggests that this complex exhibits a Twist1-dominant function in regions proximal to the autopod.
Analysis of the transgenic mice expressing Twist1-E12 via the Prx promoter resulted in phenotypes that are most similar to Twist1T125;S127A (Fig. 3 & 5). This finding suggests that there is little effect on autopod development by this dimer; however, the medial bone phenotypes observed in the ulna, humerus and clavicle from Twist1 and Twist1T125;S127A expression are recapitulated from this tethered complex. Previous interaction data shows that in tissue culture the T125;S127A mutation has a lower dimerization affinity with E12 (3) and EMSA analysis suggests that the dimers that do form do not bind CATCTG or CATATG E-box sequences as robustly as wild-type Twist1 (Fig. 1 & 6). DNA binding and transcriptional activity for the D-box is indistinguishable from wildtype Twist1 which suggests that the Twist1-E12 complex might be less active on some population of downstream target genes than on others.
The final dimer tested is the Twist1-Twist1 homodimer. Analysis indicates that unique phenotypes to this complex are encountered and are similar to phenotypes observed with Twist1T125;S127D, such as gross reduction in limb outgrowth, diminished ossification, but relatively normal development of the autopod (Fig. 3 & 5). EMSA analysis indicates that the phosphorylation mimic does not bind to the three cis-elements effectively as a homodimer or when partnered with E12. Conversely the tethered homodimer does bind to the multimerized cis-elements indicating that phosphorylation at T125 and S127 may have a more profound effect on DNA-binding then on dimer stabilization. As Twist1 function is well-established to be critical for limb outgrowth via maintaining Fgf8/Fgf10 expression within the AER (10,23), the results observed with the Twist1 homodimer expression suggest that the function of Twist1 in maintaining the Fgf-mediated outgrowth of the AER is mediated via a Twist1 heterodimer and is perhaps antagonized by Twist1 homodimers given the reduction in limb outgrowth observed.
A remaining caveat to these studies is the inability to look at the levels of Twist1 and Twist1 tethered expression within the F0 embryos used for skeletal preps. Establishing stable mouse lines would allow for both phenotypic and molecular analysis, but given the severity of the limb defects encountered this is not a practical approach. Variation in the expression levels of any monomer will clearly affect phenotypic outcome by activating/repressing genes directly and by titrating out other bHLH factors by forming either active or inactive complexes. Expression levels of tethered Twist1 complexes should not affect the balance within the endogenous bHLH factor pool; however, their artificial nature may convey novel activities or repress a required function. Ultimately using a gene targeting strategy to express specific dimers from the Twist1 endogenous locus could resolve some (but not all) of these issues. This approach has been reported for the related factor Hand1 (24) and shows promise for deducing the role of Twist-family bHLH complexes in a variety of tissues during embryonic development.
Acknowledgments
The authors would like to thank Dr. James Martin (UT IBT Houston) for providing us with the Prx promoter plasmid used in this study. We would also like to thank Bill Carter and Shaoling Jing for generating the transgenic mice as well as Leanne Mcllreavey and Karen Dionne for technical assistance in the early stages of this work. We would also like to thank the Herman B Wells Center Cardiac Developmental Biology Group for helpful input during group discussions. Infrastructural support at the Herman B Wells Center is in part supported by the generosity of the Riley Children’s Foundation. This work is supported by the AHA and NIH (ABF) and NIH (SJC).
References
- 1.Firulli AB. Gene. 2003;312C:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 2.Firulli B, Howard MJ, McDaid JR, McIlreavey L, Dionne KM, Centonze V, Cserjesi P, Virshup DMa, Firulli AB. Mol Cell. 2003;12:1225–1237. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 3.Firulli BA, Krawchuk D, Centonze VE, Virshup DE, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Nat Genet. 2005;37(4):373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massari ME, Murre C. Molec Cell Biol. 2000;20(2):429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson EN, Klein WH. Genes Dev. 1994;8(1):1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Castanon I, Von Stetina S, Kass J, Baylies MK. Development. 2001;128(16):3145–3159. doi: 10.1242/dev.128.16.3145. [DOI] [PubMed] [Google Scholar]
- 7.Jabs EW. TWIST and Saethre-Chotzen Syndrome. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. Oxford University Press; New York: 2004. [Google Scholar]
- 8.Cai J, Jabs EW. BioEssays. 2005;27:1102–1106. doi: 10.1002/bies.20313. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke MP, Tam PP. International Journal of Developmental Biology. 2002;46(4):401–413. [PubMed] [Google Scholar]
- 10.Zuniga A, Quillet R, Perrin-Schmitt F, Zeller R. Mech Dev. 2002;114:51–59. doi: 10.1016/s0925-4773(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 11.Hornik C, Brand-Saberi B, Rudloff S, Christ B, Fuchtbauer EM. Anatomy & Embryology. 2004;209(1):31–39. doi: 10.1007/s00429-004-0412-3. [DOI] [PubMed] [Google Scholar]
- 12.Tavares AT, Izpisuja-Belmonte JC, Rodriguez-Leon J. International Journal of Developmental Biology. 2001;45(5–6):707–713. [PubMed] [Google Scholar]
- 13.te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Genes & Development. 2002;16(4):421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knofler M, Meinhardt G, Bauer S, Loregger T, Vasicek R, Bloor DJ, Kimber SJ, Husslein P. Biochemical Journal. 2002;361(Pt 3):641–651. doi: 10.1042/0264-6021:3610641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firulli BA, Hadzic DB, McDaid JR, Firulli AB. Journal of Biological Chemistry. 2000;275(43):33567–33573. doi: 10.1074/jbc.M005888200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firulli BADMV, Firulli AB. Biol Proced Online. 2004;6(1):16–22. doi: 10.1251/bpo69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickman ED, Rogers R, Conway SJ. Anatomical Record. 1999;255(3):353–361. doi: 10.1002/(SICI)1097-0185(19990701)255:3<353::AID-AR11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Mol Cell Biol. 1995;15(7):3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai YS, Cserjesi P. J Biol Chem. 2002;277:12604–12612. doi: 10.1074/jbc.M200283200. [DOI] [PubMed] [Google Scholar]
- 20.Martin JF, Olson EN. genesis. 2000;26:225–229. [PubMed] [Google Scholar]
- 21.McFadden DG, McAnally J, Richardson JA, Charite’ J, Olson EN. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- 23.Moon AM, Capecchi MR. Nature Genetics. 2000;26(4):455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu D, Scott IC, Geary C, Zhao X, Cross JC. Dev Biol. 2006;295:369. [Google Scholar]