SUMMARY
Between the second and third postnatal weeks in rats, the hippocampus exhibits a transient period of hyperexcitability. To systematically assess the relationship between the onset and offset of this period and spontaneous hippocampal activity, we used silicon depth electrodes in unanesthetized head-fixed rats from postnatal day [P]2 to P18. At all ages, hippocampal sharp waves (SPWs) were prominent in the EEG. Beginning at P6, however, marked changes in SPWs and associated oscillations were detected. SPW-related “gamma tails”(60–100 Hz) and “ripples” (140–200 Hz) were first observed at P6 and P7, respectively, and both oscillations persisted through P18. Transiently, between P6 and P11, SPW width decreased and the occurrence of SPW doublets increased. In addition, between P8 and P11, a subset of rats exhibited “potentiated SPWs”characterized by double polarity reversals, enhanced likelihood of gamma tails, and population spikes. Having identified a suite of transient hippocampal features consistent with a window of increased excitability, we predicted that electrographic seizure activity would be most easily induced during this period. To test this hypothesis, we infused kainic acid (KA; 200 ng/infusion) into the hippocampus contralateral to the recording probe. KA did not induce seizure activity until P7 and reached peak effectiveness at P9. Thereafter, sensitivity to KA declined. All together, these findings provide in vivo neurophysiological support for the notion of a developmental window of heightened seizure susceptibility during the second postnatal week, and also suggest that spontaneous hippocampal activity can be used to detect the onset and offset of this period.
Keywords: ripple, gamma, interictal, fast oscillations, giant depolarizing potentials, GABA
Between the second and third postnatal weeks in rats, the hippocampus exhibits a transient period of hyperexcitability during which it is particularly prone to seizure (Jensen et al. 1991; Swann 1995, 2004). Several mediating factors have been suggested: During this period, recurrent collateral branches abruptly reach levels greater than those seen in adulthood before gradually being refined (Gomez-Di Cesare et al. 1997), proteins that mediate electrical coupling between neurons are transiently overexpressed (Rozental et al. 2000; Vogt et al. 2005), and GABA-mediated inhibition has not yet emerged (Khazipov et al. 2004). Finally, during this period several types of glutamate receptors are overexpressed (Monyer et al. 1994; Ritter et al. 2002), and the concomitant increase in susceptibility to the epileptogenic effects of glutamate receptor agonists suggests a role for this transmitter in neonatal seizure susceptibility (Jensen 1999, 2002; Sanchez and Jensen 2001).
The excitability and synchrony of the adult hippocampus are reflected in the frequency and duration of hippocampal sharp waves (SPWs) (Buzsaki 1984, 1989). Accordingly, the transiently increased tendency of the neonatal hippocampus toward excitability and synchronization (Holmes and Ben-Ari 1998) should be reflected in the expression of SPWs, and perhaps also their associated high-frequency oscillations. Two recent studies have examined the development of SPWs in vivo. In the first study, Leinekugel et al. (2002) studied rats at postnatal day (P)3 through P6, and reported that SPWs constituted the predominant form of hippocampal activity. In the second study, Buhl and Buzsaki (2005), testing P12–20 rats, reported that the SPW-associated 140–200 Hz “ripple” (Buzsáki et al. 1992; O'Keefe and Nadel 1978; Suzuki and Smith 1988) does not emerge until P14. However, because these two studies did not examine subjects during the developmentally important second postnatal week, it remains unclear how age-dependent changes in hippocampal activity relate to the developmental onset and offset of the period of hyperexcitability. A direct examination of this issue is fundamental to any hypothesis relating the developmental emergence of spontaneous high-frequency activity (> 40 Hz) to the pathological high-frequency oscillations (≥ 60 Hz) that characterize seizure activity (Khalilov et al. 2003; Khalilov et al. 2005; Le Van Quyen et al. 2006).
We therefore examined hippocampal activity in vivo in P2–P18 rats. As expected, SPWs were prominent at all ages. Beginning at P6–7, however, marked changes in SPWs and their associated oscillations were detected, including the appearance of SPW-related “gamma tails” (60–100 Hz) and ripples (140–200 Hz). In addition, several transient SPW-related features, consistent with increased excitability, were expressed between P7 and P11, leading us to predict that electrographic seizure activity would be most easily induced during this period. To test this hypothesis, we administered successive infusions of kainic acid (KA; 200 ng/infusion) into the hippocampus contralateral to the recording probe. KA did not induce interictal activity or high-frequency oscillations before P7, even after 10 infusions. In contrast, by P9, only 1–3 infusions were needed to evoke seizure activity. Thereafter, KA’s efficacy declined. All together, these findings provide a definitive in vivo developmental link between spontaneous hippocampal activity and heightened seizure susceptibility.
EXPERIMENTAL PROCEDURES
All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and were approved by the Institutional Animal Care and Use Committee of the University of Iowa. All efforts were made to minimize the number of animals used.
Subjects
Forty-nine P2–18 male Sprague-Dawley Norway rats (Rattus norvegicus) from 36 litters were used. Some of the data from 5 P2–4 subjects were reported in a previous study (Karlsson et al. 2006). When littermates were used, they were always tested at different ages. Litters were culled to 8 pups on the third day after birth (day of birth = day 0). Mothers and their litters were housed in standard laboratory cages (48×20×26 cm) in the animal colony at the University of Iowa where food and water were available ad libitum. All subjects were maintained on a 12-h light/dark schedule with lights on at 07:00 h, and all tests were conducted between 12:00 and 17:00 h.
Surgery
Under isoflurane anesthesia, the subject’s skull was bleached, dried, and coated with Vetbond (3M, St. Paul, MN, USA) to add strength. Next, to secure the subject’s head during testing, a custom-built stainless steel apparatus (Karlsson et al. 2005), designed to attach to the earbar and nosebar holders of a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA), was attached to the skull over the pretreated area using cyanoacrylate adhesive gel. EMG electrodes (50 µm diameter; California Fine Wire, Grover Beach, CA, USA) were implanted into the subject’s right nuchal muscle to identify behavioral state (Karlsson and Blumberg 2002) as well as the left vastus lateralis muscle to help identify startles (see below). Finally, to inhibit movement and calm the subject, it was wrapped gently in gauze (Corner and Kwee 1976; Karlsson et al. 2005). The subject then recovered for 1 h in a humidified incubator maintained at thermoneutrality.
Procedure
General recording procedure
After recovery from surgery, the subject was transferred to the recording apparatus. First, the subject was secured into the stereotaxic apparatus, after which the skull was leveled in the horizontal plane. After a 1-h acclimation period, the recording electrode was inserted approximately 2 mm posterior to bregma and 1–2 mm lateral to midline. All waveforms were recorded during sleep, as indicated by nuchal muscle atonia (Karlsson et al. 2005); this was done to maintain consistency in terms of the behavioral conditions in which SPWs were recorded (it should be noted, however, that behavioral state did not appear to affect the characteristics of SPWs or SPW-associated events at any age). During all experiments, body and brain temperatures were maintained at approximately 35°C and 37°C, respectively.
Recordings were performed using 16-site linear silicon probes (100 µm vertical separation between recording electrodes; NeuroNexus Technologies, Ann Arbor, MI, USA) lowered into the dorsal CA1-dentate gyrus axis. An insulated silver wire (Medwire, Mount Vernon, NY, USA; 0.25 mm diameter), inserted into the cerebellum, served as both ground and reference electrode. Silicon probes were connected to a unity-gain headstage and digital amplifier (Tucker-Davis Technologies, Alachua, FL, USA) that amplified (×10,000) and filtered (1–5,000 Hz band-pass) the neural signals. A 60 Hz notch filter was applied during all recording sessions. EMG signals were amplified (×10,000) and filtered (300–5,000 Hz band-pass) using a differential amplifier (A–M Systems, Carlsborg, WA, USA). Neural and EMG signals were sampled at 12.5 kHz using a digital interface (Cambridge Electronic Design, Cambridge, UK) and recorded synchronously to hard disk for off-line analysis using Spike2 software (Cambridge Electronic Design). Two to 4 consecutive 15-min recordings were made for each subject, and the recording for each subject that was determined to have the greatest number of SPWs was selected for analysis.
Kainic acid (KA) infusions
Thirteen of the P5–10 rats, as well as 14 additional rats (from 10 litters), received intrahippocampal infusions of KA to assess seizure susceptibility across development. After 2 consecutive 15-min baseline recording periods, a 30-gauge needle attached to a 10 µl Hamilton syringe was lowered slowly into the contralateral CA1 pyramidale-radiatum region at approximately the same coordinates as the recording electrode. Next, 1 µl of KA (200 ng/µl; dissolved in sterile saline; MP Biomedicals, Solon, OH, USA; and Tocris Cookson, Ellisville, MO, USA) was manually infused (1 µl/10 s). After each infusion, hippocampal activity was continuously recorded for 10 min. Each subject received a maximum of 10 infusions.
Data Analysis
Detection of SPWs
SPWs were detected by filtering (30 Hz low-pass) the wide-band signals and then identifying 40–120 ms events with polarity reversals across stratum pyramidale; these events had positive peaks in stratum oriens and negative peaks in stratum radiatum (Buzsaki et al. 1983; O'Keefe and Nadel 1978; Suzuki and Smith 1987). Only events with peak negative amplitudes in stratum radiatum that were ≥ 3× the baseline activity were scored as SPWs (Karlsson and Blumberg 2004). Although not quantified in the present study, SPWs were often accompanied by an increase in multi-unit activity, as reported previously in neonates (Leinekugel et al. 2002). The rate of SPW occurrence was calculated for each subject as the total number of SPWs observed per minute, and average SPW amplitude was calculated as the mean negative amplitude of the waveform at 200 µm below stratum pyramidale (i.e., in stratum radiatum). For rate and amplitude data, linear regressions were performed and the associated r2 values and best-fit lines were obtained.
Current source density (CSD) analysis
Field patterns in all recorded channels were averaged using the identified negative peaks of SPWs. One-dimensional CSD analyses (Mitzdorf 1985; Nicholson and Freeman 1975) were performed and contour maps were generated using custom scripts written for Matlab 7.0 (MathWorks, Natick, MA, USA). Although some resistivity differences have been shown to be present in different layers of the hippocampus, these differences appear not to be large enough to significantly modify the spatial distribution of sinks and sources in CSD analyses (Holsheimer 1987). Our calculations, therefore, assume that the resistivity of the extracellular medium is similar at different depths. The anatomical layers corresponding to the vertical scale of the CSD maps were reconstructed with the aid of the histologically identified electrode tracks and marking lesions.
Relationship between SPWs and startles
Because recent studies have indicated a temporal link between SPWs and behavioral “startles” in the neonatal period (Karlsson and Blumberg 2003; Karlsson et al. 2006), this temporal relationship was also assessed in the present study. Startles are sudden, spontaneous, and simultaneous contractions of multiple muscle groups throughout the body and are particularly prominent in early infancy in rats (Gramsbergen et al. 1970). We have found previously that EMG activity recorded simultaneously from multiple muscle groups can reliably be used to reveal these events (Karlsson et al. 2006). For the present study in which EMG activity was recorded from each subject’s right nuchal and left vastus lateralis muscles, a startle was defined as the co-occurrence of EMG spikes (≥ 3× baseline) in both muscle groups with an inter-spike latency ≤ 70 ms. A startle was considered to co-occur with a SPW when it preceded the SPW by ≤ 225 ms (Karlsson et al. 2006).
SPW doublets and SPW width
A SPW doublet was defined as 2 complete SPWs occurring within a 400-ms time window (Bragin et al. 1995). Average SPW width at zero height was calculated for the recording site at 200 µm below stratum pyramidale. The total number of doublets per min and the average SPW width were calculated for each subject.
Identification of SPW-related high-frequency oscillations
Observed SPW-related waveforms in the LFP recordings included 140–200 Hz ripples (Buhl and Buzsaki 2005; Buzsáki et al. 1992; Ylinen et al. 1995) and 60–100 Hz gamma “tails” that closely followed SPW negative peaks (Bragin et al. 1995; Suzuki and Smith 1988; Traub et al. 1996). Analyses thus focused on activity within the ripple- and “fast gamma”-frequency ranges (i.e., using 140–200 and 60–100 Hz band-pass filters, respectively). Ripples were considered SPW-related if they occurred within ± 50 ms of the SPW negative peak; gamma activity was considered a gamma tail if it occurred within 100 ms of the SPW negative peak. In order for a filtered waveform to be selected for analysis, it was required to (i) exhibit an amplitude ≥ 2× baseline activity, (ii) exhibit 3 complete, continuous cycles, each with clear polarity reversals (180 ± 30°) across stratum pyramidale. and (iii) propagate at least 300 µm above (i.e., into stratum oriens) and below (i.e., into stratum radiatum) stratum pyramidale. Conditional probabilities of ripples and gamma tails given the occurrence of a SPW (i.e., p(x|SPW)) were calculated for each subject.
Identification of seizure activity
The onset of seizure activity was defined as the onset of the first interictal episode, identified by the presence of interictal spikes (IISs). IISs were easily identified on the basis of their shape and laminar profile (see Results for details). Although single IISs can occur in isolation, in our experiments their initial appearance was always characterized by a series of 10 or more spikes occurring at a rate >1 Hz, thus constituting what we defined as an interictal episode. In older subjects, these interictal episodes often transition directly into tonic and/or clonic discharges typical of adult seizure activity (Khalilov et al. 1999; Khazipov et al. 2004). Latency to exhibit seizure activity was thus quantified as the number of KA infusions administered before the appearance of IISs. CSD analyses of IISs were performed as with SPWs, triggered by peaks in stratum radiatum: Associated pathological high-frequency oscillations were first identified by eye in the local field potential (LFP; 1–5,000 Hz) traces and then quantified after band-pass filtering (60–200 Hz).
Histology
Following each experiment, small marking lesions were made at the deepest and shallowest recording sites using a brief application of 50–75 µA anodal current. The subject was then overdosed with an intraperitoneal injection of sodium pentobarbital and transcardially perfused with phosphate-buffered saline, followed by a 3% formalin solution. Brains were postfixed for at least 48 h in a formalin-sucrose solution before being sliced in the coronal plane (50 µm sections), mounted, and stained with cresyl violet. Light microscopy was then used to identify the electrode tracks (as well as needle tracks, where applicable) and marking lesions for each subject, and to reconstruct the anatomical locations of the recording sites.
RESULTS
Characterizing hippocampal SPWs between P2 and P18
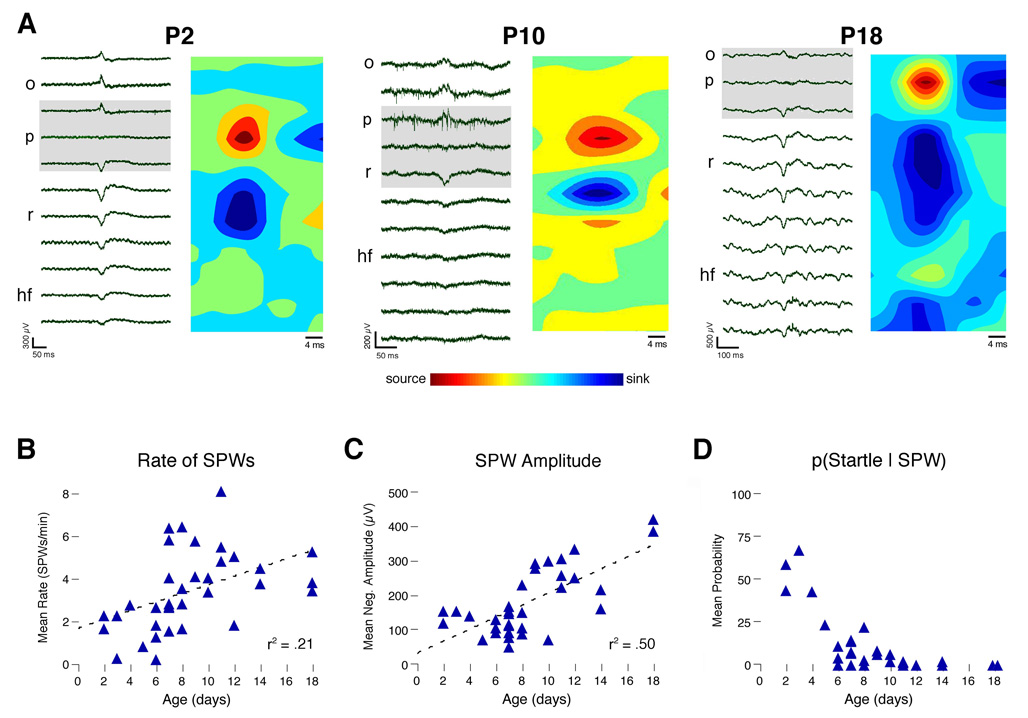
SPWs exhibited polarity reversals across stratum pyramidale, as documented by others (Leinekugel et al. 2002; Ylinen et al. 1995). Figure 1A illustrates these polarity reversals in representative P2, P10, and P18 subjects. CSD analyses revealed that, at all ages examined, neonatal SPWs exhibit an inward current (sink) in stratum radiatum and an outward current (source) in stratum pyramidale, similar to those that characterize SPWs in adults (Ylinen et al. 1995). As shown in Figures 1B and 1C, respectively, both the rate and average negative amplitude of SPWs increased with age (rate: r2 = .23, n = 35, p < .005; amplitude: r2 = .50, n = 35, p < .0001).
Figure 1. SPW Profiles Across the Early Postnatal Period.
(A) For representative subjects at P2, P10, and P18, depth profiles of single SPWs (left panels) and CSD plots (right panels) are presented. Signals were recorded using silicon electrodes (1–5000 Hz band-pass). CSD plots were constructed by averaging across all SPWs (n = 23–66 per subject). Outward currents (sources) and inward currents (sinks) are indicated by warm and cool colors, respectively. Note polarity reversals across stratum pyramidale (p) in wide-band traces (gray boxes) with corresponding sources in the CSD plots at each age; also note sinks in stratum radiatum (r) at all ages. (B) Average number of SPWs per min for each subject. The best-fit line and r2-value are indicated. (C) Average negative amplitude of SPWs, measured in stratum radiatum, for each subject. The best-fit line and r2-value are indicated. (D) For each subject, the conditional probability that a SPW was accompanied by a startle. o, stratum oriens; p, stratum pyramidale; r, stratum radiatum. hf, hippocampal fissure.
It was recently reported in P1–4 rats that SPWs are reliably preceded by behavioral “startles,” defined as phasic, simultaneous contractions of multiple skeletal muscles throughout the body (Karlsson et al. 2006). Here we found that this relationship diminished rapidly with age such that, by P9, startles persisted but now rarely co-occurred with SPWs (Figure 1D).
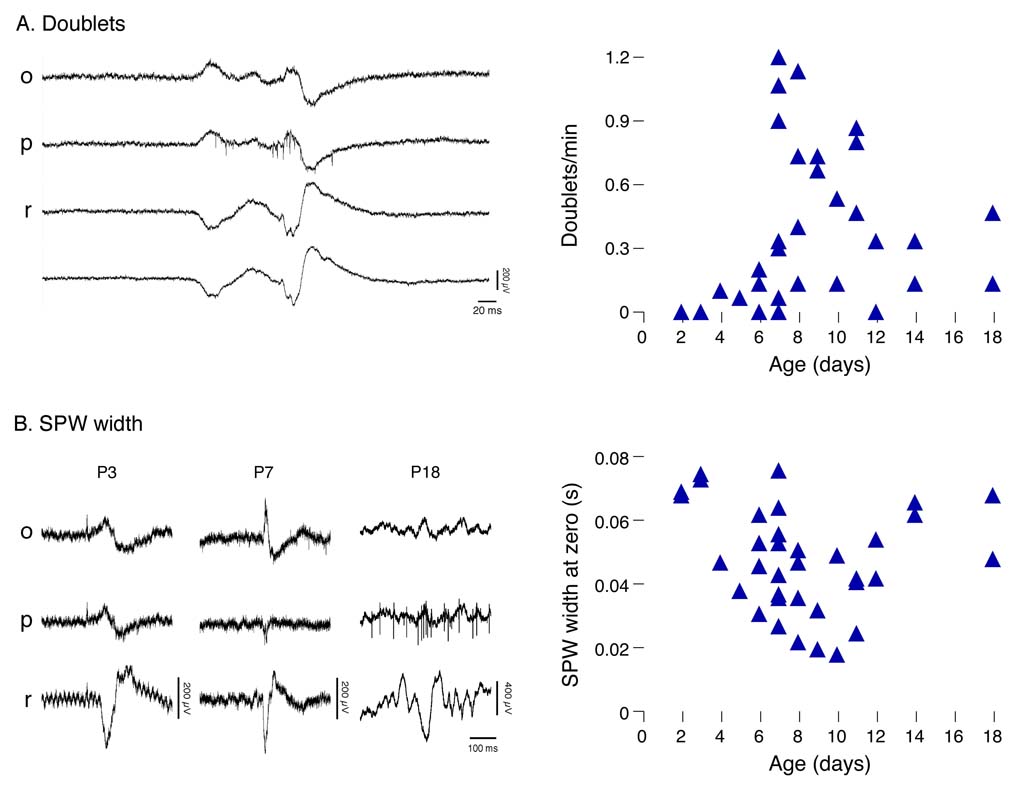
A representative SPW doublet from a P8 subject is shown in Figure 2A. Doublets were rarely observed before P6, increased abruptly between P7 and P10, and then declined after P11. Figure 2B illustrates the transient decrease in SPW width that occurred during the same developmental period.
Figure 2. Developmental Changes in the Occurrence of SPW doublets and SPW Width.
(A) Left panel: A representative SPW doublet in a P8 subject. Right panel: The number of SPW doublets per min for each subject. (B) Left panel: Representative examples of SPW width at P3, P7, and P18. Right panel: Average SPW width for each subject. Abbreviations as in Figure 1.
Developmental emergence of high-frequency oscillations in association with SPWs
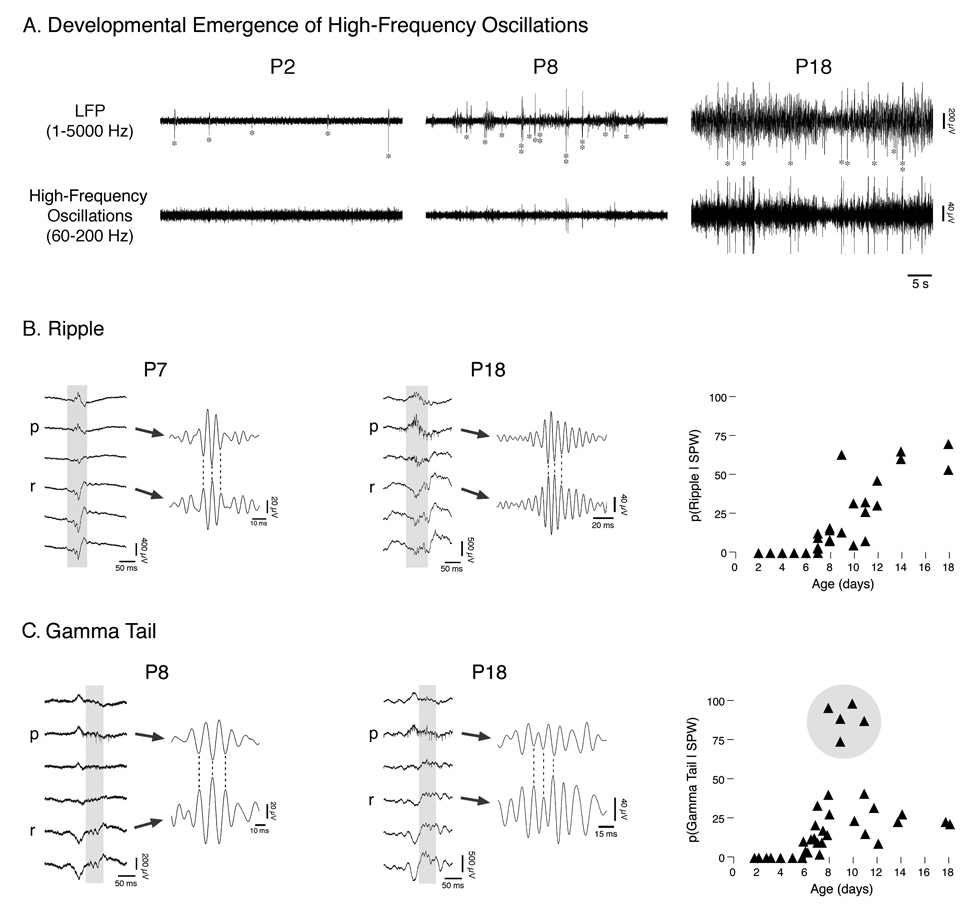
Figure 3A presents recordings from representative subjects at P2, P8, and P18. For each subject, LFP traces from stratum radiatum (top trace) are presented to indicate the occurrence of SPWs, as well as filtered data from stratum pyramidale (bottom trace; 60–200 Hz band-pass) to reveal associated bursts of high-frequency activity within the ripple- and gamma-frequency ranges. Although the P2 subject exhibited SPWs (indicated by asterisks in LFP trace), the lower trace shows that there was no associated high-frequency activity. By P8, SPWs were intermittently accompanied by low-amplitude high-frequency oscillations; note also the increased amount of non-SPW LFP activity. By P18, SPWs were more consistently associated with bursts of high-frequency activity, and high-frequency oscillations often occurred independently of SPWs. Moreover, at P18, the amplitude of both SPWs and high-frequency oscillations was greater, as was the baseline level of activity.
Figure 3. Developmental Emergence of SPW-Related High-Frequency Oscillations.
(A) Top trace: Local field potentials (LFPs, 1–5000 Hz band-pass) recorded from stratum radiatum in representative P2, P8, and P18 subjects. SPWs are indicated by asterisks and SPW doublets by vertical double asterisks. Bottom trace: Filtered (60–200 Hz band-pass) traces from stratum pyramidale to reveal developmental emergence of high-frequency oscillations. (B) Representative SPW-associated ripples in P7 and P18 subjects. At each age, LFPs are shown; gray boxes indicate regions presented at right after filtering (140–200 Hz band-pass). Plot at far right panel presents, for each subject, the average probability of detecting a ripple given a SPW. (C) Representative SPW-associated gamma tails in P8 and P18 subjects. At each age, LFPs are shown; gray boxes indicate regions presented at right after filtering (60–100 Hz band-pass). Plot at far right presents, for each subject, the average probability of detecting a gamma tail given a SPW. Five P8–11 subjects are highlighted based on their exhibiting pronounced increases in the probability of SPW-related gamma tails (see Figure 4 for details). Abbreviations as in Figure 1.
The high-frequency activity accompanying neonatal SPWs could be separated into two distinct waveforms: ripples and gamma tails. Ripple-frequency activity (i.e., 140–200 Hz) during SPWs was first observed at P7. The left panel of Figure 3B presents a laminar profile of a prominent P7 ripple. The gray box in the LFP traces indicates the location of the ripple, which is shown to the right in expanded and filtered (140–200 Hz) form. Note the polarity reversals in the filtered traces. The middle panel presents a typical P18 ripple; note the increased amplitude and number of constituent oscillations. The conditional probability of observing a ripple given the occurrence of a SPW for each subject is presented in the right panel of Figure 3B. This probability was initially low but increased steadily until P14 when it reached adult levels (Bragin et al. 1999a).
Gamma tail activity (i.e., 60–100 Hz) was first observed at P6. At earlier ages, the highest-frequency oscillation present in the hippocampal EEG was “slow gamma” activity (i.e., 20–30 Hz), which was never observed in association with SPWs (Karlsson et al. 2006). The left panel in Figure 3C presents a laminar profile of a representative gamma tail in a P8 subject. The gray box in the LFP traces indicates the location of the gamma tail, which is shown to the right in expanded and filtered (60–100 Hz) form. Note the polarity reversal of the gamma tail below stratum pyramidale, just as is the case for ripples.
The middle panel of Figure 3C illustrates gamma tail activity at P18. Similar to the developmental trend seen for ripples, the gamma tail at P18 has greater amplitude, as well as a greater number of constituent oscillations. The right panel presents the conditional probability of observing a gamma tail given the occurrence of a SPW. This probability was low at P6 and remained low across development, with the exception of the 5 subjects between P8 and P11 highlighted in the figure. Upon closer inspection, these 5 subjects exhibited a number of additional distinguishing characteristics, detailed in the next section.
“Potentiated SPWs”
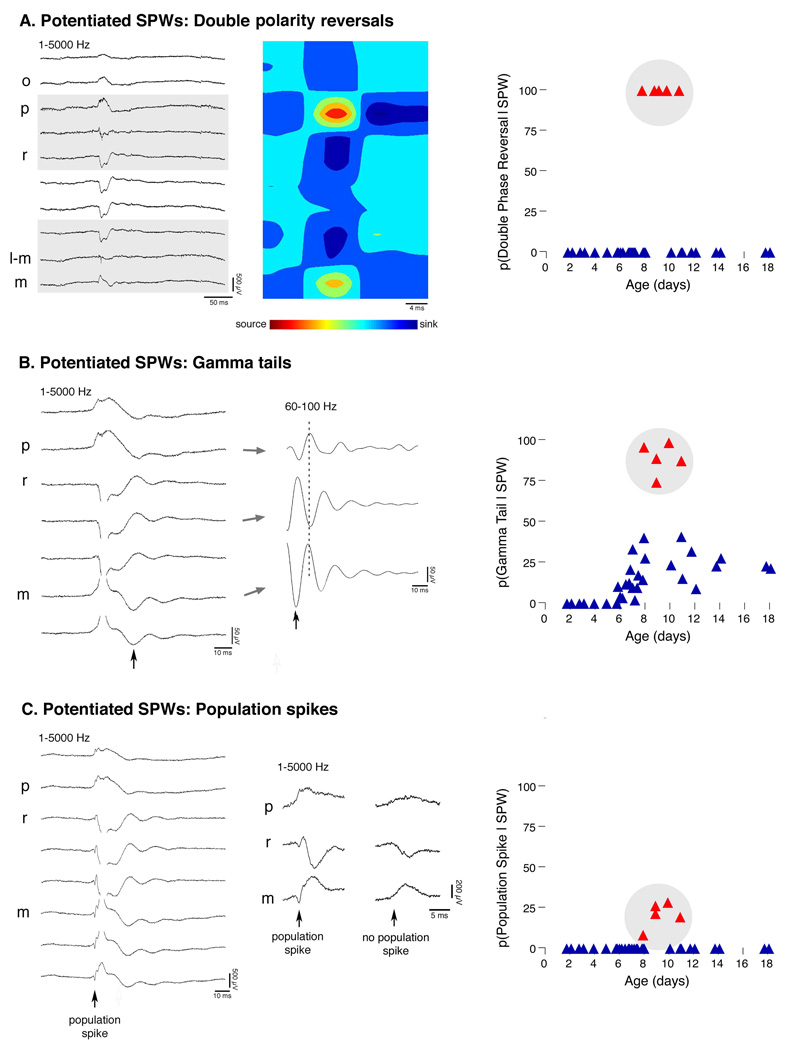
Closer examination of the 5 subjects (all from different litters) highlighted in Figure 3C revealed several distinguishing features. First, all SPWs in these subjects exhibited double polarity reversals: In addition to the typical polarity reversal observed at the pyramidale-radiatum border, there was a second polarity reversal above the hippocampal fissure, at the level of stratum lacunosum-moleculare (Figure 4A, left panel). Previous studies in adult rats have documented a shift toward positive polarity at this level (Buzsaki 1986; Buzsaki et al. 1983); however, the very prominent reversal (≥ 3× baseline) seen here appeared more similar to that observed in commisurally evoked SPWs in adults (Ylinen et al. 1995). CSD analyses revealed typical source-sink pairs at strata pyramidale and radiatum, but additionally revealed mirror sink-source pairs at the hippocampal fissure, corresponding to the second SPW polarity reversal at this level (Figure 4A, middle panel). SPW double polarity reversals were observed in every SPW for these 5 subjects, whereas they were not observed in any other subjects (Figure 4A, right panel).
Figure 4. “Potentiated SPWs”.
(A) Left panel: Local field potentials (LFPs; 1–5000 Hz band-pass) indicating a potentiated SPW (pSPW) in a P9 subject. The gray boxes highlight two polarity reversals: one across stratum pyramidale (p) and the other across stratum lacunosum-moleculare (l–m). Middle panel: CSD plot revealing two pairs of sources and sinks corresponding to the two polarity reversals in the LFPs. Right panel: For each subject, the average probability of observing a double polarity reversal given a SPW. (B) Left panel: LFPs for a P11 subject, illustrating a pSPW with associated gamma tail. To the right is a filtered (60–100 Hz band-pass) and expanded view of the same gamma tail. pSPW-related gamma tails, like pSPWs, always exhibited polarity reversals in two locations (highlighted by dashed line). Such gamma tail double polarity reversals were not observed in any other subjects. Right panel: For each subject, the average probability of observing a gamma tail given a SPW. (C) Left panel: LFPs for a P10 rat illustrating a pSPW with associated population spike (arrow). To the right is an expanded view of this population spike as well as a pSPW from the same subject that did not exhibit a population spike. The population spike appears to originate in stratum pyramidale (p), as indicated by its polarity reversal across this layer. Right panel: For each subject, the average probability of observing a population spike given a SPW. m, stratum moleculare; all other abbreviations as in Figure 1.
A second distinguishing factor, as previously indicated in Figure 3C, was the unusually high proportion of SPWs with gamma tails. An average of 89% of SPWs in these 5 subjects exhibited gamma tails (Figure 4B, right panel). These gamma tails also exhibited double polarity reversals: one below stratum pyramidale of CA1, and a second between strata moleculare and granulosum of the dentate gyrus (Figure 4B, left and middle panels) (Buzsaki et al. 1983; Csicsvari et al. 2003). Thus, while gamma tails and SPWs both reversed in polarity across stratum pyramidale, the second polarity reversal of gamma tails was approximately 100 µm lower than the second polarity reversal of SPWs. All gamma tails in these 5 subjects exhibited double polarity reversals (≥ 2× baseline), whereas gamma tail double polarity reversals were never observed in the other subjects.
Finally, SPWs in these 5 subjects occasionally contained a population spike during their initial deflection from baseline (Figure 4C, left and middle panels). As described in adults, population spikes are very brief (< 5 ms), sharp, field potential spikes that result from the near-simultaneous discharge of a homogeneous cell population (Andersen et al. 1971). Here, such events were scored as population spikes when they exhibited negative amplitudes ≥ 3× baseline in stratum radiatum (200 µm below stratum pyramidale). Population spikes always reversed in polarity near stratum pyramidale, suggesting that they were generated in the perisomatic region. The percentage of SPWs with population spikes was low in these 5 subjects (average = 20.4 ± 3.5), but their appearance is noteworthy because population spikes were never observed in other subjects (Figure 4C, right panel).
Because double polarity reversals, gamma tails, and population spikes are properties often associated with evoked (and hence, potentiated) SPW responses in CA1 (Kloosterman et al. 2004; Leung et al. 1995; Penttonen et al. 1998; Traub et al. 1996; Ylinen et al. 1995), we hereafter refer to endogenous SPWs possessing these characteristics as potentiated SPWs (pSPWs).
Age-dependent effects of repeated intrahippocampal kainic acid infusions in vivo
We hypothesized that the developmental onset and refinement of SPW-related patterns indicative of increased excitability and synchrony would be mirrored by changes in seizure susceptibility. Therefore, in a subset of the subjects just described, and in 14 additional subjects, we repeatedly infused kainic acid (KA) into the hippocampus contralateral to the recording probe while continuously monitoring electrographic activity.
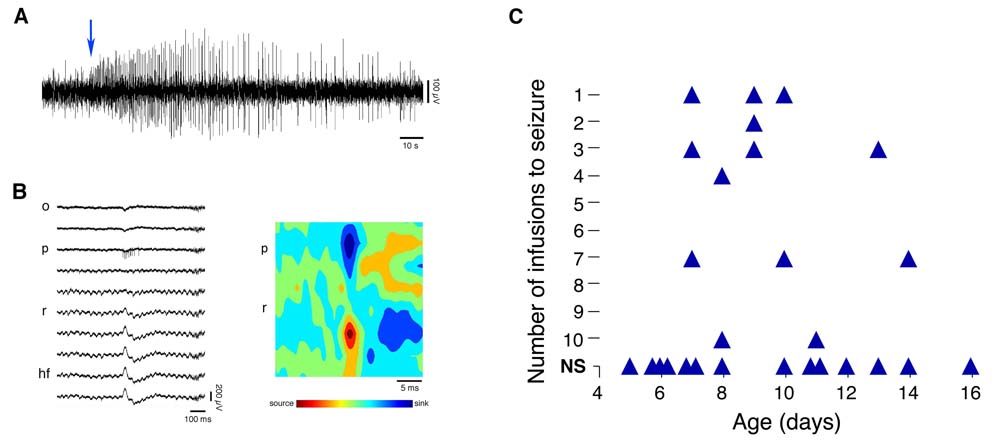
During the pre-infusion period, all subjects exhibited typical EEG activity, with normal SPWs characterized by polarity reversals across stratum pyramidale. In contrast, after KA infusion, the onset of seizure activity was characterized by the appearance of regularly recurring interictal spikes (IISs; Figure 5A) (Khalilov et al. 1999). Because the transition to seizure-like episodes was always characterized by rapid IISs, rather than an increase in the appearance of any particular oscillation, the transition was more akin to “hypersynchronous” seizure onset than to “low-voltage fast” seizure onset (Bragin et al. 2005; Bragin et al. 1999b; Velasco et al. 2000).
Figure 5. Hippocampal Activity Following Contralateral Kainic Acid Infusion.
(A) A recording illustrating the onset (indicated by arrow) of seizure activity following infusion of kainic acid (KA) in a P8 subject. (B) Left panel: representative depth profile (wide-band: 1–5000 Hz) of a type 2 IIS (and associated neuronal discharge in stratum pyramidale). Note the reversed polarity of this spike when compared to the SPWs in Figure 1A. Right panel: CSD plot derived by averaging across all IISs (n=19) comprising the interictal episode from which the IIS in the left panel was selected. Note the reversed orientation of the source and sink in comparison to the CSD profiles for the SPWs depicted in Figure 1A. (C) For each subject, the number of KA infusions required to evoke seizure activity. NS, no seizure activity detected after a maximum of 10 infusions. None of the P8–10 subjects infused with KA exhibited pSPWs. Abbreviations as in Figure 1.
In 11 of 18 subjects displaying seizure activity, IISs exhibited polarity reversals across stratum pyramidale of opposite polarity to those of SPWs, consistent with type 2 IISs (Figure 5B; (Buzsaki et al. 1991; Buzsaki et al. 1989; Leung 1990; Wadman et al. 1983). Their CSD plots indicated a source-sink pattern opposite to that exhibited by SPWs (Figure 5B; see Figure 1A for comparison). In contrast, the remaining 7 subjects displayed type 1 IISs, which have a shape and laminar profile that is very similar to that of SPWs but with duration <40 ms (Buzsaki et al. 1991; Buzsaki et al. 1989; Leung 1990; Wadman et al. 1983). The duration of type 1 IISs was typically 20–30 ms.
Using the criteria just described, we determined the number of infusions required to evoke seizure activity in each subject. The results of this analysis, presented in Figure 5C, indicate a developmental profile that mirrors those presented earlier for SPW doublets, SPW width, and pSPWs. Specifically, 200 ng/µl KA did not evoke seizure activity before P7, even after 10 infusions; indeed, before P7, no IISs or other EEG abnormalities were observed at any time during or after KA infusions. Seizure activity was first detected at P7. Sensitivity to KA infusions increased dramatically Between P7 and P10, and declined thereafter such that, in most subjects, even 10 KA infusions were no longer sufficient to evoke seizure activity.
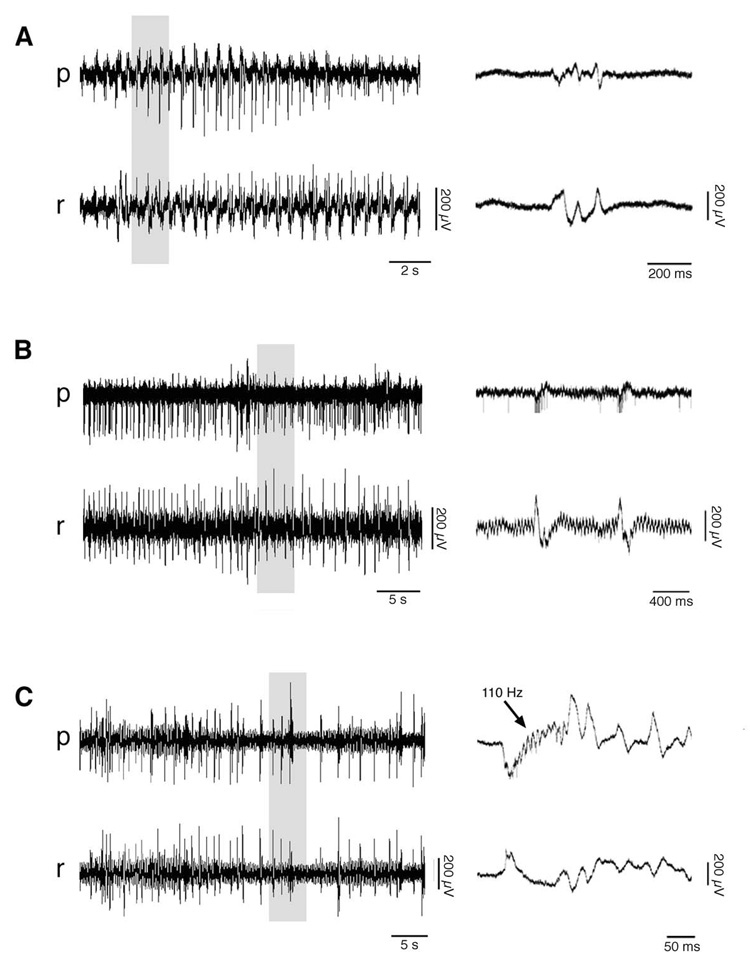
The characteristics of seizure activity also changed with age. At P7, 3 of 6 subjects exhibited seizure activity. In these 3 subjects, the activity consisted of type 2 IISs with superimposed high-frequency oscillations similar to those reported in adult rats after KA infusion (Bragin et al. 1999a; Bragin et al. 2004). After P7, these high-frequency oscillations were observed in every subject that exhibited IISs. In 1 of the 3 P7 subjects displaying seizure activity, rapid IISs were not followed by tonic or clonic components, as has been demonstrated in P2–3 rats in vitro (Khalilov et al. 1999). In the 2 other P7 subjects, the initial increase in IISs led to clonic-like multispike bursts (Figure 6A) (Bragin et al. 1999b).
Figure 6. Representative Hippocampal Seizure Activity Evoked by Kainic Acid Infusion.
Depicted are 3 general types of seizure activity displayed by subjects after kainic acid infusions into the contralateral hippocampus. In each case, local field potentials (1–5000 Hz band-pass) are presented at left from strata pyramidale (p) and radiatum (r), and gray boxes highlight portions magnified at right. (A) An episode in a P7 subject characterized by single type 2 IISs, transitioning into clonic-like bursts. (B) An episode in a P8 subject characterized only by the appearance of rapid type 2 IISs. This subject was unique in showing multiunit activity in stratum pyramidale synchronized with the IISs. Also note the continuous 30-Hz rhythm in stratum radiatum; beginning at P8, all subjects displayed this potentiated gamma activity after 1–2 infusions of kainic acid, regardless of whether they exhibited seizure activity. (C) This P8 subject exhibited a progression from single type 2 IISs with no high-frequency oscillations to single IISs with high-frequency oscillations, to IISs with high-frequency oscillations that were followed by a gamma/beta tail.
At P8, 2 of 3 subjects exhibited seizure activity. One subject displayed only rapid type 2 IISs without tonic or clonic components (Figure 6B). The other subject displayed a transition from type 2 IISs to a larger waveform characterized by 100–200 Hz bursts that were followed by a tail of activity in the slow gamma- (20–50 Hz) and beta-frequency (10–20 Hz) ranges (Figure 6C).
The seizure activity in 6 of 7 subjects at P9–10 consisted of type 1 IISs. Three of these subjects exhibited only an increase in the frequency of IISs, 1 subject exhibited rapid IISs which transitioned into clonic-like multispike bursts, and the remaining 3 subjects displayed highly synchronized tonic-clonic seizure activity, characterized by an initial interictal episode followed by alternating periods of 10–30 Hz “tonic” oscillations and clonic discharges, finally terminated by high-amplitude clonic bursts (Khalilov et al. 1999). After P10, when seizure activity occurred, it consisted of either type 1 or type 2 IISs, typically transitioning into tonic-clonic seizure activity.
DISCUSSION
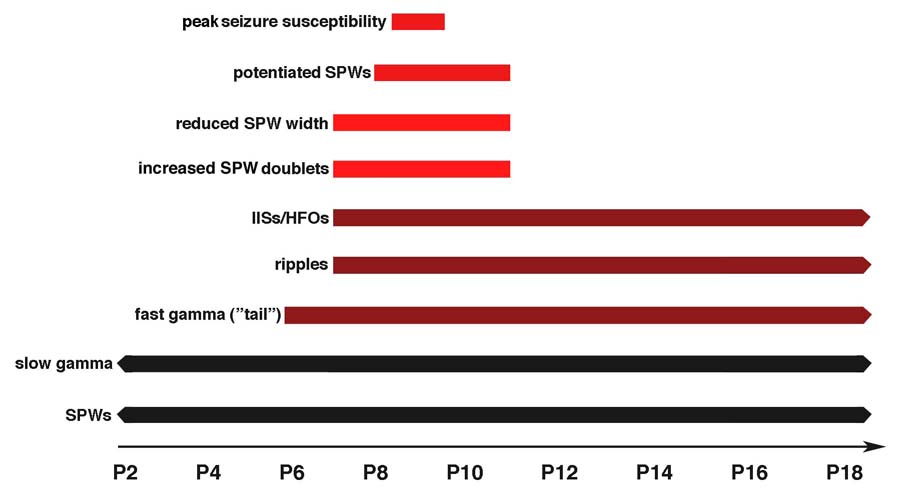
As summarized in Figure 7, this study documents the developmental onset of both persistent and transient physiological patterns in the CA1 field of the hippocampus. The persistent patterns, which include the onset of SPW-related gamma tails and ripples at P6–7, coincide with the developmental onset of KA-induced seizure activity. The transient patterns, which include decreases in SPW width, increased occurrence of SPW doublets, and the expression of pSPWs, mirror the period of increased seizure susceptibility, framing both its onset and offset. These results indicate that spontaneous hippocampal activity can be used to assess the state of excitability of the infant hippocampus.
Figure 7. Summary of Results.
Schematic presentation of the development of transient and persistent hippocampal events and oscillations based on previous research (black) and the present findings (red). SPWs and slow gamma oscillations (20–30 Hz) occurred at all ages examined. SPW-related fast gamma tails (60–100 Hz) and ripples (140–200 Hz) were first detected at P6 and P7, respectively. Interictal spikes (IISs) and associated pathological high-frequency oscillations (HFOs; 60–200 Hz) were first evoked by kainic acid infusion into the contralateral hippocampus at P7. The occurrence of SPW doublets increased and SPW width decreased transiently between P7 and P11. Potentiated SPWs were detected only in P8–11 subjects, although only in a subset of subjects at these ages. Finally, peak seizure susceptibility to kainic acid infusion, as indicated by the number of infusions required to evoke seizure activity, occurred at approximately P9.
SPW-related ripples and gamma tails
We detected ripples approximately 7 days earlier than was reported recently by Buhl and Buzsaki (2005). This inconsistency may be due to methodological differences between our two studies. In P12 and older rats, Buhl and Buzsaki (2005) used power spectral analyses to determine the age of ripple onset. This method, however, may not be adequate for detecting ripples at earlier ages when, as shown here, ripples occur with SPWs only sporadically and are expressed at low amplitude. Regardless, the present results resolve an outstanding paradox by bringing into alignment the onset of both pathological and physiological high-frequency activity (60–200 Hz) at approximately P7 (Le Van Quyen et al. 2006).
Although non-SPW-related gamma-frequency rhythms have been described in head-fixed and freely moving rats as early as P2–5 (Karlsson et al. 2006; Lahtinen et al. 2001), these bouts of gamma activity are of lower frequency (i.e., 20–60 Hz) than the SPW-related high-frequency (60–100 Hz) gamma tails that, as shown here, first appear at P6. The emergence of gamma tails at P6 may result from developmental changes in the glutamatergic network (Le Van Quyen et al. 2006). Indeed, because high-frequency gamma oscillations are dependent, in part, upon AMPA receptor-mediated excitation (Fisahn et al. 1998; Mann et al. 2005), it is noteworthy that the emergence of these oscillations at P6 is coincident with the associated up-regulation of AMPA receptors at this age (Sanchez and Jensen 2001). Although the gamma tail has been studied both in vivo and in vitro, its physiological significance remains unclear (Traub et al. 1996).
SPW width and doublets
Hippocampal SPWs occur during periods of reduced cortical and subcortical inhibition (Chrobak and Buzsaki 1994). It is for this reason that lesions of the entorhinal cortex enhance the rate of occurrence of SPWs and SPW doublets (Bragin et al. 1995), reflecting a state of increased excitability. In particular, SPW doublets are occaisonally observed when perforant path stimulation is timed to the occurrence of spontaneous SPWs (Buzsaki 1989). Doublets are also observed in rats with subcortical denervation of the hippocampus (Buzsaki et al. 1991).
SPW width in CA1 is an indicator of the degree of synchrony among CA3 pyramidal neurons (Buzsaki 1984, 1989). Because seizure itself is the product of neuronal network hypersynchrony, it is noteworthy that the period of smallest SPW width was coincident with the period of greatest seizure susceptibility reported here.
“Potentiated SPWs”
We observed a subset of subjects between P8 and P11 that exhibited SPWs with double polarity reversals, gamma tails, and population spikes. Because these are characteristics often associated with evoked (and hence, potentiated) SPW responses in adults (Kloosterman et al. 2004; Leung et al. 1995; Penttonen et al. 1998; Ylinen et al. 1995), we refer to them here as potentiated SPWs (pSPWs).
The probability of observing high-frequency gamma, in the form of SPW gamma tails, was highest in those subjects exhibiting pSPWs. It may be significant that AMPA receptors are necessary for the expression of high-frequency gamma oscillations (Mann et al. 2005), and that these receptors exhibit their highest level of expression around P10 (Sanchez and Jensen 2001). Moreover, population spikes are most closely associated with highly synchronized hippocampal activity, such as occurs with electrical stimulation of the perforant path and in epileptic adults (Andersen et al. 1971; Buzsaki et al. 1991; Buzsaki et al. 1989; Leung et al. 1995). Thus, pSPWs appear to possess characteristics indicative of increased hippocampal excitability.
pSPWs were only identified in P8–11 subjects, but not all subjects at these ages exhibited pSPWs. It remains unclear how pSPWs fit into the typical developmental trajectory of the hippocampus. It is possible that all infants exhibit pSPWs but differ in the age, duration, and/or probability of their expression. Alternatively, it is possible that only some infants exhibit pSPWs. If the latter is true, then early developmental experience, perhaps involving established effects of maternal care on hippocampal development (Meaney 2001), may determine which infants will express pSPWs. To address these unresolved questions, it will be necessary to study multiple litters and littermates, with and without cross-fostering, during the P8–11 period.
Because we did not examine the effects of KA infusion in all of the initial subjects tested, and because the probability of testing a subject with pSPWs was relatively low, we were not able to determine whether the expression of pSPWs, independent of age, is associated with a greater sensitivity to KA. It is clear, however, that expression of pSPWs is not necessary for rapid induction of seizure activity after KA infusion. Clearly, more work is needed to assess the physiological significance of pSPWs for hyperexcitability and seizure susceptibility.
Kainic acid infusion as an in vivo test of hyperexcitability
Having identified the onset of a suite of transient and persistent hippocampal features that together suggest a window of increased excitability, we predicted that seizure activity would be most readily induced during this period. Using multiple infusions of KA contralateral to the recording probe, we found that the fewest number of infusions required to produce electrographic seizure activity occurred at approximately P9, with decreased efficacy at younger and older ages. The earliest age of seizure induction was P7, coinciding with the developmental onset of SPW-related ripples, as well as decreases in SPW width and increases in SPW doublets.
The method we developed for inducing seizure activity in vivo is based on an in vitro method for induction of epileptogenic mirror foci (Khalilov et al. 1999; Khalilov et al. 2003; Khalilov et al. 2005). This method utilizes the early postnatal development of hippocampal commissural projections (Buchhalter et al. 1990) to induce seizures via contralateral KA infusion. Thus, it appears that the onset of seizure activity at P7 reported here is not attributable to delayed maturation of the hippocampal commissures. Moreover, although our findings appear inconsistent with the reported observation of IISs as early as P2 using the in vitro method (Khalilov et al. 1999), they are consistent with the finding that epileptogenic high-frequency oscillations (> 60 Hz) are not observed until approximately P7 using that same method (Khalilov et al. 2005).
Although ipsilateral KA infusions may have been preferable to the contralateral infusions used here, space limitations (i.e., the size of the electrode and syringe in relation to the surface of the infant skull) precluded this approach. It is perhaps for this reason that “fast ripples” (i.e., 250–600 Hz waveforms), which reliably indicate KA-induced epileptogenesis in adult rats, were not detectable here: such oscillations seem only to be produced after ipsilateral infusions (Bragin et al. 1999b; Bragin et al. 2002). Thus, to determine whether the infant hippocampus is capable of generating oscillations within this higher frequency range, ipsilateral infusions would have to be used.
A variety of experimental models have been developed to examine the infantile period of heightened seizure susceptibility, and all are consistent with the results reported here. Specifically, P10–12 marks the period of peak seizure susceptibility when pups are exposed to hypoxia (Jensen et al. 1991), P10–11 marks the peak seizure response to fever (Baram et al. 1997), and P11 marks the peak response to high potassium in vitro (Khazipov et al. 2004). These peaks also correspond to the period in which the cation-chloride co-transporter, KCC2, becomes fully upregulated (Rivera et al. 1999) and GABA then begins its transition from excitatory to inhibitory neurotransmission (Khazipov et al. 2004).
Conclusion
It is becoming increasingly clear that hippocampal activity in infant rats is characterized by a series of SPW-related phenomena that wax and wane during the early postnatal period—beginning with the startle-SPW relationship at birth (Karlsson et al. 2006), its decline around P6 as high-frequency oscillations appear in conjunction with SPWs, and the subsequent partial dissociation of high-frequency activity from SPWs during the third postnatal week. That the expression of pSPWs and other transient features of SPW activity coincide with the up-regulation of KCC2 suggests a mechanistic connection between the two. Specifically, if the “GABA switch” is mediated by an activity-dependent mechanism (Fiumelli and Woodin 2007; Ganguly et al. 2001), then perhaps the increased activity associated with hyperexcitability promotes a self-limiting process by which the infant transitions to a non-hyperexcitable state.
ACKNOWLEDGMENTS
Supported by National Institute of Mental Health grants MH50701 and MH66424 (M.S.B). We thank Gonzalo Viana di Prisco, Cynthia Shaw, and Adele Seelke for technical assistance.
REFERENCES
- Andersen P, Bliss TVP, Skrede KK. Unit analysis of hippocampal population spikes. Experimental Brain Research. 1971;13:208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Research Developmental Brain Research. 1997;98:265–270. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J., Jr Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and Entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic Brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999a;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999b;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, van Landeghem M, Buzsaki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. Journal of Neurophysiology. 1995;73:1691–1705. doi: 10.1152/jn.1995.73.4.1691. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. Journal of Neuroscience. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Buchhalter JR, Fieles A, Dichter MA. Hippocampal commissural connections in the neonatal rat. Brain Research Developmental Brain Research. 1990;56:211–216. doi: 10.1016/0165-3806(90)90084-c. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Buzsaki G. Developmental emergence of hippocampal fast-field "ripple" oscillations in the behaving rat pups. Neuroscience. 2005;134:1423–1430. doi: 10.1016/j.neuroscience.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: Their origin and significance. Brain Research. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Long-term changes of hippocampal sharp-waves following high frequency afferent activation. Brain Research. 1984;300:179–182. doi: 10.1016/0006-8993(84)91356-8. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for "noisy" brain States. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network Oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Hsu M, Slamka C, Gage FH, Horvath Z. Emergence and propagation of interictal spikes in the subcortically denervated hippocampus. Hippocampus. 1991;1:163–180. doi: 10.1002/hipo.450010205. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Research Reviews. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Ponomareff GL, Bayardo F, Ruiz R, Gage FH. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience. 1989;28:527–538. doi: 10.1016/0306-4522(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Selective activation of deep layer (V–VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. Journal of Neuroscience. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner MA, Kwee P. Cyclic EEG and motility patterns during sleep in restrained infant rats. Electroencephalography and Clinical Neurophysiology. 1976;41:64–72. doi: 10.1016/0013-4694(76)90215-7. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal Chloride homeostasis in development. Current Opinion in Neurobiology. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gomez-Di Cesare CM, Smith KL, Rice FL, Swann JW. Axonal remodeling during postnatal maturation of CA3 hippocampal pyramidal neurons. Journal of Comparative Neurology. 1997;384:165–180. [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of Behavioral states in the rat. Developmental Psychobiology. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Ben-Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron. 1998;21:1231–1234. doi: 10.1016/s0896-6273(00)80642-x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Electrical conductivity of the hippocampal CA1 layers and application to current-source-density analysis. Experimental Brain Research. 1987;67:402–410. doi: 10.1007/BF00248560. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Acute and chronic effects of seizures in the developing brain: experimental models. Epilepsia. 1999;40 Suppl 1:S51–S58. doi: 10.1111/j.1528-1157.1999.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. International Journal of Developmental Neuroscience. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Applegate CD, Holtzman D, Belin TR, Burchfiel JL. Epileptogenic effect of hypoxia in the immature rodent brain. Annals of Neurology. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Hippocampal theta in the newborn rat is revealed under conditions that promote REM sleep. Journal of Neuroscience. 2003;23:1114–1118. doi: 10.1523/JNEUROSCI.23-04-01114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Temperature-induced reciprocal activation of infant hippocampal field activity. Journal of Neurophysiology. 2004;91:583–588. doi: 10.1152/jn.00953.2003. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. The union of the state: Myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behavioral Neuroscience. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Mohns EJ, Viana Di Prisco G, Blumberg MS. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus. 2006;16:959–965. doi: 10.1002/hipo.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Dzhala V, Medina I, Leinekugel X, Melyan Z, Lamsa K, Khazipov R, Ben-Ari Y. Maturation of kainate-induced epileptiform activities in interconnected intact neonatal limbic structures in vitro. European Journal of Neuroscience. 1999;11:3468–3480. doi: 10.1046/j.1460-9568.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nature Neuroscience. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48:787–796. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. European Journal of Neuroscience. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, van Haeften T, Lopes da Silva FH. Two reentrant pathways in the hippocampal-entorhinal system. Hippocampus. 2004;14:1026–1039. doi: 10.1002/hipo.20022. [DOI] [PubMed] [Google Scholar]
- Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T. Postnatal development of rat hippocampal gamma rhythm in vivo. Journal of Neurophysiology. 2001;88:1469–1474. doi: 10.1152/jn.2002.88.3.1469. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Khalilov I, Ben-Ari Y. The dark side of high-frequency oscillations in the developing brain. Trends in Neurosciences. 2006;29:419–427. doi: 10.1016/j.tins.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G. Correlated bursts of activity in neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Leung LS, Roth L, Canning KJ. Entorhinal inputs to hippocampal CA1 and dentate gyrus in the rat: a current-source-density study. Journal of Neurophysiology. 1995;73:2392–2403. doi: 10.1152/jn.1995.73.6.2392. [DOI] [PubMed] [Google Scholar]
- Leung LW. Spontaneous hippocampal interictal spikes following local kindling: time-course of change and relation to behavioral seizures. Brain Research. 1990;513:308–314. doi: 10.1016/0006-8993(90)90472-n. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiological Reviews. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Freeman JA. Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. Journal of Neurophysiology. 1975;38:356–368. doi: 10.1152/jn.1975.38.2.356. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. European Journal of Neuroscience. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Vazquez DM, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor subunit expression in the rat hippocampus. Brain Research Developmental Brain Research. 2002;139:227–236. doi: 10.1016/s0165-3806(02)00572-2. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rozental R, Srinivas M, Gokhan S, Urban M, Dermietzel R, Kessler JA, Spray DC, Mehler MF. Temporal expression of neuronal connexins during hippocampal ontogeny. Brain Research Brain Research Reviews. 2000;32:57–71. doi: 10.1016/s0165-0173(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Jensen FE. Maturational aspects of epilepsy mechanisms and consequences for the immature brain. Epilepsia. 2001;42:577–585. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. I. Behavioral correlates, laminar profiles and bilateral synchrony. Electroencephalography and Clinical Neurophysiology. 1987;67:348–359. doi: 10.1016/0013-4694(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. II. Relations to synchronous burst discharges. Electroencephalography and Clinical Neurophysiology. 1988;69:532–540. doi: 10.1016/0013-4694(88)90165-4. [DOI] [PubMed] [Google Scholar]
- Swann JW. Synaptogenesis and epileptogenesis in developing cortical networks. In: Schwartzkroin PA, Moshe SL, Noebels JL, Swann JW, editors. Brain Development and Epilepsy. New York: Oxford University Press; 1995. pp. 195–233. [Google Scholar]
- Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal of Physiology. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J., Jr Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plasticity. 2000;7:49–63. doi: 10.1155/NP.2000.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Research Molecular Brain Research. 2005;141:113–120. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wadman WJ, Da Silva FH, Leung LW. Two types of interictal transients of reversed polarity in rat hippocampus during kindling. Electroencephalography and Clinical Neurophysiology. 1983;55:314–319. doi: 10.1016/0013-4694(83)90209-2. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. Journal of Neuroscience. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]