Abstract
Background
Carboxylesterases (CE) are ubiquitous enzymes responsible for the hydrolysis of numerous clinically useful drugs. Since ester moieties are frequently included in molecules to improve their water solubility and bioavailability, de facto they become substrates for CEs.
Objective
In this review, we will describe the properties of human CEs with regard to their ability to activate anticancer prodrugs and demonstrate how structure-based design can be used to modulate substrate specificity and increase efficiency of hydrolysis.
Methods
A specific example using CPT-11 and a human liver carboxylesterase will be discussed. However, these techniques can be applied to other enzymes and their associated prodrugs.
Results
Structure-guided mutagenesis of CEs can be employed to alter substrate specificity and generate novel enzymes that are efficacious at anticancer prodrug activation.
Keywords: Carboxylesterase, CPT-11, enzyme/prodrug therapy, mutagenesis, rationale enzyme design
INTRODUCTION
Carboxylesterases (CE) are a class of esterases that are ubiquitously expressed from bacteria to man. As their name implies these proteins cleave carboxyl esters into the corresponding alcohol and carboxylic acid, however, these enzymes can also hydrolyze thioesters and carbamates [1-3]. Due to this diverse substrate specificity, these proteins are frequently referred to as ‘promiscuous’ enzymes [2]. The exact function of CEs is unknown since no endogenous substrate has been definitively identified for these proteins. In general, it is thought that CEs play a protective role, detoxifying xenobiotics by cleaving these compounds to less toxic hydrolysis products [4]. This is supported in part by their pattern of expression in higher organisms, where they tend to be found in tissues that are likely to be exposed to these agents (e.g. liver, kidney small intestine, lung epithelia, etc). Interestingly, in mammals, the levels of CE expression in the blood are lower in higher genera. Indeed, humans essentially lack this activity in this fluid [5]. The exact reason for the loss of this potentially protective enzyme is unclear. Further support that these proteins may play a protective role is provided by plasma esterase-deficient mice that are viable, healthy and demonstrate a normal lifespan, despite lacking the circulating CE, Es1 [6-8]. These results suggest that if indeed CEs can metabolize endogenous compounds, it is not their primary function, and that alternative enzymes must exist that also contribute to this activity.
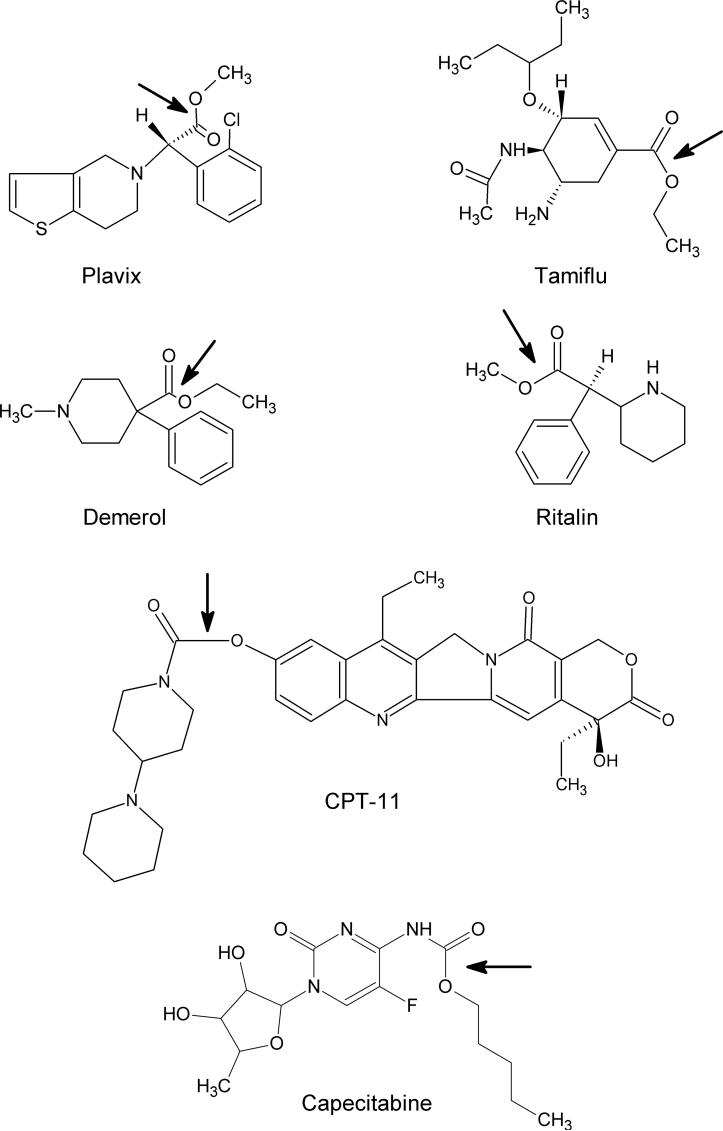
Numerous compounds contain functions that can be hydrolyzed by CEs. This includes the illegal recreational drugs heroin and cocaine [9-12], the anticancer agents capecitabine and CPT-11 (Figure 1 [13-16]), the pyrethroid class of pesticides [17-19], as well as a whole host of widely used therapeutic molecules [20-23]. Pharmaceutical companies frequently add methyl or ethyl groups to candidate drug molecules via an ester linkage to improve water solubility and/or bioavailability. As a consequence, frequently prescribed medicines such as Plavix, Tamiflu, Demerol, and Ritalin (Figure 1) all contain ester moieties and, correspondingly, are metabolized by CEs in vivo [20, 21, 23, 24]. Therefore, understanding the substrate specificity, location of expression, and potentially modulation of activity of these proteins, should allow targeting of the active drug to desired tissues and perhaps more effective therapy.
Figure 1.
Structures of clinically used agents that are subject to hydrolysis by CEs. The arrow indicates the bonds that are hydrolyzed by these enzymes.
1. Properties of Carboxylesterase
1.1 Mechanism of substrate hydrolysis
Esters can be cleaved either by a base- or acid-catalyzed mechanism. Esterases have evolved to use a base-catalyzed approach in a multi-step reaction that uses water as an intermediate nucleophile [1]. An essential catalytic triad of amino acids (Ser, His and Glu) are arranged such that a proton transfer chain can be established. This results in the formation of a serine Oγ− atom that undergoes a nucleophilic attack of the carbonyl carbon atom of the ester. The resulting tetrahedral intermediate then collapses with the loss of the alcohol and the generation of a serine ester. Water attacks this ester to yield a diol that then under goes dehydration to produce the carboxylic acid. The serine is then free to undergo another round of substrate hydrolysis [1].
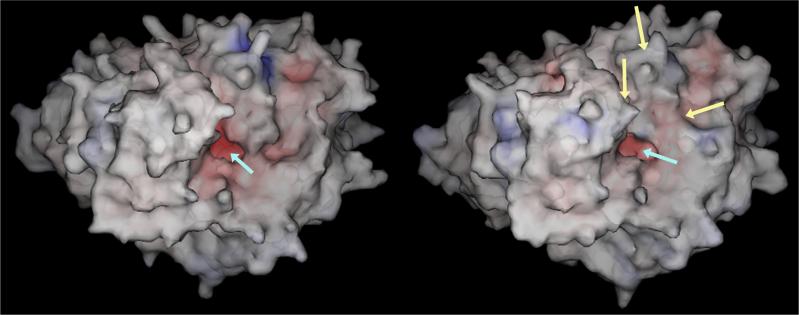
While this is elegant chemistry, as the serine nucleophile is likely to be highly reactive, this reaction must be accomplished in almost anhydrous conditions. Clearly this is not compatible with the ∼55M water concentration that is present in organisms and hence CEs have evolved to undertake these reactions at the bottom of long, deep, hydrophobic gorges that project 25−35Å into the center of the proteins (Figure 2 [1, 25-27]). The placement of the catalytic amino acids in such a position is somewhat counterintuitive for an enzyme that may have to hydrolyze a whole host of different xenobiotics that presumably, would vary in size and shape. It could be argued that to efficiently hydrolyze molecules of different complexity, the catalytic resides should be present on the surface of the enzyme such that any steric constraints would be minimized. However, this would be incompatible with the hydrolysis mechanism. Therefore, as demonstrated by the remarkable animations produced by Richard Gillilan and colleagues at the Cornell Theory Center (see the latter half of the animation using acetylcholinesterase as the hydrolytic enzyme [28]), an electrostatic gradient is established that directs the substrate molecule into the active site and forces it towards the catalytic amino acids. This mechanism allows for the efficient transport and subsequent hydrolysis of esterified molecules. However, as might be anticipated from this arrangement, the substrate specificity of CEs will be determined by a variety of parameters. Firstly, to generate the hydrophobic environment within the CE active site gorge, amino acids that line this domain tend to be aromatic in nature (Phe, Tyr, Trp, His; [1, 25-27]). As a consequence, molecules demonstrating a higher logP (octanol/water partition coefficient), i.e., those that are more hydrophobic in nature, are likely to be better substrates. Conversely, molecules demonstrating lower logP values might be expected to be poorer substrates. Secondly, larger, more bulky compounds may be physically too large to fit within the active site gorge and hence not subject to hydrolysis. Thirdly, the position of the ester chemotype within the molecule will dictate efficiency of cleavage. For example, if the carbonyl carbon atom is present at the center of a large scaffold where nucleophilic attack is hindered, such a compound would not be expected to be cleaved by these enzymes. Using these observations, the ideal substrate can be proposed for CE hydrolysis. This would include small, aromatic, unhindered esters containing an efficient leaving group e.g. nitrophenyl acetate (o-NPA). Indeed, molecules of this sort are frequently used as biochemical substrates for monitoring CE activity [29, 30].
Figure 2.
Space filling models of hCE1 (left) or hCE1m6 (right). The active site entrance is indicated by the cyan arrow and the domains that demonstrate marked differences are noted by yellow arrows.
1.2 Comparison of mammalian carboxylesterase substrate specificities
An analysis of the kinetic parameters for a series of different CE substrates has essentially confirmed the guidelines described above [30]. For example, a comparison of the Km and Vmax values for a rabbit liver (rCE) and a human liver CE (hCE1; CES1) with a panel of nitrophenyl acyl esters, indicated that as the acyl group increased in size, in general, the affinity constant decreased [30]. This is consistent with increased affinities due to increased logP values of the substrates (longer alkyl chains are more hydrophobic). However, the Vmax values also decrease (i.e. less efficient substrate hydrolysis) presumably because the more hydrophobic carboxylic acid products preferentially localize in the active site gorge, thereby reducing enzyme activity [30]. While this was a relative small series of esters, a good correlation was observed between the clogP values for the substrates and a ratio of the catalytic efficiencies (kcat/Km) for the two enzymes.
In humans, a second CE has been identified that is primarily expressed in the intestine and liver [14, 15, 31]. This enzyme, termed hiCE (human intestinal CE; CES2), appears to hydrolyze esters that are more bulky and sterically hindered than those metabolized by hCE1. For example, hiCE is at least 60-fold more efficient at activating CPT-11 than hCE1 [14, 32]. Several recent reports indicate that the major difference between these isoforms is their ability to hydrolyze compounds with different sized acyl or alcohol groups [33]. Preferential hydrolysis of substrates containing a small alcohol domain and a larger acyl moiety occurs with hCE1, whereas the converse is true with hiCE. It would appear therefore that two different enzymes have evolved with several complementary activities. This includes a general distinction in molecule size and the size of the individual domains present within substrates.
1.3 Structures of mammalian carboxylesterases
Several years ago, large-scale chromatographic techniques, that did not involve affinity resins, were developed for the purification of CEs [34]. Using this approach, milligram quantities of rCE and hCE1 were produced that allowed the determination of the X-ray crystal structures of these enzymes [25-27, 34]. A series of important points were noted from these structures. Firstly, the enzymes were comparable in structure to other esterases, notable the cholinesterases. This included a similar overall architecture, with a long deep active site gorge and the same catalytic triad of amino acids (serine, histidine and glutamic acid). Secondly, several glycosylated residues were present on the surface of the enzymes, consistent with the necessity for these proteins to be processed in the endoplasmic reticulum. Thirdly, by direct comparison of the rCE and hCE1 structures, it was apparent that two domains that formed the entrance to the active site gorge in the former protein were disordered. Since rCE is similar to the hiCE with respect to substrate specificity, this suggested that steric constraints enforced by these disordered regions might regulate the access of ester molecules to the catalytic amino acids. Hence for hCE1, large substrates would be unable to enter the gorge due to the lack of flexibility of the amino acids present in these loop domains (Figure 2). In contrast, with rCE and hiCE, that can both metabolize complex molecules including CPT-11, bulky compounds would be accommodated due to the motion of these regions. This also suggests that a gating mechanism may exist for the CEs, whereby one or other of the loop domains flexes in response to substrate interaction. Preliminary molecular dynamics studies using a bacterial CE as a model, indicates that this postulate may be correct. Therefore, the hydrolysis of different substrates is regulated by the size and structure of the substrate, and its ability to access the catalytic amino acids. Gratifyingly, the information obtained from the structural studies was entirely consistent with the results of the biochemical experiments discussed above, and has provided new insights into how these enzymes may be developed to hydrolyze novel agents, principally prodrugs.
2. Use of carboxylesterases to activate anticancer agents
2.1 Enzyme/prodrug approaches
Since CEs can activate the anticancer agents, capecitabine and CPT-11 (Figure 1), we proposed that these enzymes could be used in enzyme/prodrug strategies [1, 13, 29, 35, 36]. Ideally, the drug-activating enzyme would be specifically expressed in, or delivered to, tumor cells in vivo and following prodrug administration, selective antitumor toxicity should be apparent. To this end, we and others have developed different delivery vehicles e.g. adenovirus or antibodies [37-43], that can express or target CE to tumors. Our initial studies were performed using rCE since it is the most efficient enzyme at activating CPT-11. Results from these experiments confirmed that cells expressing this protein were sensitized to the drug and that the levels of CPT-11 that were used were similar to that which could be achieved in cancer patients. However, the application of a rabbit enzyme to a human may result in the generation of an immune response and thereby potentially negating any positive effect of the therapy. Therefore, we and others have explored the use of human CEs to activate CPT-11 [13, 44-47].
As indicated above, hiCE metabolizes CPT-11 in vivo [14, 15], however in in vitro studies in our laboratory, it became apparent that that this protein was not the best candidate for future studies [48]. This was based upon several criteria. Firstly, in cell culture experiments, the levels of intracellular hiCE expression that could be achieved were considerably reduced, as compared to hCE1 or rCE. Secondly, in cells expressing hiCE, the protein was lost more rapidly than cells containing hCE1 or rCE, for reasons unknown. Indeed, over extended periods of time, expression of hiCE was lost completely from transfected cells, even under conditions were positive selection pressure was applied [48]. Thirdly, both rCE and hCE1 are stable at room temperature for many months. In contrast, hiCE has to be stored at −80°C in glycerol to maintain its activity. This suggests that, in general, this enzyme is much less stable that the other CEs. Since this may impact the effectiveness of any prodrug/therapy approaches, we hypothesized that based upon the structural homology between hCE1 and rCE, we could generate an hCE1 variant that could efficiently metabolize CPT-11.
2.2 Development of a human carboxylesterase that is efficient at activating CPT-11
As noted previously, two domains that formed the entrance to the active site gore in rCE were disordered, yet we were able to determine the structure of the corresponding domains in hCE1 [25, 26]. Since hCE1 metabolizes CPT-11 very poorly (∼650-fold less efficiently than rCE; [30]), we postulated that this was due to an inability of the drug to access the catalytic amino acids in the human enzyme. Therefore, we overlaid the alpha carbon backbone derived from the two CE structures and determined the residues that were apparently missing in the rCE coordinates [48]. We then performed site directed mutagenesis of the hCE1 sequence to introduce amino acids that would reflect those seen in the flexible loop domains in rCE. Using this approach, a panel of six variant CEs were developed that contained up to 8 different mutations. Each mutant was transiently expressed in COS7 cells and extracts were then evaluated for CE activity, and for their ability to convert CPT-11 to SN-38 [48].
Results from these studies indicated that in a variant protein, termed hCE1m6, where the loop domains consisted entirely of amino acids derived from rCE, efficient CPT-11 was observed. We concluded that incorporating these residue changes into hCE1 makes the loops highly flexible, and as a consequence, the drug can access the catalytic amino acids [48]. Figure 2 indicates that incorporation of these mutations within hCE1 results in subtle differences in the enzyme structure that appear to reduce the steric hinderance of the active site entrance. However, these are static models based upon the crystal structure of hCE1 and are unlikely to reflect the changes in enzyme motions and dynamics that presumably occur during substrate hydrolysis.
Interestingly, the converse mutations in rCE reduced prodrug activation, but did not completely eliminate it [48]. This suggests that there are other factors, apart from steric constraints, that play a role in the hydrolysis of CPT-11 by the lagomorph protein.
2.3 Biochemical and kinetic analysis of hCE1m6
Having obtained a variant of hCE1, hCE1m6, that could activate CPT-11, we overexpressed the protein in a baculovirus expression system and purified the CE to homogeneity. Analysis of the kinetic parameters for this protein with CPT-11 indicted that the Km (i.e. the affinity constant) was identical to that seen for rCE (∼6.2μM; [48]). This confirmed the hypothesis that making the loops in hCE1 more flexible allowed increased access of the drug to the active site. While the Vmax value of hCE1m6 was ∼8-fold less than that of rCE, the levels of SN-38 produced in in vitro reactions were sufficient to induce cytotoxicity in mammalian cells [48]. The differences in the rate constants for hCE1m6 and rCE are likely due to more subtle differences within the active site gorges of the proteins. As yet, residues that play key roles in this process have not been identified (apart from the essentially catalytic triad), however we are currently undertaking mutagenesis experiments to identify such amino acids.
As indicated previously, hiCE is much less stable than hCE1, and therefore we evaluated the in vitro half-lives of the different proteins. These results indicated that hCE1m6 maintained enzymatic activity for prolonged periods of time (t½=129 days). Hence, we have now developed a stable human CE that can be expressed at high levels in cells, that is highly proficient at CPT-11 activation [48].
2.4 Sensitization of tumor cells expressing hCE1m6 to CPT-11
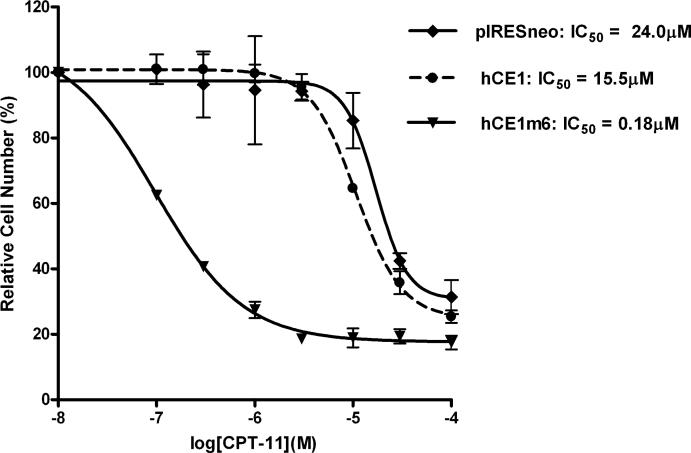
Having constructed hCE1m6 and demonstrated that this protein could efficiently activate CPT-11, we sough to evaluate its ability to modulate drug toxicity in both cell culture and in human tumor xenograft models. Both plasmid transfection and adenoviral transduction studies were performed in vitro, and results from these experiments indicated that hCE1m6 was more proficient at mediating CPT-11-induced toxicity than rCE [48]. This is indicated in Figure 3 which demonstrates the dramatic increase in sensitivity of the relatively CPT-11-resistant glioblastoma cell line U373MG expressing hCE1m6, as compared to hCE1, or cells lacking CE expression (pIRESneo). These results were corroborated using adenoviral mediated transduction which determined that rCE expression reduced the IC50 for CPT-11 by 89-fold, whereas hCE1m6 resulted in a 670-fold decrease in the same parameter (IC50=40nM [48]). It was also noted that in 3 cell lines that were analyzed, the levels of hCE1m6 that could be expressed from adenoviral vectors were consistently higher (2.9- to 5.6-fold) than that using rCE. While the reason for this is unclear, it is likely that increased expression of hCE1m6 is likely to be beneficial in any clinical application of enzyme/prodrug therapy with this CE [48]. To confirm that results seen in vitro could be translated into animal models, we established U373MG tumor xenografts designed to express hCE1m6. Animals bearing these tumors were treated with CPT-11 using a schedule that has been deemed to be most efficacious for this drug (CPT-11 was given daily for five days and this was repeated twice in a 3 week cycle). Xenografts were measured weekly and volumes were compared to tumors that did not express CEs. These studies demonstrated that tumors expressing hCE1m6 were considerably more sensitive to CPT-11, with approximately 4-fold less drug required to achieve comparable antitumor activity to that seen in control samples [48]. Furthermore, these studies were performed in a plasma esterase-deficient mouse model that we have developed, that minimizes non-specific drug activation by CEs present in the blood [6-8]. This is important since humans lack plasma CE [5] and therefore this strain of animals more accurately reflects the efficacy of esterified drugs in man. Overall, these in vitro and in vivo experiments confirm that hCE1m6 can be used for selective CPT-11 activation, and due to that fact this protein is of human origin, we believe that any immune response to the expressed enzyme would be minimized. Studies designed to evaluate the potential of using hCE1m6/CPT-11 as an effective enzyme/prodrug combination in animal models of metastatic disease are currently underway.
Figure 3.
Sensitization of U373MG cells, expressing different CEs, to CPT-11. Cells were engineered to express no exogenous CE (pIRESneo), human liver CE (hCE1), or the mutant form of this protein (hCE1m6). The IC50 values (the concentration of drug required to produce 50% growth inhibition) for each cell line are indicated.
2.5 Activation of other anticancer agents by carboxylesterases
2.5.1 Capecitabine
While the clinical use of CPT-11 has increased dramatically in the last 10 years, this is not the only anticancer drug that is activated by CEs. Capecitabine (Figure 1), a prodrug of 5-fluorouracil, that is used in the treatment of metastatic colorectal and breast cancer, is also subject to hydrolysis by this class of enzymes [16]. This drug is a pentyl-pyrimidinyl carbamate, indicating that it has same functional group as CPT-11 that is recognized by CEs i.e., the N-C(O)-O chemotype. However, capecitabine actually requires three enzymes (CE, cytidine deaminase, and thymidine phosphorylase) to yield the active metabolite. This nucleoside analogue is considerable smaller than CPT-11 and it is unlikely that steric constraints that may arise from the amino acids and domains that comprise the CE active sites would affect substrate hydrolysis. This has been validated by in vitro biochemical studies that indicate that the catalytic efficiencies of the conversion of capecitabine to 5'-deoxy-5-fluorocytidine by hCE1 or hiCE are very similar [16]. Therefore, while suitable mutations could be introduced into CE proteins to maximize the initial hydrolysis of capecitabine, since it is unclear what the rate limiting step is for drug activation (i.e. CE, cytidine deaminase or thymidine phosphorylase), it is unclear whether such an approach would be beneficial.
2.5.2 VP16 (Etoposide)
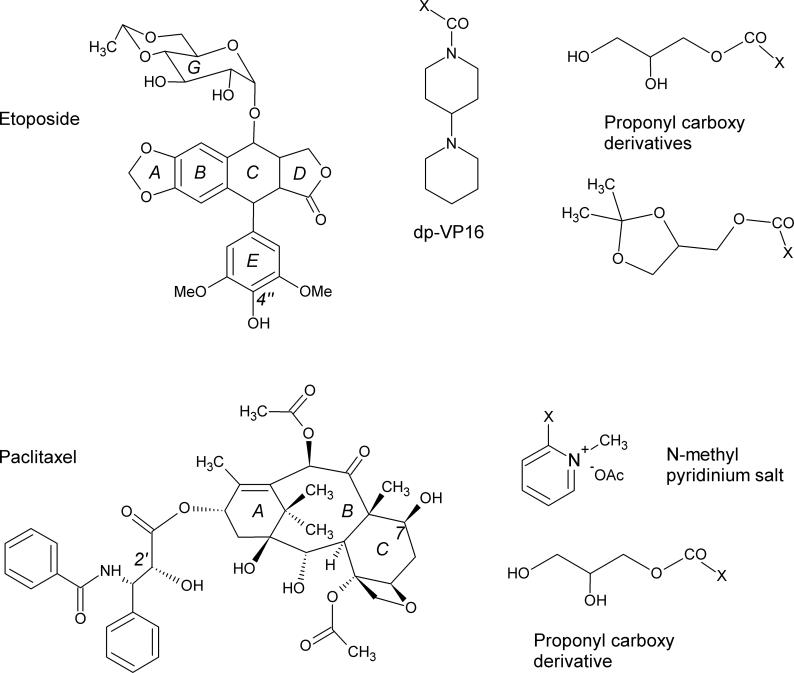
Recently, we synthesized a prodrug of VP16 (etoposide; Figure 4), termed dp-VP16, that contained a 4-piperidinopiperidine (4-PP) moiety attached to the 4” position (the para-position of the 3”,5”-dimethoxy phenol ring) of the drug [49]. Since 4-PP is the fragment that is released from CPT-11 by CEs (see Figure 1 [1, 50]), we reasoned that this group could be hydrolyzed from dp-VP16 by these enzymes, and potentially yield a prodrug with reduced toxicity and increased solubility. Biochemical studies demonstrated that rCE was the most efficient enzyme at dp-VP16 hydrolysis, with lower amounts of VP16 being produced by hiCE and virtually none by hCE1 [49]. Again, since VP16 is a relatively bulky molecule, these in vitro results are consistent the ability of the prodrug to access the catalytic amino acids at the base of the active site gorge. Cell culture analysis confirmed that cells expressing rCE were sensitized to dp-VP16 by ∼5-fold as compared to cells lacking this protein. While this was not a dramatic decrease in the IC50 values, it was noted that in combination drug studies with CPT-11, synergistic toxicity was apparent. Indeed, this combination of prodrugs reduced the growth inhibition values by 35-fold as compared to cells treated with dp-VP-16 alone and by ∼120-fold to cells lacking rCE [49]. These results suggest that combination chemotherapy using prodrugs that are activated by similar enzymes can be simultaneously applied to achieve enhanced antitumor activity. This is a relatively under-explored area of research that has potential merit, with respect to the development of new agents that might be hydrolyzed by CEs Other groups have also synthesized prodrugs of VP16 with the goal of improving the bioavailability, water solubility and pharmacokinetics of the parent drug [51-54]. In addition, some of these chemical modifications were included in an attempt to overcome the multidrug resistance phenomenon, which VP16 is though to be subject to [55]. These derivatives contain a propylcarbonoxy moiety attached at the 4” position of the 3”,5”-dimethoxy phenol ring (similar to dp-VP16) and these groups are attached by an ester chemotype [54]. Both of the prodrugs that were developed were relatively resistant to non-specific hydrolysis, however in the presence of human serum or pig liver carboxylesterase, VP16 was produced. Subsequent studies demonstrated that the prodrug demonstrated efficacy against the childhood tumor neuroblastoma, both in vitro and in a mouse model [51]. These studies suggest that by employing rational prodrug design of established anticancer agents, improved therapeutic activity, and potentially, modulation of drug resistance may be achieved. As yet, very few studies have been employed to specifically target desirable properties in prodrugs, but clearly this is an area where an improved understanding of the activation and disposition of the active drug in vivo, will assist medicinal chemists in drug design.
Figure 4.
Structures of etoposide (VP16) and paclitaxel. The rings of each molecule are labeled as well as the carbon atoms where moieties have been introduced to generate prodrugs (4”-position in etoposide and 2’- and 7-positions in paclitaxel). In addition, some of the groups that have been used to generate these prodrugs are indicated at the right, where X represents the atom of attachment.
2.5.3 Paclitaxel
More recently, a series of paclitaxel prodrugs have been synthesized that have contained a variety of ester-linked moieties at several different places within the molecule (Figure 4; [56-62]). These were primarily generated to improve the very poor water solubility of this drug. This included methyl pyridinium acetate salts [57] appended at the 2’- and 7-positions, as well as a series of benzoates, connected via a variety of alkyl, alkenyl and esters moieties [59]. Unfortunately, not all of the prodrugs were active in biochemical assays and some were subject to non-specific hydrolysis in aqueous buffers. The most promising candidate that has arisen from these studies is a C-7 hydroxy propyl derivative [61]. However, it appears that drug activation is mostly pH dependent and it is not known whether CEs can activate this compound. Furthermore, the parent paclitaxel molecule contains 3 phenyl rings, cyclohexyl and cyclohexane moieties, as well as an 8 membered cyclo-octane core (Figure 4). This makes the molecule very bulky and unlikely to fit within the active site of the CEs. In addition, it is not obviously apparent how mutagenesis of these proteins could be accomplished to accommodate the molecule within the catalytic gorge and also to arrange the ester group within several Å of the serine residue, a necessity for substrate hydrolysis. We believe therefore, that prodrugs derived from these sorts of compounds would have to be activated by non-esterase-mediated mechanisms.
2.5.4 Doxorubicin
Doxorubicin is an anthracycline-based antibiotic that is used for the treatment of wide variety of human malignancies. It intercalates within DNA and thus likely disrupts several crucial processes in DNA replication and transcription, with topoisomerase II being considered the primary target. Several different prodrugs of doxorubin have been designed including a formaldehyde conjugate that is selectively activated by hiCE [63], peptidic-based analogues [64], as well as a panel of glucuronides and diacetoxypent-1-yl derivatives [65-67]. The latter contain the diacetoxy group appended to the 5-position of the daunosamine sugar and these molecules were designed to be activated by hydrolases. However, since these drugs have not been assayed against the purified human enzymes, it is unclear whether hCE1 or hiCE can activate these compounds. These doxorubicin prodrugs demonstrated good activity in cell culture models, but it is not clear if these drugs have been tested in in vivo models [68]. However given the widespread use of doxorubicin in the clinic, it is likely that prodrugs based on this compound are likely to be useful against human tumors.
3. Design of novel anticancer prodrugs that are activated by carboxylesterases
3.1 Strategy
As indicated in the previous sections, several key issues must be considered in the design of prodrugs that would be efficiently activated by CEs. Firstly, the compound must be small enough fit within the CE active site. Secondly, the molecule must have an unhindered ester or carbamate group that can be subject to nucleophilic attack by the catalytic serine residue. Thirdly, the hydrolysis must not give rise to a highly reactive intermediate, or product that could react with the amino acids in the protein. This might be an issue with prodrugs derived from alkylating agents or platinum-containing compounds. If this occurred, this would likely result in an inactivation of the enzyme and dramatically reduced drug hydrolysis. Fourthly, by evaluating the pattern of expression of CEs in tissues, it may be possible to target specific agents to different locations. For example, since hCE1 is found in lymphocytes and lung epithelia [69], cells that do not express hiCE, the design of a prodrug that specifically uses hCE1 as the activating enzyme may show enhanced selectivity for cytotoxicity towards these tissues. Finally, since prodrug development has typically been undertaken to circumvent problems with water solubility and bioavailability, the choice of the acyl group might significantly impact biodistribution. In a situation analogous to the use of radioactive iodine for thyroid cancer, potentially, moieties within the prodrug might be used target specific subgroups of cells. While this approach has been proposed and tested both in in vitro and preclinical experiments, with agents such as the folic acid receptor and drug molecules containing folate [70-74], this area of research is still very much in its infancy. However, with the development of proteomic array data for different tumors and a comparison with normal tissues, it is possible that such information may be used to improve prodrug design.
3.2 Brute-force approach
While we have identified some key guidelines for anticancer prodrug design using CEs as the activating enzyme, it is likely that with the development and application of parallel chemistry, it may be simpler, although more time consuming and expensive, to generate large libraries of desired compounds containing varying acyl moieties. These could then be rapidly screened for hydrolysis using purified CEs in vitro and subsequent hits evaluated in cell culture and in appropriate animal models. While these studies may not be as elegant as intuitive drug design based upon knowledge of the biochemical properties of the target enzyme, this approach has been previously employed with great success. For example, CPT-11 was identified after development of ∼50 or so prodrugs of 7-ethyl-10-hydroxy camptothecin, and evaluation of their antitumor activity against human tumor xenografts [75, 76]. The principle goal of the chemistry was to improve water solubility of the parent molecule, and since these studies were undertaken in the 1980s, little was known about the candidate esterases involved in drug hydrolysis. In the subsequent animal studies, CPT-11 demonstrated the greatest antitumor efficacy and has since gone on to generate sales of ∼$550M in 2007, in the US alone. Whether such an approach would be considered by a pharmaceutical company today is doubtful, but it is likely that this technique would be applied by academic investigators. Overall, this brute-force strategy, while expensive and technically demanding, will clearly play a part in future prodrug design.
Conversely, the same methods could be applied to designing enzymes that metabolize currently used prodrugs. For example, Pancook et al, undertook random mutagenesis to improve the ability of human butyrylcholinesterase to activate CPT-11 [77]. Clones demonstrating an increase in drug hydrolysis were then subjected to further rounds of mutagenesis until an enzyme was generated that was ∼3000-fold more efficient at CPT-11 activation than the wildtype protein. While very labor intensive, these studies demonstrate that even in the absence of structural information and/or a directed evolution approach, enzymes with altered biochemical activities can be generated.
3.3 Drug choice
The choice of the active drug to use as a starting point for prodrug design should also be carefully considered. We chose CPT-11 in our studies for several reasons. Firstly, this drug already has anticancer activity and we hypothesized that even small improvements in drug hydrolysis might yield enhanced antitumor effects. Secondly, the parent molecule is effective against a broad spectrum of tumors including pediatric malignancies [78-81], suggesting that any enzyme/prodrug technology could be applied to several different disease histiotypes. Thirdly, CPT-11 can be given on an out patient basis, the toxicity (delayed diarrhea) is manageable [82-85], and rarely requires hospitalization. Finally, this agent has just lost patent protection in the US and its use against other malignancies is likely to increase. Therefore, evaluation of the desirable (and undesirable) properties of suitable active drugs should always be considered prior to prodrug development.
In summary, the rationale development of novel anticancer prodrugs that can be activated by specific CEs, is unlikely to be trivial and will probably result in failures. However, based upon the improved efficacy and universal applicability of the CE/CPT-11 system that we have developed, it is clear that such approaches are valid and warrant further research.
EXPERT OPINION
The studies presented here represent a variation of a typical enzyme/prodrug therapy approach. Typically, an enzyme from a foreign source (e.g. E.coli, Herpes simplex virus) is used to activate a prodrug (5-fluorocytosine, CB1954, ganciclovir), protein/drug combinations that have been efficacious in in vitro models [86-98]. However, while this approach works well in cell culture and in animals (typically immune-deprived), the expression of these proteins in humans is very likely to elicit an immune response. Hence, the initial therapy could potentially be compromised and subsequent administration would depend upon the presence of neutralizing antibodies within the patient. We have opted for an alternate strategy, initially using a mammalian protein (rCE; [29]), however we realized that this may be just as immunogenic as enzymes derived from lower organisms. Indeed, this had been a major criticism of studies using the rCE/CPT-11 approach. hiCE could have been used for these approaches since it can activate CPT-11 [14, 15], but both the biochemical and cellular properties of this enzyme were such that we thought that it was unlikely that this enzyme would be suitable for in vivo applications. Therefore, we used structure-based design to develop a human CE, based upon hCE1, that was very efficient at prodrug activation [48].
Identifying CEs that can activate CPT-11 most efficiently is necessary, but not sufficient for the successful clinical application of these proteins in enzyme/prodrug therapy approaches. Another important component is the ability to target expression of the CEs to tumor cells. This would allow high levels of prodrug activation at the tumor site, resulting in increased preferential cytotoxicity even after systemic administration of the prodrug. If tumor-specific activation of CPT-11 to SN-38 by CEs could be achieved, this could lead to improved antitumor efficacy, or potentially allow the reduction of the drug dose without compromising the therapeutic activity.
Currently, we envisage two specific applications of a CE/CPT-11 based enzyme/prodrug therapy approach that might be successful in clinical applications. Firstly, we propose an adenovirus (Ad) driven therapy that could be used for the purging of tumor cells from the bone marrow of high-risk neuroblastoma (NB) patients [41-43]. The need for a highly efficient purging protocol is based on the observation that autologous stem cell grafts, that are used in standard therapy, are often contaminated with histologically undetectable amounts of tumor cells that lead to relapse [99]. Since Ad transduces NB cells with a significantly higher efficiency than hematopoietic cells [41, 42], these viruses can be used to preferentially deliver a transgene encoding a CE to the tumor cells. Subsequent exposure of the mixture of bone marrow samples to CPT-11, would result in selective cytotoxicity in tumor cells (i.e., those expressing CE). The feasibility and success of this protocol has been demonstrated both in vitro and in mouse models, and conditions that allowed complete eradication of NB cells without cytotoxicity to the hematopoietic cells have been determined [41-43]. Furthermore, since purging would take place ex vivo, using replication-deficient Ad, safety concerns associated with this protocol would be significantly reduced.
Secondly, the CE/CPT-11 enzyme/prodrug combination can be employed using neural stem cells (NSCs) or progenitor cells (NPCs) as delivery vehicles for the treatment of metastatic, disseminated solid tumors [100-102]. This approach termed NDEPT (Neural progenitor cell Directed Enzyme Prodrug Therapy), is based upon the observation that NPCs and NSCs when administered systemically, migrate selectively to sites of pathology, including tumor cells. Furthermore, this tumor-tropism was observed to target different tumor types, such as prostate cancer, breast cancer, melanoma, glioma and neuroblastoma [103]. Thus, these cell types could be used as vehicles to deliver the CE encoding transgene selectively to tumor cells. Expression of the CE followed by systemic administration of CPT-11 should produce tumor-specific drug activation, and antitumor activity. Using a disseminated NB mouse model, Aboody and others reported that expression of a rabbit liver CE as a transgene did not affect the tumor-tropic potential of NPCs, migrating to disseminated tumor cells in different tissues including liver and bone marrow [101, 104]. In contrast, the transgene carrying NPCs were not detected in most normal tissues. They also observed that upon CPT-11 administration, plasma levels of SN-38 were similar to control mice, and the amount of the active drug in the systemic circulation was not increased. These findings were important to establish that this method would minimize systemic toxicity. Finally, it was observed that mice that had been injected with tumor cells and that had received NDEPT treatment, demonstrated significantly increased disease-free survival as compared to mice receiving CPT-11 alone [101, 104]. Overall, these results demonstrate the feasibility and the possible clinical application of the CE/CPT-11 enzyme/prodrug combination. Clearly the use of hCE1m6 as the activating enzyme is the most appropriate for clinical studies, since any immune response to the protein should be minimized. In addition, since CPT-11 has already demonstrated antitumor activity against a variety of solid tumors, this approach should be applicable to many malignancies where the tumor burden is low. We believe that this approach is unlikely to work with bulky solid masses due to the logistical problems of administering large numbers of transduced NPCs. However, since this therapeutic approach has not been tested in mice bearing macroscopic xenografts, potentially this NDEPT method may be effective against larger tumors.
In summary, we have used structure-based design of a human CE (hCE1) to modify its substrate specificity to make it effective at activating CPT-11. This enzyme can sensitize tumor cells and xenografts to the drug both in vitro and in vivo, and we envisage that this enzyme/prodrug therapy approach can be clinically applied, both ex vivo and in vivo, using two different delivery vehicles (Ad and NSC/NPC, respectively). Any potential immune response should be minimized, increasing the likelihood of success. As the FDA has recently approved the use of NSCs in patients diagnosed with cancer, it is probable that these enzyme/prodrug techniques will be used for the treatment of metastatic tumors, with the anticipation that they will improve the outcome of individuals diagnosed with this disease.
Acknowledgements
The Potter lab is supported by grants from the NIH (CA76202, CA79763, CA98468, CA108775, CA113446, DA018116, Cancer Center Core grant CA21765) and by the American Lebanese Syrian Associated Charities.
Footnotes
This is an electronic version of an article published in Expert Opinion on Drug Metabolism & Toxicology which is available online at: www.expertopin.com.
Declaration of Interest
No conflicts for any author.
REFERENCES
- *1.Potter PM, Wadkins RM. Carboxylesterases – detoxifying enzymes and targets for drug therapy. Curr. Med. Chem. 2006;13:1045–1054. doi: 10.2174/092986706776360969. [DOI] [PubMed] [Google Scholar]
- *2.Redinbo MR, Potter PM. Mammalian Carboxylesterases: From drug targets to protein therapeutics. Drug Discov. Today. 2005;10:313–325. doi: 10.1016/S1359-6446(05)03383-0. [DOI] [PubMed] [Google Scholar]
- *3.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to function. Annu. Rev. Pharmacol. Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. These previous reviews provide a great deal of information concerning the biology of carboxylesterases. [DOI] [PubMed] [Google Scholar]
- 4.Cashman J, Perroti B, Berkman C, et al. Pharmacokinetics and molecular detoxification. Environ. Health Perspect. 1996;104:23–40. doi: 10.1289/ehp.96104s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Sedlacek M, Manoharan I, et al. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Morton CL, Iacono L, Hyatt JL, et al. Metabolism and antitumor activity of CPT-11 in plasma esterase-deficient mice. Cancer Chemother. Pharmacol. 2005;56:629–636. doi: 10.1007/s00280-005-1027-y. [DOI] [PubMed] [Google Scholar]
- 7.Morton CL, Taylor KR, Iacono L, et al. Metabolism of CPT-11 in esterase deficient mice. Proc. Am. Assoc. Cancer Res. 2002;43:248. [Google Scholar]
- 8.Morton CL, Wierdl M, Oliver L, et al. Activation of CPT-11 in mice: Identification and analysis of a highly effective plasma esterase. Cancer Res. 2000;60:4206–4210. [PubMed] [Google Scholar]
- 9.Pindel EV, Kedishvili NY, Abraham TL, et al. Purification and cloning of a broad substrate specificity human liver carboxylesterase that catalyzes the hydrolysis of cocaine and heroin. J. Biol. Chem. 1997;272:14769–14775. doi: 10.1074/jbc.272.23.14769. [DOI] [PubMed] [Google Scholar]
- 10.Kamendulis LM, Brzezinski MR, Pindel EV, et al. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J. Pharmacol. Expt. Therap. 1996;279:713–717. [PubMed] [Google Scholar]
- 11.Brzezinski MR, Abraham TL, Stone CL, et al. Purification and characterization of a human liver cocaine carboxylesterase that catalyzes the production of benzoylecgonine and the formation of cocaethylene from alcohol and cocaine. Biochem. Pharmacol. 1994;48:1747–1755. doi: 10.1016/0006-2952(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 12.Brzezinski MR, Spink BJ, Dean RA, et al. Human liver carboxylesterase hCE-1: binding specificity for cocaine, heroin, and their metabolites and analogs. Drug Metab Dispos. 1997;25:1089–1096. [PubMed] [Google Scholar]
- 13.Danks MK, Morton CL, Krull EJ, et al. Comparison of activation of CPT-11 by rabbit and human carboxylesterases for use in enzyme/prodrug therapy. Clin. Cancer Res. 1999;5:917–924. [PubMed] [Google Scholar]
- 14.Humerickhouse R, Lohrbach K, Li L, et al. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60:1189–1192. [PubMed] [Google Scholar]
- 15.Khanna R, Morton CL, Danks MK, et al. Proficient metabolism of CPT-11 by a human intestinal carboxylesterase. Cancer Res. 2000;60:4725–4728. [PubMed] [Google Scholar]
- 16.Quinney SK, Sanghani SP, Davis WI, et al. Hydrolysis of capecitabine to 5′-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. J Pharmacol Exp Ther. 2005;313:1011–1016. doi: 10.1124/jpet.104.081265. [DOI] [PubMed] [Google Scholar]
- 17.Crow JA, Borazjani A, Potter PM, et al. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol Appl Pharmacol. 2007;221:1–12. doi: 10.1016/j.taap.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross MK, Borazjani A, Edwards CC, et al. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 2006;71:657–669. doi: 10.1016/j.bcp.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Ross MK, Potter PM, Borazjani A. Hydrolytic metabolism of pyrethroids by human carboxylesterases and rodent and human liver microsomes. Tox. Sci. 2005;84:S1. A1569. [Google Scholar]
- 20.Zhang J, Burnell JC, Dumaual N, et al. Binding and hydrolysis of meperidine by human liver carboxylesterase hCE-1. J. Pharmacol. Exp. Ther. 1999;290:314–318. [PubMed] [Google Scholar]
- 21.Sun Z, Murry DJ, Sanghani SP, et al. Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther. 2004;310:469–476. doi: 10.1124/jpet.104.067116. [DOI] [PubMed] [Google Scholar]
- 22.Takai S, Matsuda A, Usami Y, et al. Hydrolytic profile for ester- or amide-linkage by carboxylesterases pI 5.3 and 4.5 from human liver. Biol. Pharm. Bull. 1997;20:869–873. doi: 10.1248/bpb.20.869. [DOI] [PubMed] [Google Scholar]
- 23.Shi D, Yang J, Yang D, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319:1477–1484. doi: 10.1124/jpet.106.111807. [DOI] [PubMed] [Google Scholar]
- 24.Tang M, Mukundan M, Yang J, et al. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J Pharmacol Exp Ther. 2006;319:1467–1476. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- **25.Bencharit S, Morton CL, Howard-Williams EL, et al. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat. Struct. Biol. 2002;9:337–342. doi: 10.1038/nsb790. This article represents the first structure of a mammalian carboxylesterase and provides the groundwork for developing the hCE1m6 mutant. [DOI] [PubMed] [Google Scholar]
- 26.Bencharit S, Morton CL, Hyatt JL, et al. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine. From binding promiscuity to selective inhibition. Chem. & Biol. 2003;10:341–349. doi: 10.1016/s1074-5521(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 27.Bencharit S, Morton CL, Xue Y, et al. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat. Struct. Biol. 2003;10:349–356. doi: 10.1038/nsb919. [DOI] [PubMed] [Google Scholar]
- *28.Gillilan R. Acetylcholinesterase molecular dynamics. http://opendx.sdsc.edu/animations/chemistry/chem-ctc.mpg This amazing movie demonstrates how esterases direct substrates into the catalytic gorges of these proteins.
- 29.Potter PM, Pawlik CA, Morton CL, et al. Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug Irinotecan (CPT-11). Cancer Res. 1998;52:2646–2651. [PubMed] [Google Scholar]
- 30.Wadkins RM, Morton CL, Weeks JK, et al. Structural constraints affect the metabolism of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11) by carboxylesterases. Mol. Pharmacol. 2001;60:355–362. doi: 10.1124/mol.60.2.355. [DOI] [PubMed] [Google Scholar]
- 31.Schwer H, Langmann T, Daig R, et al. Molecular cloning and characterization of a novel putative carboxylesterase, present in human intestine and liver. Biochem. Biophys. Res. Comm. 1997;233:117–120. doi: 10.1006/bbrc.1997.6413. [DOI] [PubMed] [Google Scholar]
- 32.Sanghani SP, Quinney SK, Fredenburg TB, et al. Hydrolysis of irinotecan and its oxidative metabolites, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxycamptothecin and 7-ethyl-10-[4-(1-piperidino)-1-amino]-carbonyloxycamptothecin, by human carboxylesterases CES1A1, CES2, and a newly expressed carboxylesterase isoenzyme, CES3. Drug Metab Dispos. 2004;32:505–511. doi: 10.1124/dmd.32.5.505. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13:412–431. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton CL, Potter PM. Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda and COS7 cells for recombinant gene expression: Application to a rabbit liver carboxylesterase. Mol. Biotechnol. 2000;16:193–202. doi: 10.1385/MB:16:3:193. [DOI] [PubMed] [Google Scholar]
- 35.Danks MK, Potter PM. Enzyme-prodrug systems: carboxylesterase/CPT-11. Meth. Mol. Med. 2004;90:247–262. doi: 10.1385/1-59259-429-8:247. [DOI] [PubMed] [Google Scholar]
- 36.Potter PM, Danks MK. Carboxylesterase-mediated activation of irinotecan. Cancer Res. Alert. 2000;2:80–83. [Google Scholar]
- 37.Senter PD, Springer CJ. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv. Drug. Deliv. Rev. 2001;53:247–264. doi: 10.1016/s0169-409x(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 38.Senter PD, Beam KS, Mixan B, et al. Identification and activities of human carboxylesterases for the activation of CPT-11, a clinically approved anticancer drug. Bioconjug. Chem. 2001;12:1074–1080. doi: 10.1021/bc0155420. [DOI] [PubMed] [Google Scholar]
- 39.Senter PD. Activation of prodrugs by antibody-enzyme conjugates: a new approach to cancer therapy. Faseb J. 1990;4:188–193. doi: 10.1096/fasebj.4.2.2404820. [DOI] [PubMed] [Google Scholar]
- 40.Senter PD. Antitumor effects of antibody enzyme conjugates in combination with prodrugs. Front. Radiat. Ther. Oncol. 1990;24:132–141. discussion 161−135. [PubMed] [Google Scholar]
- 41.Meck M, Wierdl M, Wagner L, et al. A VDEPT approach to purging neuroblastoma cells from hematopoeitic cells using adenovirus encoding rabbit carboxylesterase and CPT-11. Cancer Res. 2001;61:5083–5089. [PubMed] [Google Scholar]
- 42.Wagner LM, Guichard SM, Burger RA, et al. Efficacy and toxicity of a virus-directed enzyme prodrug therapy purging method: preclinical assessment and application to bone marrow samples from neuroblastoma patients. Cancer Res. 2002;62:5001–5007. [PubMed] [Google Scholar]
- 43.Wierdl M, Morton CL, Weeks JK, et al. Sensitization of human tumor cells to CPT-11 via adenoviral-mediated delivery of a rabbit liver carboxylesterase. Cancer Res. 2001;61:5078–5082. [PubMed] [Google Scholar]
- 44.Oosterhoff D, Overmeer RM, de Graaf M, et al. Adenoviral vector-mediated expression of a gene encoding secreted, EpCAM-targeted carboxylesterase-2 sensitises colon cancer spheroids to CPT-11. Br J Cancer. 2005;92:882–887. doi: 10.1038/sj.bjc.6602362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oosterhoff D, Pinedo HM, van der Meulen IH, et al. Secreted and tumour targeted human carboxylesterase for activation of irinotecan. Br J Cancer. 2002;87:659–664. doi: 10.1038/sj.bjc.6600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oosterhoff D, Pinedo HM, Witlox MA, et al. Gene-directed enzyme prodrug therapy with carboxylesterase enhances the anticancer efficacy of the conditionally replicating adenovirus AdDelta24. Gene Ther. 2005;12:1011–1018. doi: 10.1038/sj.gt.3302492. [DOI] [PubMed] [Google Scholar]
- 47.Oosterhoff D, Witlox MA, van Beusechem VW, et al. Gene-directed enzyme prodrug therapy for osteosarcoma: sensitization to CPT-11 in vitro and in vivo by adenoviral delivery of a gene encoding secreted carboxylesterase-2. Mol Cancer Ther. 2003;2:765–771. [PubMed] [Google Scholar]
- **48.Wierdl M, Tsurkan L, Hyatt JL, et al. An improved human carboxylesterase for use in enzyme/prodrug therapy with CPT-11. Cancer Gene Therap. 2008;15:183–192. doi: 10.1038/sj.cgt.7701112. This paper describes the development of hCE1m6 and its ability to activate CPT-11. [DOI] [PubMed] [Google Scholar]
- 49.Yoon KJ, Qi J, Remack JS, et al. An etoposide prodrug for development of a dual prodrug-enzyme approach to antitumor therapy. Mol Cancer Therapeut. 2006;5:1577–1584. doi: 10.1158/1535-7163.MCT-06-0090. [DOI] [PubMed] [Google Scholar]
- 50.Yoon KJ, Potter PM, Danks MK. Development of prodrugs for enzyme-mediated, tumor-selective therapy. Curr Med Chem Anticancer Agents. 2005;5:107–113. doi: 10.2174/1568011053174837. [DOI] [PubMed] [Google Scholar]
- 51.Lange B, Schroeder U, Huebener N, et al. Rationally designed hydrolytically activated etoposide prodrugs, a novel strategy for the treatment of neuroblastoma. Cancer Lett. 2003;197:225–230. doi: 10.1016/s0304-3835(03)00106-x. [DOI] [PubMed] [Google Scholar]
- 52.Jikai J, Shamis M, Huebener N, et al. Neuroblastoma directed therapy by a rational prodrug design of etoposide as a substrate for tyrosine hydroxylase. Cancer Lett. 2003;197:219–224. doi: 10.1016/s0304-3835(03)00104-6. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder U, Bernt KM, Lange B, et al. Hydrolytically activated etoposide prodrugs inhibit MDR-1 function and eradicate established MDR-1 multidrug-resistant T-cell leukemia. Blood. 2003;102:246–253. doi: 10.1182/blood-2002-07-2268. [DOI] [PubMed] [Google Scholar]
- 54.Wrasidlo W, Schroder U, Bernt K, et al. Synthesis, hydrolytic activation and cytotoxicity of etoposide prodrugs. Bioorg Med Chem Lett. 2002;12:557–560. doi: 10.1016/s0960-894x(01)00801-0. [DOI] [PubMed] [Google Scholar]
- 55.Meresse P, Dechaux E, Monneret C, et al. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11:2443–2466. doi: 10.2174/0929867043364531. [DOI] [PubMed] [Google Scholar]
- 56.Senter PD, Marquardt H, Thomas BA, et al. The role of rat serum carboxylesterase in the activation of paclitaxel and camptothecin prodrugs. Cancer Res. 1996;56:1471–1474. [PubMed] [Google Scholar]
- 57.Wrasidlo W, Gaedicke G, Guy RK, et al. A novel 2′-(N-methylpyridinium acetate) prodrug of paclitaxel induceds superior antitumor responses in preclinical cancer models. Bioconjug. Chem. 2002;13:1093–1099. doi: 10.1021/bc0200226. [DOI] [PubMed] [Google Scholar]
- 58.Bhat L, Liu Y, Victory SF, et al. Synthesis and evaluation of paclitaxel C7 derivatives: solution phase synthesis of combinatorial libraries. Bioorg Med Chem Lett. 1998;8:3181–3186. doi: 10.1016/s0960-894x(98)00551-4. [DOI] [PubMed] [Google Scholar]
- 59.Boge TC, Wu ZJ, Himes RH, et al. Conformationally restricted paclitaxel analogues: macrocyclic mimics of the ”hydrophobic collapse” conformation. Bioorg Med Chem Lett. 1999;9:3047–3052. doi: 10.1016/s0960-894x(99)00522-3. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Ali SM, Boge TC, et al. A systematic SAR study of C10 modified paclitaxel analogues using a combinatorial approach. Comb Chem High Throughput Screen. 2002;5:39–48. doi: 10.2174/1386207023330615. [DOI] [PubMed] [Google Scholar]
- 61.Niethammer A, Gaedicke G, Lode HN, et al. Synthesis and preclinical characterization of a paclitaxel prodrug with improved antitumor activity and water solubility. Bioconjug Chem. 2001;12:414–420. doi: 10.1021/bc000122g. [DOI] [PubMed] [Google Scholar]
- 62.Seligson AL, Terry RC, Bressi JC, et al. A new prodrug of paclitaxel: synthesis of Protaxel. Anticancer Drugs. 2001;12:305–313. doi: 10.1097/00001813-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Barthel BL, Torres RC, Hyatt JL, et al. Identification of human intestinal carboxylesterase as the primary enzyme for activation of a doxazolidine carbamate prodrug. J Med Chem. 2008;51:298–304. doi: 10.1021/jm7011479. [DOI] [PubMed] [Google Scholar]
- 64.Ravel D, Dubois V, Quinonero J, et al. Preclinical toxicity, toxicokinetics, and antitumoral efficacy studies of DTS-201, a tumor-selective peptidic prodrug of doxorubicin. Clin Cancer Res. 2008;14:1258–1265. doi: 10.1158/1078-0432.CCR-07-1165. [DOI] [PubMed] [Google Scholar]
- 65.Cherif A, Farquhar D. N-(5,5-diacetoxypent-1-yl)doxorubicin: a new intensely potent doxorubicin analogue. J Med Chem. 1992;35:3208–3214. doi: 10.1021/jm00095a017. [DOI] [PubMed] [Google Scholar]
- 66.Farquhar D, Newman RA, Zuckerman JE, et al. Doxorubicin analogues incorporating chemically reactive substituents. J Med Chem. 1991;34:561–564. doi: 10.1021/jm00106a013. [DOI] [PubMed] [Google Scholar]
- 67.Zwelling LA, Altschuler E, Cherif A, et al. N-(5,5-diacetoxypentyl)doxorubicin: a novel anthracycline producing DNA interstrand cross-linking and rapid endonucleolytic cleavage in human leukemia cells. Cancer Res. 1991;51:6704–6707. [PubMed] [Google Scholar]
- 68.Farquhar D, Cherif A, Bakina E, et al. Intensely potent doxorubicin analogues: structure-activity relationship. J Med Chem. 1998;41:965–972. doi: 10.1021/jm9706980. [DOI] [PubMed] [Google Scholar]
- 69.Munger JS, Shi GP, Mark EA, et al. A serine esterase released by human alveolar macrophages is closely related to liver microsomal carboxylesterases. J. Biol. Chem. 1991;266:18832–18838. [PubMed] [Google Scholar]
- 70.Leamon CP, Reddy JA, Vlahov IR, et al. Synthesis and biological evaluation of EC140: a novel folate-targeted vinca alkaloid conjugate. Bioconjug Chem. 2006;17:1226–1232. doi: 10.1021/bc060145g. [DOI] [PubMed] [Google Scholar]
- 71.Leamon CP, Reddy JA, Vlahov IR, et al. Synthesis and biological evaluation of EC72: a new folate-targeted chemotherapeutic. Bioconjug Chem. 2005;16:803–811. doi: 10.1021/bc049709b. [DOI] [PubMed] [Google Scholar]
- 72.Leamon CP, Reddy JA, Vlahov IR, et al. Preclinical antitumor activity of a novel folate-targeted dual drug conjugate. Mol Pharm. 2007;4:659–667. doi: 10.1021/mp070049c. [DOI] [PubMed] [Google Scholar]
- 73.Reddy JA, Dorton R, Westrick E, et al. Preclinical evaluation of EC145, a folate-vinca alkaloid conjugate. Cancer Res. 2007;67:4434–4442. doi: 10.1158/0008-5472.CAN-07-0033. [DOI] [PubMed] [Google Scholar]
- 74.Reddy JA, Westrick E, Santhapuram HK, et al. Folate receptor-specific antitumor activity of EC131, a folate-maytansinoid conjugate. Cancer Res. 2007;67:6376–6382. doi: 10.1158/0008-5472.CAN-06-3894. [DOI] [PubMed] [Google Scholar]
- 75.Kunimoto T, Nitta K, Tanaka T, et al. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothecin, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987;47:5944–5947. [PubMed] [Google Scholar]
- 76.Tsuji T, Kaneda N, Kado K, et al. CPT-11 converting enzyme from rat serum: purification and some properties. J. Pharmacobiodyn. 1991;14:341–349. doi: 10.1248/bpb1978.14.341. [DOI] [PubMed] [Google Scholar]
- 77.Pancook JD, Pecht G, D'Arigo K, et al. Optimization of butyrylcholinesterase for the targeted activation of CPT-11. Proc. Am. Assoc. Cancer Res. 2004;45:2198. [Google Scholar]
- 78.Simon M, Argiris A, Murren JR. Progress in the therapy of small cell lung cancer. Crit. Rev. Onc./Hema. 2004;49:119–133. doi: 10.1016/S1040-8428(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 79.Langer CJ. The global role of irinotecan in the treatment of lung cancer: 2003 update. Oncology. 2003;17:30–40. [PubMed] [Google Scholar]
- 80.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a Phase I trial in children. J. Clin. Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 81.Von Hoff DD, Rothenberg ML, Pitot HC, et al. Irinotecan (CPT-11) therapy for patients with previously treated metastatic colorectal cancer (CRC): overall results of FDA-reviewed pivotal US clinical trials (Meeting abstract). Proc. Annu. Meet. Am. Soc. Clin. Oncol. 1997;16:A803. [Google Scholar]
- 82.Kurita A, Kado S, Kaneda N, et al. Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemotherapy and Pharmacology. 2000;46:211–220. doi: 10.1007/s002800000151. [DOI] [PubMed] [Google Scholar]
- 83.Ikegami T, Ha L, Arimori K, et al. Intestinal alkalization as a possible preventive mechanism in irinotecan (CPT-11)-induced diarrhea. Cancer Res. 2002;62:179–187. [PubMed] [Google Scholar]
- 84.Takeda Y, Kobayashi K, Akiyama Y, et al. Prevention of irinotecan (CPT-11)-induced diarrhea by oral alkalization combined with control of defecation in cancer patients. Int.J. Cancer. 2001;92:269–275. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1179>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 85.Saliba F, Hagipantelli R, Misset JL, et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. J. Clin. Oncol. 1998;16:2745–2751. doi: 10.1200/JCO.1998.16.8.2745. [DOI] [PubMed] [Google Scholar]
- 86.Green NK, Youngs DJ, Neoptolemos JP, et al. Sensitization of colorectal and pancreatic cancer cell lines to the prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by retroviral transduction and expression of the E. coli nitroreductase gene. Cancer Gene Ther. 1997;4:229–238. [PubMed] [Google Scholar]
- 87.Trinh QT, Austin EA, Murray DM, et al. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808–4812. [PubMed] [Google Scholar]
- 88.Fillat C, Carrió M, Cascante A, et al. Suicide gene therapy mediated by the Herpes simplex virus thymidine kinase gene/ganciclovir system: Fifteen years of application. Curr. Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 89.Grove JI, Searle PF, Weedon SJ, et al. Virus-directed enzyme prodrug therapy using CB1954. Anticancer Drug Des. 1999;14:461–472. [PubMed] [Google Scholar]
- 90.Searle PF, Weedon SJ, McNeish IA, et al. Sensitisation of human ovarian cancer cells to killing by the prodrug CB1954 following retroviral or adenoviral transfer of the E. coli nitroreductase gene. Adv Exp Med Biol. 1998;451:107–113. doi: 10.1007/978-1-4615-5357-1_17. [DOI] [PubMed] [Google Scholar]
- 91.Bridgewater JA, Knox RJ, Pitts JD, et al. The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite. Hum Gene Ther. 1997;8:709–717. doi: 10.1089/hum.1997.8.6-709. [DOI] [PubMed] [Google Scholar]
- 92.Bailey SM, Hart IR. Nitroreductase activation of CB1954--an alternative ’suicide’ gene system. Gene Ther. 1997;4:80–81. doi: 10.1038/sj.gt.3300400. [DOI] [PubMed] [Google Scholar]
- 93.Bailey SM, Knox RJ, Hobbs SM, et al. Investigation of alternative prodrugs for use with E. coli nitroreductase in ’suicide gene’ approaches to cancer therapy. Gene Ther. 1996;3:1143–1150. [PubMed] [Google Scholar]
- 94.Garcia-Sanchez F, Pizzorno G, Fu SQ, et al. Cytosine deaminase adenoviral vector and 5-fluorocytosine selectively reduce breast cancer cells 1 million-fold when they contaminate hematopoietic cells: a potential purging method for autologous transplantation. Blood. 1998;92:672–682. [PubMed] [Google Scholar]
- 95.Hanna NN, Mauceri HJ, Wayne JD, et al. Virally directed cytosine deaminase/5-fluorocytosine gene therapy enhances radiation response in human cancer xenografts. Cancer Res. 1997;57:4205–4209. [PubMed] [Google Scholar]
- 96.Bouali-Benazzouz R, Laine M, Vicat JM, et al. Therapeutic efficacy of the thymidine kinase/ganciclovir system on large experimental gliomas: a nuclear magnetic resonance imaging study. Gene Ther. 1999;6:1030–1037. doi: 10.1038/sj.gt.3300921. [DOI] [PubMed] [Google Scholar]
- 97.Hwang HC, Smythe WR, Elshami AA, et al. Gene therapy using adenovirus carrying the herpes simplex-thymidine kinase gene to treat in vivo models of human malignant mesothelioma and lung cancer. Am J Respir Cell Mol Biol. 1995;13:7–16. doi: 10.1165/ajrcmb.13.1.7598939. [DOI] [PubMed] [Google Scholar]
- 98.Vile RG, Nelson JA, Castleden S, et al. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 99.Rill D, Santana V, Roberts W, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–383. [PubMed] [Google Scholar]
- 100.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **101.Aboody KS, Bush RA, Garcia E, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;1:1–10. doi: 10.1371/journal.pone.0000023. This article demonstrate that NPCs can be used to selectively deliver carboxylesterase to tumor cells in vivo and that this results in an increased anticancer response following CPT-11 administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aboody KS, Najbauer J, Schmidt NO, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro-Oncol. 2006;8:119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 104.Danks MK, Yoon KJ, Bush RA, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]