Abstract
Purpose
Health of the general population is improving along a number of major health dimensions. Using a cumulative deficits approach we investigated whether such improvements were evident at the level of minor health traits.
Methods
We selected 37 small-effect traits consistently measured in the 9th (performed in 1964) and 14th (1974) Framingham Heart and 5th (1991–1995) Offspring Study exams to construct indices of cumulative deficits (DIs).
Results
We identified deficits-specific DIs characterizing health dimensions associated with no health changes (DINHC), health worsening (DIWRS), and health improving (DIIMP) between the 1960s and 1990s. The risks of death attributable to the DINHC dominate within shorter time horizons. For longer time horizons, both the DINHC and DIIMP provide the same contribution to the risks of death. The mortality risks associated with the DIWRS are the weakest and least significant.
Conclusions
The analyses show that the cumulative deficits approach might be an efficient tool for analyzing the effects of a large number of health characteristics for which the individual effects are small, inconsistent, or non-significant. They show favorable trends such that health of the Framingham studies participants either did not change or improved over time for the most serious small-effect traits.
Keywords: Trends in health, Population characteristics, Age patterns
INTRODUCTION
Numerous studies have documented improvements in the health of the general population during the 20th century (reviewed in [1]). These improvements were documented along a number of dimensions of health reflecting the process of population health change (e.g., a simplified pathway is: risk factors → disease → loss of functioning → disability → death [1]). Most studies of health trends (apart from mortality changes) in recent years have emphasized positive changes in disability prevalence among older individuals (e.g., [2, 3]). Improvements in physical, cognitive, and sensory limitations were recently summarized in [3]. Trends in diseases are not so positive; most studies suggest an increasing chronic disease burden, including consistent estimates of upward trends in heart disease prevalence during the 1970-to-1990 period from several major studies (e.g., National Health Interview Survey, Framingham Heart [FHS] and Offspring [FHSO] Studies, Minnesota Heart Survey) [1]. Studies of temporal changes in disease risk factors were largely focused on heart diseases and cancer and provided mixing evidences [1, 4].
Another approach to the assessment of health status is based on global health characteristics. One such characteristic is self-rated health, which is viewed as a summary of overall health status due to its high predictive power of death. Measures of self-rated health show a consistent decline in the prevalence of individuals who rate their health as poor during the 1980s and 1990s, a trend that was more pronounced among the elderly than among the younger population [5–7].
Major health dimensions provide some indications of trends in severe health conditions (e.g., disease, disability, self-perceived health). Will these trends continue in the future? To answer this question, a mechanism driving changes in severe health conditions has to be understood. This is a motivation for studies of trends in risk factors. Understanding the importance of trends in minor health conditions leads to yet another focus of recent research which is not simply on risk factors but on symptoms and signs [8]. Insights on changes in these factors might provide more precise clues on future changes in population health. The challenge facing such studies is the large number of various symptoms and signs and the small or inconsistent effect of each on health/mortality risks. The aggregate effect of several such small-effect factors, however, might be more informative. This is an underlying paradigm of recent developments of a new promising instrument which is called a frailty index [9–11] or an index of cumulative deficits [12, 13]. The concept of a cumulative health deficits index (DI) also appears to be useful in studies of aging, health, and survival for which the DI is a promising alternative to chronological age for characterizing aging-associated processes in individuals and for improved predictions of chances of adverse events [11–20]. Consequently, the DI can be an indicator of changes in health on the level of small-effects traits (e.g., signs, symptoms), and, simultaneously, can serve as a characteristic of global health/well-being.
This study investigates trends in the health status in sample of adult and elderly individuals participating in the FHS/FHSO using a new instrument, the DI, which aggregates small-effect variables routinely collected during the 1960-to-1990 period. Unlike other studies, the focus of this work is on a broad spectrum of such traits.
METHODS
The FHS and FHSO data
Beginning in 1948, 5,209 respondents (46% male) aged 28–62 years residing in Framingham, Massachusetts were enrolled in the famous Framingham Heart Study. The FHSO dataset consists of a sample of 3,514 biological descendants of the FHS Cohort, 1,576 of their spouses and 34 adopted offspring for a total sample of 5,124 subjects; 48% male. The FHSO subjects were enrolled in 1971–1975 using research protocols similar to those of the FHS so that comparisons of the results from the FHSO and the FHS could be made. Selection criteria and study design have been described [21, 22]. These cohorts have been followed for the occurrence of certain diseases (e.g., heart disease, cancer, diabetes mellitus) and death. Examination also included an interview, physical examination, and laboratory tests.
The cumulative deficits approach
In traditional analyses, traits with small, inconsistent, or non-significant contributions to risks of adverse health outcomes are usually ignored. When the number of such traits is large enough, however, their cumulative effect on chances of future adverse events may become significant and, thus, an integrative or cumulative measure (i.e., the DI) might be more informative compared to individual traits [12–15, 20]. Therefore, the DI is designed to gather different manifestations of health deterioration with aging (regardless of their individual significance) from a wide set of deficits into a single measure. An important advantage of the DI is that it can be constructed using the set of deficits typically collected in majority of the aging-related studies. This is because such studies collect wide sets of the aging-related traits and statistical properties of the DI (e.g., age patterns) and its effect on other outcomes (e.g., mortality) are weakly sensitive to the selection of specific set of deficits. Robustness of the DI is confirmed in several studies using different sets of deficits [11, 15, 17, 18]. In addition, the DI might be a good characterization of the level of aging-associated decline in health status at this age [12–15, 20]. If the DI is constructed from a set of small-effect traits, it can capture small decrements in declining health with aging, hopefully, informing about health problems long before clinically manifested conditions.
The conceptual framework behind the DI can be summarized in a simplified scheme in which the individual’s vulnerability state can be characterized by a proportion of failed units out of a large number, N, of such units (subsystems). The failure of each unit is associated with a “deficit”. The proportion of deficits accumulated by age x characterizes individual’s health/wellbeing status and affects chances of further health deterioration and death. The data often do not allow for observing failures of all the N units. Therefore, an empirical estimate of this proportion in a given individual, i.e., the DI(x), can be calculated by selecting a set of M units out of a list with N units, summing the number of failed units from the selected set M up to age x, m(x), and dividing this sum by M, i.e., DI(x)=m(x)/M [15, 23–25]. Prior studies suggest that the properties of the DI are weakly sensitive to the choice of the subset M [18].
Analyses
The evaluation of trends in the age patterns of DI is constrained by several factors. First, ideally, the DIs have to be constructed using a wide set of heath-related conditions (see above Section). Second, survey instruments have to be comparable over time. Third, the range of intersecting ages should be as large as possible. Fourth, the surveys/exams should be well separated in time. Finally, selected samples have to be of adequate size. To address all these constraints, the same sets of 37 deficits (Table 1) with comparable diagnostic procedures across all years was selected from two representative exams of the FHS (9th FHS exam performed in 1964; N=3833; age range is 44–78 years; mean age (MA) ± standard error=59.0 ± 0.13 years and 14th FHS exam performed in 1974; N=2871; age range is 55–88 years; MA=67.5 ± 0.14) and one representative exam of the FHSO (5th FHSO exam performed in 1991–1995; N=3799; age range is 31–78 years; MA=55.0 ± 0.16). Seventeen deficits were either dichotomous (yes, or no) or dichotomized for the sake of consistency between exams. The remaining 20 deficits were rescaled to the unit interval to reflect the degree of abnormality, e.g., the urinary sugar level was recoded as negative (0 or no deficit), doubtful (0.5) and positive (1 or yes deficit).
Table 1.
List of 37 deficits used in the analyses
| Group | N | Deficit |
|---|---|---|
| 1 | 1 | urinary sugar |
| 2 | chronic cough | |
| 3 | trouble with wheezing | |
| 4 | increase in dyspnea | |
| 5 | orthopnea | |
| 6 | paroxysmal nocturnal dyspnea | |
| 7 | chest discomfort | |
| 8 | frequent coldness in one hand/foot | |
| 9 | arcus senilis | |
| 10 | xanthelasma | |
| 11 | xanthomata | |
| 12 | distended neck veins | |
| 13 | localized breast mass | |
| 14 | axillary breast nodes | |
| 15 | peripheral pulses: dorsal pedis | |
| 16 | peripheral pulses: posterior tibial | |
| 17 | peripheral pulses: femoral | |
| 18 | peripheral pulses: radial | |
| 2 | 19 | ankle edema |
| 20 | discomfort in lower limbs while walking | |
| 21 | abnormal breast | |
| 22 | left ankle edema | |
| 23 | right ankle edema | |
| 3 | 24 | dyspnea or exertion |
| 25 | thyroid exam: scar | |
| 26 | thyroid exam: single nodule | |
| 27 | thyroid exam: multiple nodules | |
| 28 | thyroid exam: diffuse enlargement | |
| 29 | other manifestation of thyroid disease | |
| 30 | abnormal breath sounds | |
| 31 | rales | |
| 32 | abnormal heart sounds | |
| 33 | liver enlarged | |
| 34 | premature beats on ECG | |
| 35 | pulmonary disease | |
| 4 | 36 | increased antero-posterior diameter |
| 37 | venous insufficiency or varicose veins |
The construction of the DI handles the problem of missing answers by counting only those questions which were explicitly answered. To ensure that missing answers do not bias the weight of deficits, however, all analyses were performed with individuals, for whom information on any of the selected 37 deficits was missing, excluded. The age range in all analyses was limited to that which is common for all exams, i.e., from 55 to 78 years. These yielded samples of N=2117 (MA=63.9 ± 0.13) for the 9th FHS, N=2471 (MA=65.6 ± 0.12) for the 14th FHS, and N=1274 (MA=63.1 ± 0.15) for the 5th FHSO exams.
For more reliable estimates, the patterns of the respective characteristics were plotted for 5-year age cohorts in each exam. They were computed for each deficit to elucidate whether there were consistent trends on an individual-deficit level as well as for the DIs composed of different numbers of deficits. The Cox proportional hazard regression model was used to evaluate the effects of the DIs—all as measured in the baseline exams—on the hazard of death considering deaths that occurred within the maximum follow-up period for the 14th exam, i.e., up to 24 years (the last known vital status assessment was at the 25th exam performed in 1998). The regression models were sex and age adjusted.
RESULTS
These analyses show, first, that the traditional approach of considering trends over time in individual traits associated with selected medical or lab exams (Table 1) generally fails. Specifically, only two deficits out of 37 (i.e., increased antero-posterior diameter [IAPD] and venous insufficiency or varicose veins [VV]; Table 1, group 4) showed consistent and significant downward trends over time in the 5-year age patterns. No definitive conclusions (except trivial on inconsistent trends) can be made about trends in the age patterns for other deficits. An advantage of the approach based on the DI is that the cumulative effect of traits with such non-consistent behaviors may be more informative.
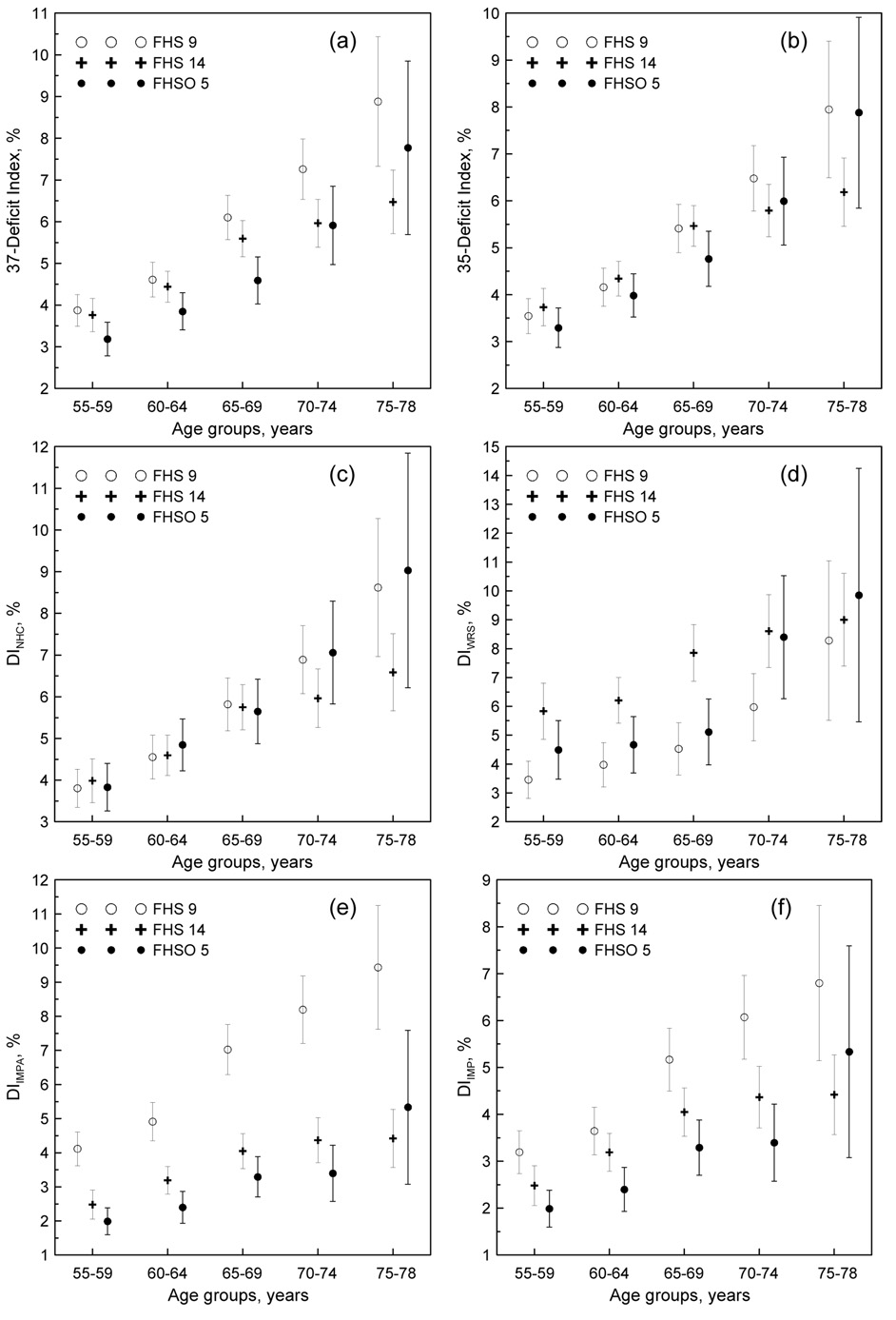
Figure 1 shows age patterns for (a) the full set of 37 deficits DI (DI37) as well as for (b) the 35-deficits DI (DI35) with IAPD and VV excluded. Downward trends indicating improvements in health are more pronounced for the DI37 than for the DI35 between the mid 1960s and the mid 1990s. This effect is, however, attributed to significant trends in the IAPD and VV.
Figure 1.
(a–b) Age patterns of the DIs constructed using (a) all 37 deficits selected for the analyses and (b) reduced set of 35 deficits with group 4 (see Table 1) excluded for participants of the 9th and 14th exams of the FHS and 5th exam of the FHSO as denoted in the inset. (c–f) Age patterns of the DIs characterizing health dimensions associated with (c) no health changes (DINHC), (d) health worsening (DIWRS), and (e and f) health improving (DIIMPA and DIIMP, respectively) over time. Bars show 95% confidence intervals.
Analysis of trends in the age patterns for individual deficits allowed us to identify subgroups of deficits with qualitatively different time behaviors. Specifically, we identified 18 deficits (Table 1, group 1) each of which exhibits no trend. These deficits were gathered into the respective DI which characterizes health dimensions associated with no health changes (DINHC). Five deficits (Table 1, group 2) showed inconsistent increasing trends over time. The respective DI is associated with worsening-over-time health dimension (DIWRS). A third group of 12 deficits was characterized by non-consistent trends of decline over time. This group does not include the IAPD and VV and characterizes improving-over-time health dimension (DIIMP). We also constructed alternative DI with these two deficits (i.e., IAPD and VV) included (DIIMPA).
As expected, Figure 1c shows no changes in health characterized by the DINHC at younger ages (55–69 years). For older ages, the results are inconsistent: the DINHC for ages 70–78 years tends to be lower at the 14th FHS exam compared to the 9th FHS and 5th FHSO exams. The DIWRS (Figure 1d) shows a pattern of increase over time (although due to small number of deficits included, this is not entirely convincing). The DIIMPA (Figure 1e) and DIIMP (Figure 1f) exhibit significant downward trends from the mid 1960s to the mid 1990s for all age groups except 75–78 years. It is important to recognize that the DIIMP is constructed without the IAPD and VV deficits.
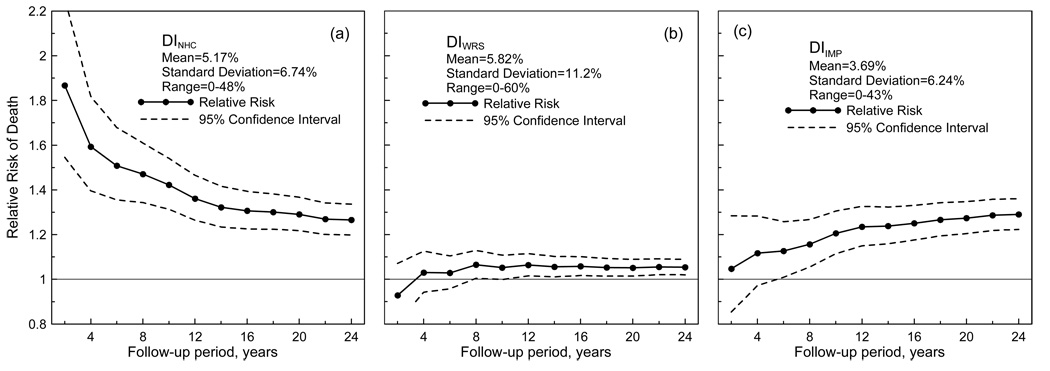
Are the respective DIs relevant to health deterioration with aging? To address this question, we evaluated the relative risks of death attributable to the DINHC, DIWRS, and DIIMP in multivariate Cox regression analyses of the pooled sample of participants of the 9th and 14th FHS and 5th FHSO exams with all three indices included. Figures 2a–c show that each of these indices can significantly predict death within certain periods of follow-up. The strongest determinant of the short-term risks of death is the DINHC. Its contribution into the hazard rate, however, declines when the follow-up time increases. In contrast, the contribution of the DIIMP increases when the follow-up time increases. The risks of death attributable to the DINHC and DIIMP converge in the long-term perspective. The relative risk attributable to the DIWRS is insignificant for the short-term follow-ups. It first increases and than quickly saturates. The DIWRS provide the weaker and less significant estimates than the other two indices.
Figure 2.
Relative risks of death evaluated for a 10% increase in (a) the DINHC, (b) the DIWRS, and (c) the DIIMP. Insets show means, standard deviations, and range for the respective DIs.
DISCUSSION AND CONCLUSIONS
The results show a high potential of an approach of cumulative deficits for characterizing the aggregate effect of small-effect health-related traits. Specifically, traditional analysis of trends in the age patterns of individual deficits identified only two (i.e., IAPD and VV) out of 37 deficits that showed consistent trends over time. The DI constructed on the basis of all 37 deficits shows trends for improvements in health status for the 5-year age groups ranging from 55 to 75 years between the mid 1960s and the mid 1990s (Figure 1a). These trends, however, were attributed to the effect of these two deficits, because no convincing trends were seen for the DI35 (i.e., with the IAPD and VV excluded).
The analyses reveal that the non-convincing results for the DI35 are due to this index aggregating small-effect traits for which the changes over time are of an opposite nature. Decomposing the set of 35 deficits (i.e., excluding the effect of the IAPD and VV) according to potential trends for each deficit (i.e., no, upward, or downward), we constructed the respective DIs characterizing health dimensions associated with no health changes (DINHC), health worsening (DIWRS), and health improving (DIIMP) over time to elucidate whether such aggregations of small-effect traits can be reliably informative. Aggregation of the 12 deficits with inconsistent downward trends into the DIIMP shows that such an index is capable of a more informative characterization; the DIIMP exhibits significant downward trends indicating improvements in health for the 5-year age groups ranging from 55 to 75 years between the 1960s and 1990s (Figure 1f). The results for the DIWRS, which was intended to characterize worseningover-time health dimension, are not as convincing as for the DIIMP because of small number of deficits used for construction of the DIWRS.
Although the DINHC, DIWRS, and DIIMP are significantly predictive of the mortality risks within different time horizons (Figure 2), their relationships to the risks are not identical. The mortality risks associated with the DIWRS, i.e., health worsening over time, are the weakest and least significant. The mortality risks attributable to the DINHC, which characterizes no changes in health over time, dominate within shorter time horizons. For longer time horizons, both the DINHC and DIIMP provide the same contribution to the risks of death.
Thus, the analyses show that a cumulative deficits approach might be an efficient tool for analyzing the effect of a large number of traits for which individual effects on survival are small, inconsistent, or non-significant. They show favorable trends such that health of the FHS/FHSO participants either did not change or improved over time for the most serious small-effect traits. This corroborates early findings [8] and provides a broader perspective on health trends because of the wide spectrum of the deficits that was considered.
ACKNOWLEDGEMENTS
The research reported in this paper was supported by the National Institute on Aging grants 1R01 AG028259, 1R01-AG-027019, R01 AG030612-01 and 5P01-AG-008761. The Framingham Heart Study (FHS) is conducted and supported by the NHLBI in collaboration with the FHS Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the FHS or the NHLBI.
LIST OF ABBREVIATIONS
- FHS
the Framingham Heart Study
- FHSO
the Framingham Heart Study Offspring
- DI
deficit index
- MA
mean age
- IAPD
increased antero-posterior diameter
- VV
venous insufficiency or varicose veins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Crimmins EM. Trends in the health of the elderly. Annu Rev Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- 2.Freedman VA, Crimmins E, Schoeni RF, Spillman BC, Aykan H, Kramarow E, Land K, Lubitz J, Manton K, Martin LG, Shinberg D, Waidmann T. Resolving inconsistencies in trends in old-age disability: Report from a technical working group. Demography. 2004;41:417–441. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- 3.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the united states: A systematic review. JAMA. 2002;288:3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 4.Cronin KA, Krebs-Smith SM, Feuer EJ, Troiano RP, Ballard-Barbash R. Evaluating the impact of population changes in diet, physical activity, and weight status on population risk for colon cancer (united states) Cancer Causes Control. 2001;12:305–316. doi: 10.1023/a:1011244700531. [DOI] [PubMed] [Google Scholar]
- 5.Andersen FK, Christensen K, Frederiksen H. Self-rated health and age: A cross-sectional and longitudinal study of 11,000 danes aged 45–102. Scand J Public Health. 2007;35:164–171. doi: 10.1080/14034940600975674. [DOI] [PubMed] [Google Scholar]
- 6.Martin LG, Schoeni RF, Freedman VA, Andreski P. Feeling better? Trends in general health status. J Gerontol B Psychol Sci Soc Sci. 2007;62:S11–S21. doi: 10.1093/geronb/62.1.s11. [DOI] [PubMed] [Google Scholar]
- 7.Allaire SH, LaValley MP, Evans SR, O'Connor GT, Kelly-Hayes M, Meenan RF, Levy D, Felson DT. Evidence for decline in disability and improved health among persons aged 55 to 70 years: The framingham heart study. Am J Public Health. 1999;89:1678–1683. doi: 10.2105/ajph.89.11.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa DL. Understanding the twentieth-century decline in chronic conditions among older men. Demography. 2000;37:53–72. [PubMed] [Google Scholar]
- 9.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 10.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051. doi: 10.1093/gerona/60.8.1046. [DOI] [PubMed] [Google Scholar]
- 12.Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity. Rejuvenation Res. 2007;10:75–86. doi: 10.1089/rej.2006.0500. [DOI] [PubMed] [Google Scholar]
- 13.Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007;55:935–940. doi: 10.1111/j.1532-5415.2007.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K, Yashin AI. Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol. 2007 doi: 10.1016/j.exger.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, Manton K. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: Results from analyses of the national long term care survey. Mech Ageing Dev. 2007;128:250–258. doi: 10.1016/j.mad.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitnitski A, Graham J, Mogilner A, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood K. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 18.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 19.Woo J, Goggins W, Sham A, Ho SC. Public health significance of the frailty index. Disabil Rehabil. 2006;28:515–521. doi: 10.1080/09638280500215867. [DOI] [PubMed] [Google Scholar]
- 20.Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L, Ukraintseva SV. Health decline, aging and mortality: How are they related? Biogerontology. 2007;8:291–302. doi: 10.1007/s10522-006-9073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: The framingham study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Kulminski A, Yashin A, Ukraintseva S, Akushevich I, Arbeev K, Land K, Manton K. Accumulation of health disorders as a systemic measure of aging: Findings from the nltcs data. Mech Ageing Dev. 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitnitski A, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59:M627–M632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev. 2004;125:517–519. doi: 10.1016/j.mad.2004.05.003. [DOI] [PubMed] [Google Scholar]