Abstract
Study Objectives:
Epidemiological studies have demonstrated an association between short sleep times and obesity as defined by body mass index (BMI). We wanted to determine whether this association occurs in patients with chronic medical diagnoses since the number of confounding factors is likely higher in patients than the general population.
Methods:
Two hundred patients attending internal medicine clinics completed a survey regarding sleep habits, lifestyle characteristics, and medical diagnoses. An independent surveyor collected the information on the questionnaires and reviewed the medical records. Height and weight were measured by clinic personnel. Data were analyzed with multivariate logistic regression.
Results:
Subjects with short sleep times (< 7 hours) had an increased likelihood of obesity as defined by a BMI ≥ 30 kg/m2 when compared to the reference group of (8, 9] hours (odds ratio 2.93; 95% confidence interval, 1.06–8.09). There was a U-shaped relationship between obesity and sleep time in women but not in men. Young age (18 to 49 years), not smoking, drinking alcohol, hypertension, diabetes, and sleep apnea were also associated with obesity in the overall model.
Conclusions:
This study demonstrates an association between short sleep times and obesity in undifferentiated patients attending an internal medicine clinic using models adjusting for age, lifestyle characteristics, and some medical diagnoses. The U-shaped relationship in women suggests that sleep patterns may have gender specific associations. These observations provide the background for therapeutic trials in weight loss in patients with established medical problems.
Citation:
Buscemi D; Kumar A; Nugent R; Nugent K. Short sleep times predict obesity in internal medicine clinic patients. J Clin Sleep Med 2007;3(7):681–688.
Keywords: Sleep time, lifestyle habits, obesity, observational study, logistic regression
Epidemiological studies have demonstrated that shorter sleep times are associated with an increased likelihood of obesity as defined by body mass index (BMI). Gangwisch, et al. analyzed the data collected in the NHANES-1 Study, a cross-sectional study using a probability sample of civilian non-institutionalized adults in the United States.1 This study demonstrated that subjects in the age range of 32 to 49 years who slept <7 hours/night were more likely to be obese than those who slept >7 hours/night. Singh, et al. reported results from a population-based study in which subjects responding to a telephone survey self-reported sleep times over a 2-week period.2 After calculating a weighted total sleep time average, they found that subjects with sleep times of ≤6 hours had a higher probability of obesity than subjects who slept 7 hours. Vorona, et al. have reported the only information available from a primary care clinic population.3 In this study, women slept more than men, and overweight and obese patients slept less than patients with a normal BMI. This study did not find any relationship between total sleep time and several medical diagnoses. We now report a clinic-based survey of adult patients attending internal medicine resident clinics who completed a supervised questionnaire regarding sleep time, lifestyle characteristics, and medical diagnoses. We hypothesized that obese patients with chronic medical problems frequently associated with obesity would have shorter sleep times than nonobese patients.
METHODS
Subjects
The Internal Medicine residency program at Texas Tech University Health Science Center in Lubbock, Texas, has 29 residents. These residents see continuity-of-care clinic patients in the departmental clinics on Mondays through Fridays in the afternoon. These clinics have approximately 3400 visits per year. The most frequent diagnoses are hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), coronary artery disease, congestive heart failure, rheumatoid arthritis, renal insufficiency, chronic renal failure, systemic lupus erythematosus, and asthma. The ethnic backgrounds include 43% Hispanic, 40% Caucasian (non-Hispanic), 14% African American, 0.9% American Indian, 0.2% other and 1.6% unknown (no reply on information request). The patients have the following health care insurance: commercial (8%), Medicaid (18%), Medicare (37%), self-pay (36%), and other government programs (1%). Overall clinic information on diagnoses, ethnicity, and payer status was obtained using composite records from the business office.
Subjects were identified prospectively using a convenience sample from the Internal Medicine clinic during March and April 2005. Two hundred people agreed to participate; four refused. Patients were visiting the clinic for scheduled medical care. Participants were administered a lifestyle questionnaire by an independent individual surveyor who collected all responses. The clinic records provided medical diagnoses. Two authors (DB, AK) worked as attending physicians during this study and were on-site for supervision. We did not record information on medication use. This study was approved by the Institutional Review Board at Texas Tech University Health Science Center.
Measures
The outcome of interest was the patient's body mass index (BMI), weight in kilograms divided by the square of height in meters − kg/m2, at time of clinic visit. Heights and weights were measured by clinic staff. The World Health Organization suggests the following international BMI classification: underweight (BMI < 18.5 kg/m2), normal (18.5 kg/m2 < BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), obese (30 kg/m2 ≤ BMI < 35 kg/m2), very obese (35 kg /m2 ≤ BMI < 40 kg/m2), and morbidly obese (40 kg/m2 < BMI). However, for this analysis, we focused only on the clinical definition of obesity; patients were classified as either not obese (BMI < 30 kg/m2) or obese (BMI ≥ 30 kg/m2). The predictor variable of interest was the patient's number of hours of sleep obtained each night or total sleep time (TST). Self-reported regular bed times (to the nearest quarter hour) and wake times (to the nearest quarter hour) were recorded. The exact questions were “what time do you go to sleep?” and “what time do you get up?” Bedtime was defined as the time the patient went to bed with the intent to fall asleep. Patients were asked about the regularity of their bed and wake times. If these times varied, additional questions were asked regarding the patient's range of bedtimes and wake times during the week prior to the clinic visit. The patients were also questioned about nap frequency and length. However, for this analysis, we used total sleep time at night, defined as the number of hours between the self-reported regular bedtime and self-reported regular wake time. This approach was used in earlier studies.1,2,4 No questions were asked about the quality of sleep.
Control variables in this study included age, gender, work status, disabled status, number of daily meals, diet status, physical activity, smoking, alcohol consumption, and the presence of the following diagnoses: diabetes (n=80), hypertension (124), coronary artery disease (35), heart failure (26), arthritis (101), sleep apnea (17), and COPD (36). One hundred and seventy-eight patients had at least one of these diagnoses. Smoking status was defined by three categories: current, former, and never smokers. Physical activity was classified as either active (regular exercise not further quantified) or inactive. Alcohol consumption was categorized as zero drinks per week or one or more drinks per week. All status and medical condition variables were binary (Yes/No).
Statistical Analyses
All statistical analyses were conducted using the statistical software package R version 1.8.1. General demographics, lifestyle characteristics, and medical diagnoses in the study population were summarized with means and standard deviations, medians and ranges, or categorical counts. One male patient of excessive BMI (76.4) was excluded as an outlier. For the univariate analysis, t-tests were used to test for significant subgroup differences in both the BMI means and TST means.
We used multivariate logistic and linear regression analyses to model the relationship between obesity and the total sleep time quartiles adjusting for gender, age, work status, disabled status, number of meals, diet status, physical activity, alcohol consumption, and the presence of all aforementioned medical diagnoses. For the logistic regression, significant variables were determined by 95% confidence intervals for odd ratios; for the linear regression, p-values were used. Since the results of the linear regression mirrored those of the logistic regression, we focused the remainder of our analyses on predicting obesity status using logistic regression. The overall significance of the models was determined by Wald chi-square tests. In subgroup analyses, we separately stratified the patients by gender (M/F) and by age group (18–49/>50) and performed multivariate logistic regression analyses on these subgroups.
RESULTS
The final study population included 74 men (37.2%) and 125 women (62.8%). The median age was 54 with a range of 18 to 89. The mean BMI was 30.1 ± 7.9 kg/m2. Eighty-two patients (41.2%) were obese (BMI ≥ 30 kg/m2). Univariate analyses indicated several statistically significant differences in mean BMI: women are heavier than men, young patients (18–49) are heavier than older patients (≥ 50), patients eating three or more meals per day are lighter than patients who eat fewer than three, patients on a diet are heavier than those who are not, and patients with sleep apnea are heavier than those without this diagnosis (p < 0.05; Appendix). Population and lifestyle characteristics are summarized in Table 1.
Table 1.
Study Population and Lifestyle Characteristics
| Gender | |
| Men | 74 (37.2)a |
| Women | 125 (62.8) |
| Age (yrs) | 54 (18–89)b |
| Weight (lbs) | 183.69 (53.55)c |
| Height (in) | 65.35 (3.77)c |
| Body Mass Index (kg/m2) | 30.10 (7.93)c |
| BMI categories | |
| < 18.5 | 7 (3.5)a |
| [18.5, 25) | 42 (21.1) |
| [25,30) | 68 (34.2) |
| [30,35) | 41 (20.6) |
| [35,40) | 19 (9.5) |
| ≥ 40 | 22 (11.1) |
| Working | |
| Yes | 47 (23.6)a |
| No | 152 (76.4) |
| Disabled | |
| Yes | 65 (32.7)a |
| No | 134 (67.3) |
| Smoking Status | |
| Never | 85 (42.7)a |
| Former | 49 (24.6) |
| Current | 65 (32.7) |
| Exercise | |
| Yes | 77 (38.7)a |
| No | 122 (61.3) |
| Alcohol | |
| Yes | 47 (23.6)a |
| No | 152 (76.4) |
| No. of Meals | |
| 1–1.5 | 52 (26.1)a |
| 2 | 71 (35.7) |
| ≥ 3 | 76 (38.2) |
| Diet | |
| Yes | 23 (11.6)a |
| No | 175 (87.9) |
n (%)
Median (Range)
Mean (SD)
The mean total sleep time was 7.89 ± 1.91 hours. The median sleep time was 8.00 hours (Q1=7, Q3=9). Eighty patients (40.2%) took naps with a median nap length of one hour. Univariate analyses indicated the following statistically significant differences in mean TST: working patients slept less than those not working, current smokers slept less than former or never smokers, and patients with coronary artery disease slept less than those without coronary artery disease (p < 0.05; Appendix). Sleep time and its variability are summarized in Table 2.
Table 2.
Sleep and its Variability
| Total Sleep Time (hrs) | |
| Overall | 7.89 (1.91)a |
| by Gender | |
| Men | 7.72 (1.71)a |
| Women | 7.99 (2.02) |
| by Age | |
| 18–49 | 7.92 (1.87)a |
| 50–59 | 7.65 (2.19) |
| 60–89 | 8.06 (1.68) |
| by BMI category | |
| < 18.5 | 7.42 (3.60)a |
| [18.5,25) | 7.99 (1.74) |
| [25,30) | 8.09 (1.77) |
| [30, 35) | 7.48 (1.32) |
| [35, 40) | 7.74 (1.84) |
| ≥ 40 | 8.11 (2.79) |
| Total Sleep Time Quartiles | |
| < 7 | 56 (28.1)c |
| [7,8] | 63 (31.7) |
| (8,9] | 43 (21.6) |
| > 9 | 37 (18.6) |
| Regular Bed Time | |
| Yes | 132 (66.3)c |
| No | 67 (33.7) |
| Bed Time Range (hrs)d | 3.00 (2.00 – 4.00)b |
| Regular Wake Time | |
| Yes | 163 (81.9)c |
| No | 36 (18.1) |
| Wake Time Range (hrs)d | 2.00 (2.00 – 4.25)b |
| Nap | |
| Yes | 80 (40.2)c |
| No | 119 (59.8) |
| Nap Length if Yes (hrs) | 1.00 (0.50 – 1.50)b |
Mean (SD)
Median (1st Q – 3rd Q)
n (%)
Patients were asked about differences in bed and wake times. If these times varied, a range of times was provided by the patient for the most recent week.
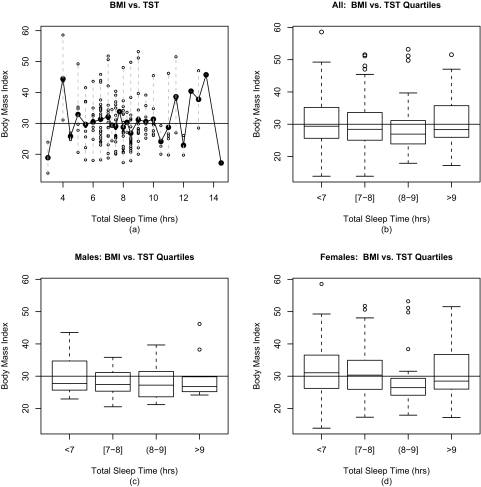
Prior to any modeling, we explored the relationship between total sleep time and body mass index (Figure 1). In Figure 1a, the individual and mean BMIs are graphed for each unique TST value. The TST subgroups clearly do not demonstrate a linear trend in BMI. The change in BMI associated with a one-hour increase in TST is dependent on the number of hours of sleep and not constant as would be assumed by a model with TST as a continuous variable. In addition, logistic regression analyses predicting obesity (<30/≥30) with each hour of sleep as a predictor variable generated log odds that were far from linear (results not shown). Figure 1a also indicates a wide range of sample sizes for the TST subgroups. Since very few subjects reported either very low or high total sleep times, we would not be confident in the generated odds ratios for those extreme TST values. For these reasons, total sleep time ultimately was categorized by quartiles to create roughly equal-sized groups of adequate size to support multivariate models; quartiles also generated easily relatable categories of total sleep time: <7; [7–8]; (8–9]; >9 hours of sleep per night (distribution in Table 2; notation: “[“ indicates inclusion of interval endpoint in the quartile). The distribution of BMI by total sleep time quartiles is graphed for all patients (Figure 1b) and by gender (Figure 1c, 1d). Stratifying by gender reveals different distributions across quartiles; men have a slightly negative relationship, and women have a U-shaped relationship.
Figure 1.
In Figure 1a, the individual BMIs are plotted versus the TSTs in open circles. The mean BMI for each TST value is plotted in black; the BMI range for each TST value is marked by a dashed grey line. In Figure 1b, boxplots illustrate the BMI distribution for each TST quartile for all patients. The top of the box is the 3rd quartile BMI; the black line across the box is the median BMI; and the bottom of the box is the 1st quartile BMI. Outliers are plotted as circles. Figure 1c and Figure 1d illustrate the BMI distributions for each TST quartile for men and women respectively. In all four subfigures, a horizontal line is graphed at BMI = 30 kg/m2, the obesity cut point.
Logistic regression was used to model the relationship between all factors of interest and obesity status (BMI ≥ 30 kg/m2) (Table 3). In the unadjusted analyses, the likelihood of obesity significantly increased with short sleep times (< 7 vs. (8–9]) (Odds Ratio [OR] 2.41), young age (18–49) (OR 2.75), a positive response to “Are you on a diet?” (OR 3.77), never smoking (vs. currently smoking) (OR 2.00), and a clinical diagnosis of apnea (OR 3.84). In the multivariate adjusted model, the factors significantly associated with obesity included sleep times ≤8 hours (<7 vs. (8–9], OR 2.93; [7–8] vs. (8–9], OR 2.88), female gender (OR 2.44), young age (OR 4.62), and selected lifestyle characteristics and medical diagnoses (Table 3). The overall model was significant (p < 0.0001). The interaction between gender and TST first noted in Figure 1 was also modeled in a more complex multivariate adjusted logistic regression (results not shown). While a significant difference in the TST quartile distributions exists, the complexity of the model decreased its ease of interpretability and was not well supported by the size of the study.
Table 3.
Logistic Regression Results for Predicting Obesity (BMI ≥ 30 kg/m2)a
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)b | |
|---|---|---|
| TST (hrs): | ||
| < 7 | 2.41 (1.03, 5.62) | 2.93 (1.06, 8.09) |
| [7,8] | 2.20 (0.96, 5.06) | 2.88 (1.02, 8.10) |
| (8,9] | reference | reference |
| > 9 | 1.57 (0.61, 4.03) | 1.73 (0.52, 5.73) |
| Gender: | ||
| Men | ref | ref |
| Women | 1.80 (0.99, 3.29) | 2.44 (1.05, 5.64) |
| Age: | ||
| 18–49 | 2.75 (1.51, 5.03) | 4.62 (1.88, 11.36) |
| ≥ 50 | ref | ref |
| Working: | ||
| Yes | 1.51 (0.78, 2.92) | 0.82 (0.30, 2.23) |
| No | ref | ref |
| Disabled: | ||
| Yes | 1.48 (0.82, 2.70) | 1.49 (0.61, 3.63) |
| No | ref | ref |
| No. of Meals: | ||
| 1–2 | ref | ref |
| ≥ 3 | 0.68 (0.38, 1.23) | 0.45 (0.21, 0.98) |
| Diet: | ||
| Yes | 3.77 (1.48, 9.66) | 7.24 (2.14, 24.49) |
| No | ref | ref |
| Exercise: | ||
| Yes | 1.22 (0.68, 2.17) | 1.01 (0.48, 2.14) |
| No | ref | ref |
| Smoker: | ||
| Never | 2.00 (1.03, 3.90) | 3.58 (1.40, 9.17) |
| Former | 1.04 (0.48, 2.27) | 1.54 (0.58, 4.11) |
| Current | ref | ref |
| Alcohol: | ||
| Yes | 1.35 (0.70, 2.61) | 4.05 (1.55, 10.62) |
| No | ref | ref |
| Diabetes: | ||
| Yes | 1.19 (0.67, 2.12) | 2.34 (1.05, 5.21) |
| No | ref | ref |
| Hypertension: | ||
| Yes | 1.55 (0.86, 2.81) | 2.62 (1.17, 5.86) |
| No | ref | ref |
| Coronary Artery Disease: | ||
| Yes | 0.94 (0.45, 1.98) | 1.98 (0.68, 5.81) |
| No | ref | ref |
| Heart Failure: | ||
| Yes | 0.48 (0.19, 1.20) | 0.19 (0.05, 0.78) |
| No | ref | ref |
| Arthritis: | ||
| Yes | 1.03 (0.59, 1.82) | 1.17 (0.54, 2.54) |
| No | ref | ref |
| Apnea: | ||
| Yes | 3.84 (1.30, 11.37) | 8.71 (2.11, 35.89) |
| No | ref | ref |
| COPD: | ||
| Yes | 0.89 (0.42, 1.86) | 0.57 (0.18, 1.82) |
| No | ref | ref |
Odds Ratios Significance: bold (p < 0.05); italics (0.05≤ p < 0.10)
Adjusted model significant; p< 0.0001
The patients were stratified by gender and age for additional exploratory subgroup analyses (Table 4). A U-shaped relationship in women emerged; both short and long sleep time quartiles (<7; [7–8]; >9) were associated with obesity when compared to the reference quartile of (8–9]. Men were more likely to be obese if they were between the ages 18 and 49. Use of alcohol was associated with obesity in men and older patients (≥50). Men were more likely to be obese if diabetic; women were more likely to be obese if hypertensive. Sleep apnea was associated with an increased likelihood of obesity in women and older patients. All subgroup models were significant (p < 0.0001).
Table 4.
| Overall | Women | Men | 18–49 | 50–89 | |
|---|---|---|---|---|---|
| TST: | |||||
| < 7 hrs |
2.93 (1.06, 8.09) |
11.06 (2.15, 56.74) |
1.49 (0.21, 10.66) |
4.60 (0.45, 46.82) |
2.15 (0.59, 7.81) |
| [7,8] hrs |
2.88 (1.02, 8.10) |
7.25 (1.48, 35.52) |
1.52 (0.18, 12.79) |
2.98 (0.33, 27.20) |
3.16 (0.82, 12.15) |
| (8,9] hrs | reference | reference | reference | reference | reference |
| >9 hrs | 1.73 (0.52, 5.73) |
6.97 (1.17, 41.52) |
0.53 (0.03, 8.07) |
13.75 (0.75, 251.57) |
0.83 (0.18, 3.85) |
| Gender: | 2.44 | ----- | ----- | 1.87 | 3.31 |
| Women vs. Men | (1.05,5.64) | (0.22, 15.62) | (1.04, 10.50) | ||
| Age: | 4.62 | 3.16 | 25.31 | ----- | ----- |
| 18–49 vs. ≥ 50 | (1.88, 11.36) | (0.99, 10.06) | (2.24, 285.61) | ||
| Working: | 0.82 | 1.43 | 0.19 | 5.39 | 0.30 |
| Yes vs. No | (0.30, 2.23) | (0.37, 5.54) | (0.02, 1.88) | (0.46, 62.85) | (0.07, 1.41) |
| Disabled: | 1.49 | 4.71 | 0.17 | 1.03 | 2.45 |
| Yes vs. No | (0.61,3.63) | (1.33, 16.71) | (0.02, 1.26) | (0.08, 13.03) | (0.82, 7.30) |
| No of Meals: | 0.45 | 0.51 | 0.21 | 0.07 | 0.65 |
| ≥ 3 vs. < 3 | (0.21, 0.98) | (0.18, 1.47) | (0.03, 1.24) | (0.01, 0.50) | (0.23, 1.89) |
| Diet: | 7.24 | 11.40 | 2.77 | 1.30 | 16.56 |
| Yes vs. No | (2.14, 24.49) | (2.16, 60.18) | (0.16, 48.01) | (0.07, 22.72) | (3.38, 81.03) |
| Exercise: | 1.01 | 1.19 | 0.35 | 0.61 | 1.10 |
| Yes vs. No | (0.48, 2.14) | (0.42, 3.35) | (0.06, 1.88) | (0.11, 3.49) | (0.39, 3.14) |
| Smoker Status: | |||||
| Never |
3.58 (1.40, 9.17) |
6.76 (1.68, 27.25) |
2.33 (0.31, 17.30) |
9.74 (1.05, 89.97) |
2.71 (0.79, 9.33) |
| Former | 1.54 (0.58, 4.11) |
2.09 (0.44, 9.87) |
1.20 (0.20, 7.09) |
2.23 (0.13, 37.36) |
1.16 (0.33, 4.01) |
| Current | reference | reference | reference | reference | reference |
| Alcohol: | 4.05 | 4.18 | 11.03 | 5.41 | 8.19 |
| Yes vs. No | (1.55, 10.62) | (0.86, 20.26) | (2.01, 60.55) | (0.59, 50.01) | (2.11, 31.79) |
| Diabetes: | 2.34 | 1.46 | 12.74 | 3.37 | 2.20 |
| Yes vs. No | (1.05, 5.21) | (0.51, 4.18) | (1.80, 90.26) | (0.48, 23.85) | (0.77, 6.27) |
| Hypertension: | 2.62 | 3.21 | 3.44 | 2.83 | 2.64 |
| Yes vs. No | (1.17, 5.86) | (1.12, 9.17) | (0.52, 22.50) | (0.54, 14.78) | (0.83, 8.47) |
| Coronary Artery Disease: | 1.98 | 1.12 | 3.96 | 0.50 | 1.91 |
| Yes vs. No | (0.68, 5.81) | (0.19, 6.46) | (0.52, 30.21) | (0.00, 32.95) | (0.54, 6.79) |
| Heart Failure: | 0.19 | 0.12 | 0.14 | c | 0.31 |
| Yes vs. No | (0.05, 0.78) | (0.02, 0.73) | (0.01, 2.57) | (0.06, 1.52) | |
| Arthritis: | 1.17 | 1.12 | 1.20 | 3.34 | 1.18 |
| Yes vs. No | (0.54, 2.54) | (0.39, 3.21) | (0.25, 5.77) | (0.30, 37.42) | (0.44, 3.13) |
| Apnea: | 8.71 | 8.64 | 3.07 | c | 9.72 |
| Yes vs. No | (2.11, 35.89) | (1.30, 57.60) | (0.19, 48.36) | (1.75, 53.82) | |
| COPD: | 0.57 | 1.23 | 0.85 | 10.44 | 0.19 |
| Yes vs. No | (0.18, 1.82) | (0.24, 6.22) | (0.08, 8.90) | (0.53, 204.03) | (0.04, 0.99) |
Odds Ratios Significance: bold (p < 0.05); italics (0.05≤ p < 0.10)
All models significant; p< 0.0001.
Small sample size in variable's reference group gives uninterpretable odds ratios (zero or infinity)
DISCUSSION
This study found that sleep time, patient demographics, lifestyle characteristics, and some medical diagnoses have a significant association with obesity as defined by a BMI ≥ 30 kg/m2. In the overall model sleep times less than the reference quartile of (8, 9] hours increased the likelihood of obesity. Young age (18–49), acknowledging dieting, never smoking, drinking alcohol, and the diagnoses of hypertension, diabetes and sleep apnea also increased the likelihood of obesity in this model. Our results demonstrate that short sleep times have a significant association with obesity in internal medicine patients with common chronic medical problems. Moreover, they indicate that these chronic diseases and attendant therapies and/or changes in physical activity do not obscure this relationship.
Vorona, et al. have reported the only information currently available on the relationship between sleep time and obesity in patients attending primary care clinics.3 This study included 924 subjects with an age range of 18 to 91. Mean BMI in this population was 30 ± 7 kg/m2. Thirty-nine percent of the subjects had hypertension, 17% diabetes, 9% CAD, and 33% arthritis. Men slept less than women (7.5 vs. 7.9 hours, p = 0.009). Obese patients had a significantly shorter total sleep time than patients with a normal weight, but there was no significant interaction between gender and BMI in the total sleep time analysis. The average difference in total sleep time between the normal and obese groups was 1.86 hours per week. The average difference in BMI was 10 kg/m2. Consequently, a one hour decrease in sleep time per week was associated with an increase in BMI of 5.4 kg/m2 in this cohort. These authors found no clear relationship between medical diagnoses and sleep time but did find an increased BMI in patients with some diagnoses. Our univariate analyses mirror these results. In addition, we used multivariate logistic regression to model the relationship between sleep time and obesity while controlling for other factors, including clinical diagnoses, and found a significant difference between men and women. Furthermore, in our models stratified by gender, both short sleep time and long sleep time quartiles (when compared to the reference quartile) had an association with obesity in women.
Gangwisch, et al. analyzed the information pertinent to sleep and obesity collected in the NHANES-1 Survey.1 This survey included both longitudinal (8073 subjects) and cross-sectional (9588 subjects) data from a probability sample of the civilian non-institutional population in the United States. They found that subjects in the age range of 32 to 49 who slept <7 hours per night were more likely to be obese than subjects who slept ≥7 hours per night. The odds ratio for obesity for subjects sleeping 2 to 4 hours per night was 2.35 (95% CI 1.36–4.05) when compared to subjects sleeping seven hours per night after adjustment for potential confounding variables. When gender was analyzed, the association between short sleep duration at night and obesity occurred only in women. Subjects with short sleep times (2–4 h) tended to gain weight over time, but this trend was not statistically significant. There was a nonsignificant trend towards increased obesity in men who slept >9 hours per day and in women who slept >8 hours per day. We found a statistically significant association between obesity and both short and long sleep times in females. Furthermore, our patients did not have the same degree of sleep restriction, as only 5% slept <4 hours. This result suggests that less sleep restriction in patients has the potential for greater adverse effects on weight gain than in normal subjects from population studies.
Singh, et al. collected information using telephone interviews with a population-based sample of 3158 subjects.2 These participants reported their sleep times at night over the previous 2 weeks which were then converted to a weighted total sleep time average by the investigators. The overall prevalence of obesity in the sample was 24.8%. Subjects who slept <6 hours had a higher prevalence of obesity than subjects in the reference group (7–8 hr). After correction for confounding factors, including age, sex, snoring, hypertension, and alcohol intake, their odds ratio was 1.7 (95% CI 1.3–2.3). These investigators did not report any differences between men and women in their analysis. The prevalence for obesity plotted against total sleep time appeared to have a U-shaped distribution but was not significant at the upper end. In contrast, the Wisconsin Sleep Cohort Study (1024 subjects) did find a significant U-shaped relationship between the average sleep time at night and BMI after adjustment for age and gender.4 The minimum BMI occurred around 7.7 hours of nightly sleep. Other studies, including our study with clinic patients, have also reported U-shaped associations between total sleep times and BMI, especially in women.5–7 The overall effect of sleep restriction on BMI is modest, and the BMI in the Wisconsin cohort increased approximately one kg/m2 when the average nightly sleep decreased from 8 hours to 5 hours. The effect of a change in sleep time on BMI reported in our study with patients was much less pronounced than the effect calculated by Vorona, et al; this difference probably reflects the complex set of factors which influence BMI.
Several factors could explain the relationship between short sleep times and increased BMIs and/or obesity in patients. This association may reflect mere caloric opportunity as patients with shorter sleep periods are awake for longer periods and have more opportunities to eat. It may also reflect habitual inactivity associated with short sleep periods in some patients resulting in decreased energy expenditure. For example, Vioque, et al. reported that obese subjects spend more time watching television than non-obese subjects, and Cournot, et al. found that an increased BMI is associated with low physical activity and television watching.8,9 Chronic medical problems could influence both sleep time and weight gain and possibly explain these observations. Sleep apnea, for example, could reduce sleep times and increase body weight. Multivariate analysis allows for adjustment for these potential confounders but requires the inclusion of all relevant factors in the model. Unknown confounders omitted from data collection would prevent corresponding adjustments. Finally, disease chronicity could create complex interactions not apparent in a cross-sectional study.
Interactions among hormones, appetite regulation, and sleep time could also explain the association between obesity and short sleep periods. Taheri, et al. studied 1,024 subjects in the Wisconsin cohort who provided a 6-day diary, completed a questionnaire regarding sleep times, and had overnight polysomnography followed by blood sampling in the morning. This study demonstrated that short sleep periods were associated with reduced leptin levels and elevated ghrelin levels.4 The hormones ghrelin and leptin modulate appetite; in particular, elevated ghrelin levels and reduced leptin levels should increase appetite. Experimental studies have demonstrated that acute sleep restriction decreases serum leptin levels, increases ghrelin levels, and increases appetite, especially for carbohydrate rich food.10–12 The data reported by Taheri (Table 2, Reference 4) also indicate that men and women of the same age and BMI have different leptin and ghrelin levels. The higher ghrelin levels in female subjects may help explain the differences in sleep time associations between men and women found in our and other studies. Alternatively, sex hormones and reproductive events could explain the differences between men and women. In particular, women have reduced sleep time and sleep efficiency during the post partum period.13–15 These changes and maladaptive sleep schedules associated with family responsibilities could cause chronic sleep restriction in some women, resulting in changes in leptin and ghrelin and chronic weight gain.
Our study has several important limitations and illustrates the inherent complexity in studies with patients with chronic medical problems. The self-reported information clearly depends on patient recall. Patients may have subconscious preferences for the time they report or report “what's expected.” However, other reports have described a good correlation between sleep questionnaires and other measures of sleep.4 In addition, we do not know whether short sleepers voluntarily restrict their sleep or involuntarily restrict their sleep secondary to medical or environmental disturbances; this difference could have important implications in pathogenesis. Most studies on sleep time and obesity have focused on short sleep times and have examined physiological pathways that could explain the effect of sleep restriction on BMI. Long sleepers likely differ substantially from short sleepers, and the explanation between long sleep times and an increased BMI is uncertain. Chronic poor health, depression, and/or low socioeconomic status could be causal factors in this group.16 Clearly, the metabolic studies reported by Taheri, et al do not provide a plausible hypothesis since leptin levels increased and ghrelin levels decreased with longer sleep times. Finally, our study, like most others, did not provide any information about the quality of sleep which may also influence the health effects related to sleep.
In summary, this study found that shorter sleep times in patients with chronic medical problems are associated with an increased likelihood of obesity. The subgroup analyses reveal a U-shaped curve in women with an increased prevalence of obesity in both short sleep periods and longer sleep periods. Future clinical studies should focus on narrower questions (such as the effect of sleep in a single disease states, e.g., diabetes17), on each gender separately, on the causes of sleep restriction (voluntary or involuntary), on therapeutic interventions, and/or on data collection which does not completely depend on patient recall.18 Physicians probably should include this information in patient management. In particular, optimal sleep time may promote weight loss.
ACKNOWLEDGMENT
We thank Miranda Peek for her excellent work on the patient surveys.
Appendix
Analysis of BMI and TST by Patient Characteristics
| Average BMI (kg/m2) (SD) | Average TST (hrs) (SD) | |||
|---|---|---|---|---|
| All participants | (n=199) | 30.11 (7.93) | 7.89 (1.91) | |
| Gender: | Men | (74) | 28.80 (5.34) | 7.72 (1.71) |
| Women | (125) | 30.88 (9.06) | 7.99 (2.02) | |
| Age: | 18–49 | (68) | 32.76 (8.76) | 7.92 (1.87) |
| 50–59 | (60) | 29.45 (7.57) | 7.65 (2.19) | |
| 60–89 | (71) | 28.12 (6.67) | 8.06 (1.68) | |
| 18–49 vs. 50+ | ||||
| Work Status: | Yes | (47) | 30.56 (6.36) | 7.18 (1.26) |
| No | (152) | 29.96 (8.37) | 8.11 (2.02) | |
| Disabled: | Yes | (65) | 31.68 (9.68) | 8.14 (2.35) |
| No | (134) | 29.35 (6.84) | 7.78 (1.65) | |
| Meals: | 1–1.5 | (52) | 30.27 (7.71) | 7.53 (1.70) |
| 2 | (71) | 31.49 (8.36) | 8.10 (2.02) | |
| ≥ 3 | (76) | 28.70 (7.52) | 7.94 (1.92) | |
| 1–2 vs. ≥ 3 | ||||
| Diet: | Yes | (23) | 34.15 (7.36) | 8.47 (1.63) |
| No | (175) | 29.58 (7.89) | 7.81 (1.93) | |
| Exercise: | Yes | (77) | 29.69 (6.99) | 7.79 (1.72) |
| 1–3/week | (31) | 29.67 (7.19) | 7.48 (1.53) | |
| 4–5/week | (46) | 29.70 (6.93) | 7.99 (1.82) | |
| No | (122) | 30.37 (8.49) | 7.96 (2.02) | |
| Smoking: | Never | (85) | 31.86 (8.72) | 8.01 (1.89) |
| Former | (49) | 29.03 (6.20) | 8.17 (1.87) | |
| Current | (65) | 28.63 (7.66) | 7.52 (1.93) | |
| former/never vs. current | former/never vs. current | |||
| former/current vs. never | ||||
| Alcohol: | Yes | (47) | 30.48 (7.26) | 7.57 (1.22) |
| 1–3/week | (22) | 32.65 (8.59) | 7.83 (1.19) | |
| ≥ 4/week | (25) | 28.57 (5.32) | 7.35 (1.23) | |
| No | (152) | 29.99 (8.15) | 7.99 (2.07) | |
| 1–3 vs. ≥ 4 | yes vs. no | |||
| Diabetes: | Yes | (80) | 30.41 (7.97) | 7.84 (2.08) |
| No | (119) | 29.90 (7.93) | 7.92 (1.79) | |
| Hypertension: | Yes | (124) | 30.44 (7.74) | 7.85 (1.99) |
| No | (75) | 29.57 (8.26) | 7.94 (1.78) | |
| Coronary | Yes | (35) | 29.28 (7.67) | 7.30 (1.88) |
| Artery Disease: | No | (164) | 30.38 (7.99) | 8.02 (1.89) |
| Heart Failure: | Yes | (26) | 29.99 (8.20) | 8.73 (2.32) |
| No | (173) | 30.13 (7.96) | 7.76 (1.81) | |
| Arthritis: | Yes | (101) | 30.37 (8.20) | 7.79 (1.94) |
| No | (98) | 29.84 (7.68) | 8.00 (1.88) | |
| Apnea: | Yes | (17) | 37.16 (11.47) | 7.88 (2.56) |
| No | (182) | 29.45 (7.22) | 7.89 (1.84) | |
| COPD: | Yes | (36) | 31.33 (10.23) | 7.79 (1.76) |
| No | (163) | 29.84 (7.34) | 7.91 (1.94) |
T-test for difference between the two subgroup means: bold (p < 0.05); italics (0.05≤ p < 0.10)
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES-1. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 2.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 3.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware C. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 4.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:210–17. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 6.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–36. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep. 2005;28:1217–20. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- 8.Vioque J, Torres A, Quiles Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes. 2004;24:1683–8. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 9.Cournot M, Ruidavets J, Marquie J, Esquirol Y, Baracat B, Ferrieres J. Environmental factors associated with body mass index in a population of Southern France. Eur J Cardiovas Prev Rehabil. 2004;11:291–7. doi: 10.1097/01.hjr.0000129738.22970.62. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Cauter EV. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metabl. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 12.Mullington JM, Chan JL, Van Dongen A, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang M, Matsumoto K, Shinkoda H, Mishima M, Seo Y. Longitudinal study for sleep-wake behaviours of mothers from pre-partum to post-partum using actigraph and sleep logs. Psychiatry Clin Neurosci. 2002;56:251–2. doi: 10.1046/j.1440-1819.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Zaffke M, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 15.Parry B, Martinez L, Maurer e, Lopez A, Sorenson D, Meliska C. Sleep, rhythms and women's mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–44. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Malhotra A, Gottleib D, White D, Hu F. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Applied Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 18.Tworoger SS, Davis D, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–9. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]