Abstract
Rationale:
The treatment of choice for obstructive sleep apnea (OSA) is nasal continuous positive airway pressure (nCPAP) during sleep, but dryness of the upper airway compromises compliance. Heated humidifiers may mitigate such noncompliance; however, recent observations suggest that their use, particularly if not cleaned, increases the risk of respiratory infections. Humidifier water may be contaminated, but the long-held view that passive humidifiers cannot aerosolize water may obscure the perception of risk of infection.
Objectives:
This study challenges the long-held view that “passover” humidifiers do not aerosolize water. With such evidence, this study characterizes the performance of filters to reduce the potential risk of contamination.
Methods:
Heated humidifier water contaminated with bacteria was studied under conditions simulating week-long use of nCPAP for OSA.
Results:
Bacteria were recovered in 9 of 11 tests from the breathing tubes of CPAP devices fitted with heated humidifiers with water contaminated with Brevundimonas diminuta or Serratia marcescens. Recoverable bacteria ranged from tens to thousands of colony forming units when tested at air flow rates of 60 liters per minute for 90 minutes. Neither organism was recovered from the circuit tubing when a hydrophobic breathing-circuit filter was positioned between the humidifier and face-mask tubing with a commercially available nCPAP machine tested under simulated-use conditions. Conclusion: Data suggest that patients with OSA being treated with nCPAP fitted with humidifiers may be aerosolizing bacteria, putting them at risk for developing respiratory infections and that the use of a hydrophobic filter may attenuate the passage of microbes from contaminated humidifier water.
Citation:
Ortolano GA; Schaffer J; McAlister MB et al. Filters reduce the risk of bacterial transmission from contaminated heated humidifiers used with CPAP for obstructive sleep apnea. J Clin Sleep Med 2007;3(7):700–705.
Keywords: Breathing filter, heated humidifier, CPAP, Obstructive Sleep Apnea, respiratory infection
Obstructive sleep apnea (OSA), aside from resulting in the debilitating effect of fatigue derived from disrupted sleep, can lead to increased morbidity and mortality.1,2 The nonsurgical treatment of choice for OSA is nasal continuous positive airway pressure (nCPAP) ventilatory support.3–5 Unfortunately, noncompliance is an issue in about 10% of patients due to drying and irritation of the mucosal lining of the respiratory tract.6–8 Heated humidification is effective in promoting compliance and is increasingly being recommended for patients receiving nCPAP.9,10
Factors known to predispose patients to healthcare-associated infections include the immune status of the patient, as well as the dose and virulence of the infectious agent.11 Ventilated patients are at higher risk of developing pneumonia (ventilator-associated pneumonia, VAP) because the protective effect of an intact upper airway is bypassed with intubation.12–14 VAP is a serious concern in that it occurs in up to 20% of patients ventilated for more than 48 hours and causes the length of hospital stay to increase over 6 days at a cost of over $10,000 per incident.15,16 Although nonintubated ventilated patients, such as those treated with mechanical ventilation for sleep apnea, appear to be at lower risk for developing respiratory infections, the risk is not zero.17
In a recent report of 206 patients treated with CPAP for OSA, compared with a retrospectively collected cohort of 40 who received non-CPAP therapy, through a 165-week period of study, there were more infections among CPAP patients than controls with OSA who did not use CPAP. Infectious diseases among controls were 25%, and in those with CPAP averaged 43%. Among CPAP users, the incidence of those reporting upper airway infections was higher in those using humidifiers (22% vs 2%). Finally, and most germane to the current study, patients using heated humidifiers without regard to good hygienic practices in maintaining their humidifiers by thorough cleansing and replacing their breathing tubes showed a dramatic increase in upper airway infections, compared with those who cared for their equipment regularly, when examined over a 6-month interval (52.4% vs 13.3%; p < 0.05).18
This observation is consistent with increasing recognition of tap water as a source of bacteria that can cause infections among hospitalized patients.19 However, far less is known of the consequences of contaminated tap water in homes. Therefore, the potential for respiratory infections among those using heated humidification with nCPAP for OSA may be an unrecognized risk.
This study serves to challenge the widely held view that passive humidifiers do not form aerosols and therefore do not aerosolize bacteria from contaminated humidifier water. This study focuses upon the ability to aerosolize bacteria from a “passover” heated humidifier with nCPAP machines set to conditions simulating home use for the treatment of OSA with nCPAP. Upon establishing the potential to aerosolize bacteria, the efficacy of bacteria-retaining hydrophobic filters was also investigated for their potential to reduce the risk of infection when positioned between the humidifier and the breathing tube leading to the mask.
MATERIALS AND METHODS
Materials
Microorganisms used in the study included Brevundimonas diminuta (American Type Culture Collection; Manassas, VA ATCC # 17598), Serratia marcescens (ATCC 13477), and MS-2 bacteriophage (ATCC # 15597-B1). B. diminuta and S. marcescens were prepared by culture in trypticase soy broth (TSB) at 30°. B. diminuta is one of the smallest known bacterial species, MS-2 bacteriophage is a virus that infects bacteria, and their combined use represents a rigorous challenge to the filters under study. S. marcescens was employed because of its ease of detection as easily visualized pink colonies on agar plates. MS-2 was prepared by the agar overlay method, using Escherichia coli (ATCC 15597) as the host. Following overnight incubation of the bacteriophage and host at 37°C, lysis of the bacterial cells occurred, and the bacteriophage was recovered by centrifugation at 3000 × G for 20 minutes at 4°C. MS-2 bacteriophage represents the industry standard of challenge with viruses. The size of the MS-2 bacteriophage (25-nm diameter) approximates that of many viruses and represents a severe particle size challenge to breathing filters.
Quantification of the microorganisms was performed by serial dilutions (1:10) in phosphate buffered saline (40 mM) and then using the spread plate technique to plate volumes (0.2 mL) of the appropriate dilutions onto trypticase soy agar (TSA) plates in duplicate. Enumeration of MS-2 was by the agar overlay method, in which serial dilutions were used, but these were placed into sterile tubes containing soft agar and the E. coli host cells. Incubation of bacteria was at 30°C, whereas the MS-2 was incubated at 37°C.
The bacteria were grown in TSB until the late exponential phase, as determined by optical density measurements at 540 nm, and then spiked into the carrier fluid to achieve the appropriate inoculum level. The MS-2 stock solution prepared (as described above) was diluted in the carrier fluid to achieve the appropriate inoculum level. Plate counts yielding 20 to 200 colony forming units (CFU) or plaque forming units (PFU), for bacteriophage, were used to enumerate microbial levels. All samples in the study were serially diluted up to 9 times, and the remaining original sample volume was recorded and passed through a 0.2-um filter, and then the filter was placed onto TSA in an effort to quantify any samples with nondetectable counts on plates. The values were reported as CFU per milliliter or PFU per milliliter.
Nine nCPAP machines (ResMed S7™ [cat no. 30011], ResMed, Inc.; Poway, CA) were fitted with HumidAire 2i (cat no. 326201; ResMed, Inc.) heated humidifiers. Resistance to air flow simulating the use of properly fitted masks was performed by affixing a plastic plug to the end of the supplied breathing tubes in which a 6-mm hole was created with a drill. Flow rates were set to 20 cm water pressure, providing flow rates of 60 to 70 liters per minute (LPM) and confirmed by air-flow meter measurements at the beginning and end of each experiment.
Calibrated equipment in conformance with ISO 9001 standards was used for all measurements, including air flow meters (Dwyer S22G, Dwyer Instruments Inc., Michigan City, IN), manometers, weighing balances (MCI Analytical AC2105, Sartorius Corporation Inc., Bohemia, NY), and reagent-grade chemicals obtained from Sigma. Co. (St. Louis, MO) or from VWR International (West Chester, PA). Hydrophobic heat and moisture exchange filters were used (Pall BB50T filters; Pall Corp; East Hills, NY).
Aerosolization of Bacteria
B. diminuta and S. marcescens were recovered from TSB and diluted into 500 mL of sterile water to an anticipated concentration of 5 to 10 × 107 CFU/mL. A heated humidifier was filled with 350 mL of the suspension of both B. diminuta and K. pneumoniae, and the exact concentration was confirmed by spread plating 1:10 serial dilutions of samples recovered prior to, and at the end of, the experiment from the humidifier reservoir.
The heated humidifier was attached to an nCPAP machine and a 2-m corrugated breathing hose (supplied with the device and sterilized by ethylene oxide) was affixed to the humidifier. After setting the temperature and allowing it to achieve levels of 37°C, a flow-restriction plastic cap was fitted on the distal end of the breathing tube, and the breathing tube was attached to the humidifier. The length of the breathing tube was immersed, at its middle, into an ice bath to promote condensation, and the entire apparatus was operated within the confines of a biohazard hood.
Initiation of air flow (approximately 60 LPM for 90 minutes per experiment; n = 11) was followed by recovery of the condensate by pouring the breathing-tube contents into a 50-mL sterile conical centrifuge tube. Duplicate samples (200 μL) of serial dilutions (1:10 v/v) were plated onto TSA and incubated at 30°C overnight. The remaining volume was recorded and passed through a sterile 0.2-micron filter, and the filter was placed onto a TSA plate and incubated along with the remaining plates. Plates with colony counts from 20 to 200 were taken for quantification of levels of bacteria, adjusted for volume, and reported as the amount of recovered bacteria. In the event colony counts were below 20 at all solution samples, the count was recorded from the filtered concentrated sample.
Filter Humidification Cycles
Filters, if used in the setting of heated humidification with nCPAP for OSA, would be expected to be subjected to the humidity of the heated humidifier output throughout the normal sleep duration, for which an upper limit of 10 hours was selected in this study. Filters were therefore positioned on each of 9 heated humidifiers (27 filters in all, with 3 groups designated as 1, 4, and 7, representing the number of 10-hour humidification cycles to which they were exposed). Filters were positioned with the long end perpendicular to the laboratory work bench (Figure 1) and exposed to the maximal temperature setting of the heated humidifier with air flow at 60 to 70 LPM (20 cm water pressure) for 10 consecutive hours, constituting 1 cycle of humidification. The terminal end of the corrugated breathing tube was positioned at least 1 meter above the outlet of the humidifier to ensure condensate would flow back to the filter, representing the most severe humidification challenge to the filter. These preconditioning humidification cycles were repeated with the same filter on successive days if they were designated as requiring preconditioning for 4 or 7 cycles.
Figure 1.

Photograph of apparatus used to condition filters with humidification. Nine ResMed S7™ nasal continuous positive airway pressure machines were fitted with Pall BB50T breathing filters at the outlet of the ResMed HumidAire™ 2i humidifiers. The filters were arbitrarily but consistently positioned with the long axis of the filter perpendicular to the lab bench. The breathing hoses were attached to the filters on one side, and the other side was suspended 1 to 1.25 m above the outlet to ensure that condensate would flow backward into the downstream side of the filter.
To gauge the extent of condensation that occurred within the breathing tube, the volume of condensate in the downstream side of the filter and in the length of the breathing tube was observed and codified to an ordinal scale of 0, 1, and 2, representing no water, water in either the filter or tube, and water in both, respectively. Flow rates were measured at the beginning and end of each humidification period (i.e., 1, 4, or 7 cycles) using a calibrated low-impedance air flow meter (Fisher-Porter, tube #FP-1/2 27-G-10/82, and float 3 1/2-GNSVT-48-T60) were reported in liters per minute.
Filters exposed to 1, 4, or 7 daily humidification cycles were then subjected to 1 of 3 tests (n = 3/test): pressure drop, bacteria (B. diminuta), or bacteriophage (MS-2) log reduction values. The log reduction value is defined as the log of the total challenge – log recovered downstream of the filter.
Pressure Drop
At the end of humidification episodes 1, 4 and 7, pressure across the filter (i.e., pressure drop) was measured using a water manometer (n = 3 each), and air flow rate was controlled with the aid of a needle valve and an air flow meter set at 70 LPM.
Microbial Retention with Filters
The efficiency of filtration was tested with monodispersed B. diminuta and MS-2 bacteriophage separately (n = 3 each) using a previously described method.20
Briefly, the method dictates that approximately 5 mL of a suspension of microbes at a concentration from 1 to 10 × 107 CFU (or PFU in the case of MS-2 bacteriophage) per milliliter is carried with air flow at 28 LPM through a DeVilbiss nebulizer into a chamber through which dry air is passed to convert the aerosolized microbial suspension into discrete dry monodispersed microbial particles. Sample impingers are positioned upstream and downstream of filters, and microbes accumulate in TSB within the impingers. An aliquot from each impinger is then serially diluted, and all dilutions are spread plated, incubated for 1 to 3 days, and counted.
Static Microbial Challenge to Preconditioned Filters
In another test of microbial barrier integrity, 5 filters were exposed to a 10-hour episode of humidification, and sterile water was filled to the bottom of the inflow and outflow ports. A final concentration of 107 CFU of B. diminuta was added to 1 side, and the filters were sealed with Parafilm M (Alcan, Inc., Neenah, WI) to retain the water and left for 7 days at room temperature. Following incubation, aliquots were removed from either side to assess the concentration of bacteria on either side of the filter.
Statistical Analyses
Standard methods of statistical analyses were performed with Prism (version 3.02; GraphPad, Inc., San Diego, CA), and, when necessary, data transformation employed the use of Excel (Microsoft; Redmond, WA). Bartlett test for homogeneity of variance and the F-max test of normality were employed to determine if parametric tests were appropriate. Previous experience of microbial removal efficiency data support the used of parametric analyses, including 1-way analysis of variance with Newman-Keuls multiple comparisons. For ordinal data, or if the violation of the assumptions of normality and homogeneity of variance were revealed, the nonparametric equivalents, Kruskal-Wallis test followed by Dunn’s multiple range tests, were employed.
RESULTS
Aeroslization of Bacteria
The duration of air flow expressed as mean ± standard deviation (SD) was 1.3 ± 0.3 hours (n = 11), and the condensate volume recovered was 14.2 ± 0.6 mL. The initial and final reservoir temperatures were 18.9°C ± 3.9°C and 35.2°C ± 1.7°C, respectively. The initial and final bacteria counts in the reservoir were 6.1 ± 1.7 × 107 and 4.4 ± 2.4 × 107 CFU/mL (t test; p = 0.074) for S. marcescens and 10.3 ± 5.5 × 107 and 5.6 ± 5.1 × 107 CFU/mL (p = 0.002) for B. diminuta, respectively. Of the 11 trials performed, bacteria were recovered in all except 2. The remaining 9 trials showed recoverable S. marcescens and B. diminuta that averaged 17.0 ± 20.4 × 103 and 9.2 ± 9.6 × 103 CFU, respectively.
Effects of Number of Humidification Cycles on Filter Performance
There are data to suggest that filters subjected to testing should be exposed to simulated clinical use conditions, most notably humidification.21 To ensure compliance with the humidification protocol, a comparison of the ordinal scale results showed that filters had accumulated water comparable to levels after 1, 4, and 7 humidification cycles by a statistical comparison of ordinal scale medians (Kruskal-Wallis p>0.05, data not shown) with a median value of 1.3 out of a possible 2. The number of humidification cycles was shown to have no substantial impact on flow rate (Figure 2) after the filter was affixed to the output of the humidifier. The number of humidification cycles did not have any effect on the pressure drop across the filter at the end of 1, 4, or 7 humidification cycles (Figure 3). Finally, microbial challenges presented to the filter after 1, 4, or 7 daily humidification cycles did not compromise the efficiency of filters to retain microbes represented by either B. diminuta or MS-2 bacteriophage challenge as monodispersed cells (Figure 4).
Figure 2.

Effect of number of humidification cycles on air-flow rate measured at the end of each cycle. Flow rates from the breathing tubes of ResMed S7 nasal continuous positive airway pressure machines were taken with or without BB50T filters and just prior to the end of episodes of humidification with BB50T filters. Box and whisker symbols illustrate the median (line within the box), 25th, and 75th percentile (lower and upper borders of the box) and the range (error bars) of values measured.
In general, statistically significant differences were shown using Kruskal-Wallis followed by Dunn multiple range test, but they were largely limited to comparisons in the presence and absence of filters. No systematic impact of the number of humidification cycles on flow rate was apparent, suggesting no accumulation in resistance was imparted by the filter over 7 consecutive days of episodic (10-h) use.
Figure 3.
Effect of number of humidification cycles on pressure drop measured immediately afterward (n = 3). One-way analysis of variance with 3 per group with mean and SD depicted returned a p value of 0.3188.
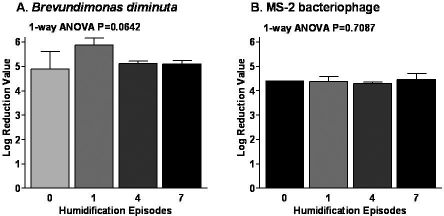
Figure 4.
Removal of monodispersed bacteria and bacteriophage challenge to Pall BB50T breathing filters following 1, 4, and 7 humidification cycles under simulated use of nCPAP for OSA.
Data are represented at log reduction values for B. diminuta (Panel A) and MS-2 bacteriophage in Panel B (MS-2 bacteriophage was used because the size at 25 nm approximates those of the smallest mammalian viruses). The monodispersed microbial challenge represents the most stringent challenge to breathing filters in that, unlike salt challenges, it employs the use of microbes. Furthermore, unlike aerosolized microorganisms, the method also ensures that each microbe is presented individually to the filter, not as an aggregate of microbes on an aerosol droplet.
Static Microbial Challenge
Filters left at room temperature for 7 days with sterile water on 1 side and high concentrations of B. diminuta on the opposite side showed microbial retention characteristics exceeding 99.99% or 4 log reduction value (Table 1). This effect was similar in magnitude to that observed with monodispersed challenges in this study (Figure 4).
Table 1.
Summary Statistics for the Concentrations of B. Diminuta Across a Filter Following 7 Days of Incubation at Room Temperature
| CFU/mL |
% removal | LRV | ||
|---|---|---|---|---|
| Entry Port | Exit Port | |||
| Mean | 1.13E+07 | 54.2 | 99.9995 | 5.32 |
| SD | 1.26E+07 | 115.1 | 99.9991 | 5.04 |
| Min | 1.00E+06 | 0 | ||
| Max | 2.60E+07 | 260 | ||
Mean, SD, min and max are expressed in CFU/mL for 5 filters. LRV is the log reduction value.
DISCUSSION
There continues to be debate over the likelihood that the use of heated humidification in chronically ventilated patients puts them at risk for ventilator-associated pneumonia and whether or not the use of a breathing-circuit filter may mitigate that risk.14,22 Important distinctions should be made between heated humidification used for chronically ventilated patients and those using nCPAP for OSA. The former group have their upper airway bypassed and are treated in the hospital, where attention to infection control practices may be considered to be rigorous. In contrast, those on nCPAP for OSA largely receive their treatment at home, where the principles of infection control may be viewed as absent or at least not adhered to as strictly as in the hospital setting. In fact, the impact of nCPAP for OSA on infectious complications is relatively unstudied.18 Moreover, there is increasing awareness of the microbial content of tap water both in and out of hospitals and its potential as a source of infection.19 Therefore, it is not unlikely to expect that the microbial content of heated humidification used with nCPAP in home settings may be higher than that seen in a more controlled, infection control-sensitive environment. This is the basis for the current investigation.
There is a widely held view that passive (“passover”) humidifiers do not aerosolize water but merely help to produce, and allow for the passage of, water vapor. Supporting such a view are the results of a recent study.23 In this study, the positive control was the use of a nebulizer, filled with 400 MBq of 99mTc-diethylenetriamine penta-acetic acid (labeled DTPA) in 5 mL of water, from which only 28% of all radioactivity could be trapped and detected in just the first of 2 Pall BB50T breathing circuit filters in series, with the second containing no measurable radiolabel. Then a humidifier reservoir was spiked with the same amount of labeled DTPA, and, at the highest air flow tested (46 LPM), no radioactivity was detected in a breathing filter positioned downstream of the humidifier. Given that only 28% of the label was detectable in the control run, the limit of detection of the assay cannot be greater than 28-fold or less than 2 orders of magnitude
Data reported here suggest that, although the amount of bacteria in the heated humidification reservoir was high (approximating 5 × 107 CFU/mL), this is indeed a level at which bacteria can sometimes be found in contaminated water. We recovered levels as high as 103 CFU in the breathing tube. The difference of 4 orders of magnitude is one that cannot be detected in the labeled DTPA study. It is fair to say, however, that contaminated water used in a home environment may persist, particularly if water is added to the humidifier without rinsing between refills. Our study also employed slightly higher flow rates of 60 to 70 LPM, in contrast to the 46 LPM reported in the referenced study. This could also account for observable differences.
Having established that it is possible to aerosolize bacteria, we show that the hydrophobic breathing circuit filter used in this study does not demonstrate drastic pressure drops or decreases in air flow rates when used repeatedly over the course of 7 daily exposures to 10-hour periods of nCPAP use with heated humidification. This suggests the device can be used under the conditions studied and those conditions that simulate clinical use.
Humidification is handled differently by breathing filters of varying types. Hygroscopic materials absorb water and result in an increase in pressure drop, whereas hydrophobic media is unaffected by humidity and maintains low pressure drops.24 Therefore, these results cannot be extended to other filters, particularly those of different design and/or composition of functional filtration material. The filter efficiency for retaining microbes with sizes ranging from small bacteria (B. diminuta) to viruses (MS-2 bacteriophage) are unaffected by humidification episodes over the course of 7 days and remain high, approximating four orders of magnitude or more.
Care should be taken to position a hydrophobic filter in such a way as to prevent the accumulation of water on the upstream side of the filter, for, if the filtration media is totally covered, the pressures used will not drive the water through the hydrophobic media and air will not be permitted to pass to the patient. Water condensing on the downstream side does not present a problem because air will simply bubble through the accumulated water. The test conditions used in this study apply to the make and model of humidifier and nCPAP machine studied. The results of this study may extend to devices with the same orientation of filter-to-humidifier outlet that will prevent the accumulation of water condensate on the upstream side of the filter such that the water does not completely cover the filter media.
In conclusion, patients treated with nCPAP using heated humidification for OSA may be at risk for respiratory infections caused by contaminated water present in heated humidifiers.18 Such patients may benefit from a properly positioned hydrophobic breathing filter positioned in between the heated humidifier outlet and breathing tube. Data provided here support the view that a properly positioned hydrophobic breathing filter may reduce the transmission of bacteria from inadvertently contaminated water that may be present in heated humidifier water used with nCPAP for OSA.
ACKNOWLEDGMENTS
The authors express their gratitude for the commitments made by analysts of Pall Corporation’s Division of Scientific and Laboratory Services who ensured compliance with the humidification protocol by carrying out facets of the protocol in the evenings and on weekends. Notable among them are Lois Hayon, Biomedical Lab Manager; Microbiology Supervisor; Kristin Acosta; Melanie Adams; Kyle Nevins; Brian Lang and Kurt Lempin. The nCPAP equipment used in this study was graciously provided by Mr. S. Campbell-Smith of ResMed, Inc.
Statement of prior publication: This work has been published in abstract form and presented at an annual meeting of the AARC: Ortolano GA, Schaffer J, McAlister MB, Canonica FP, Satti F, Maguire JM, Cervia JS. Hydrophobic filter retains microbial barrier characteristics when used with heated humidifier for CPAP in sleep apnea. American Association for Respiratory Care, San Antonio, TX. Dec 3–6, 2005 – Respiratory Care 2005;50(11):1546 (Abstr # OF-05-130).
Footnotes
Disclosure Statement
This study was funded by Pall Corporation. All authors are, or were at the time of the study, employees of the Pall Corporation.
REFERENCES
- 1.Aloia MS, Arnedt JT, Riggs RL, Hecht J, Borrelli B. Clinical management of poor adherence to CPAP: motivational enhancement. Behav Sleep Med. 2004;2:205–22. doi: 10.1207/s15402010bsm0204_3. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT, Ryan S. Obstructive sleep apnoea syndrome: translating science to clinical practice. Respirology. 2006;11:136–44. doi: 10.1111/j.1440-1843.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 4.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins De Araujo MT, Vieira SB, Vasquez EC, Fleury B. Heated humidification or face mask to prevent upper airway dryness during continuous positive airway pressure therapy. Chest. 2000;117:142–7. doi: 10.1378/chest.117.1.142. [DOI] [PubMed] [Google Scholar]
- 7.Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116:403–8. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 8.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest. 2005;128:2151–8. doi: 10.1378/chest.128.4.2151. [DOI] [PubMed] [Google Scholar]
- 9.Wiest GH, Lehnert G, Bruck WM, Meyer M, Hahn EG, Ficker JH. A heated humidifier reduces upper airway dryness during continuous positive airway pressure therapy. Respir Med. 1999;93:21–6. doi: 10.1016/s0954-6111(99)90072-0. [DOI] [PubMed] [Google Scholar]
- 10.Gay P, Weaver T, Loube D, Iber C, Positive Airway Pressure Task Force. Standards of Practice Committee. American Academy of Sleep Medicine Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 11.Duncan HE, Edberg SC. Host-microbe interaction in the gastrointestinal tract. Crit Rev Microbiol. 1995;21:85–100. doi: 10.3109/10408419509113535. [DOI] [PubMed] [Google Scholar]
- 12.Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82:172–8. doi: 10.1136/pgmj.2005.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32:1396–405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 14.Ricard JD, Boyer A, Dreyfuss D. The effect of humidification on the incidence of ventilator-associated pneumonia. Respir Care Clin N Am. 2006;12:263–73. doi: 10.1016/j.rcc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 16.Gay P, Weaver T, Loube D, Iber C, Positive Airway Pressure Task Force. Standards of Practice Committee. American Academy of Sleep Medicine Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 17.Benhamou D, Cuvelier A, Muir JF. [Prevention of infections transmitted by CPAP and noninvasive ventilation] Rev Pneumol Clin. 2001;57:73–8. [PubMed] [Google Scholar]
- 18.Sanner BM, Fluerenbrock N, Kleiber-Imbeck A, Mueller JB, Zidek W. Effect of continuous positive airway pressure therapy on infectious complications in patients with obstructive sleep apnea syndrome. Respiration. 2001;68:483–7. doi: 10.1159/000050555. [DOI] [PubMed] [Google Scholar]
- 19.Ortolano GA, McAlister MB, Angelbeck JA, Schaffer J, Russell RL, Maynard E, Wenz B. Related Hospital water point-of-use filtration: a complementary strategy to reduce the risk of nosocomial infection. Am J Infect Control. 2005;33(5) Suppl 1:S1–19. doi: 10.1016/j.ajic.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Duberstein R, Howard G. Sterile filtration of gases: a bacterial aerosol challenge test. J Parenter Drug Assoc. 1978;32:192–8. [PubMed] [Google Scholar]
- 21.Wilkes AR. Assessing breathing-system filters. Med Device Technol. 2004;15:12–4. [PubMed] [Google Scholar]
- 22.Branson RD. Humidification of respired gases during mechanical ventilation: mechanical considerations. Respir Care Clin N Am. 2006;12:253–61. doi: 10.1016/j.rcc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel M, Klauke M, Gessenhardt F, et al. Sterile water is unnecessary in a continuous positive airway pressure convection-type humidifier in the treatment of obstructive sleep apnea syndrome. Chest. 2005;128:2138–40. doi: 10.1378/chest.128.4.2138. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull D, Fisher PC, Mills GH, Morgan-Hughes NJ. Performance of breathing filters under wet conditions: a laboratory evaluation. Br J Anaesth. 2005;94:675–82. doi: 10.1093/bja/aei091. [DOI] [PubMed] [Google Scholar]