Abstract
Study objectives:
To evaluate the psychometric properties and clinical significance of a new scale for measuring daytime fatigue associated with insomnia: The Flinders Fatigue Scale (FFS).
Methods:
The 7-item FFS was used in two separate studies. Study 1 was an on-line validation study involving 1093 volunteers (mean [SD] age = 38.6 [14.7] y, 626 poor sleepers, 467 good sleepers) in a cross-sectional design; Study 2 investigated the clinical sensitivity of the FFS on 113 insomnia patients (mean [SD] age = 48.3 [15.0] y) in response to a 5-week cognitive-behavior therapy for insomnia (CBT-I) program.
Results:
The FFS had an internal consistency of 0.91; it comprised a single factor, accounting for 67% of the total variance. Poor sleepers in Study 1 scored significantly higher than good sleepers on the FFS (p < 0.0001). In Study 2, significant reductions in FFS scores were found in response to CBT-I (p < 0.0001). These reductions in fatigue correlated with improvements on subjective sleep parameters (all p < 0.0001). The FFS showed good discriminant validity with the Epworth Sleepiness Scale.
Conclusions:
The Flinders Fatigue Scale is a brief, clinically sensitive measure with strong psychometric properties.
Citation:
Gradisar M; Lack L; Richards H; Harris J; Gallasch J; Boundy M; Johnston A. The flinders fatigue scale: preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med 2007;3(7):722–728.
Keywords: Daytime fatigue, insomnia, daytime functioning, psychometrics, daytime sleepiness
Insomnia can be characterized not only by difficulty initiating and/or maintaining sleep leading to insufficient sleep, but also poor daytime functioning. The Diagnostic and Statistical Manual for Mental Disorders 4th edition1 and the recent second edition of the International Classification of Sleep Disorders (ICSD-2)2 both specify that a diagnosis of insomnia includes both poor sleep and associated daytime impairment. Despite the dual emphasis placed on both sleep and daytime consequences from both of these diagnostic systems, traditionally there has been a stronger emphasis on the measurement of insomnia patients' habitual sleep episode. For instance, insomnia patients' sleep quality has been measured via self-report questionnaires,3,4 sleep diaries,5 actigraphy,6,7 polysomnography,8,9 and clinical interviews.10 However, there has been relatively little focus on the assessment of insomnia patients' daytime functioning, despite this being the cause for many seeking treatment.11,12 As such, many expert working groups have now recommended the research of insomnia include the measurement of daytime functioning.13,14
Both the DSM-IV and the ICSD-2 indicate a number of typical daytime consequences of insomnia. These include fatigue, malaise, poor attention and concentration, memory impairment, social or vocational dysfunction, irritability, mood disturbance, daytime sleepiness, lack of motivation or energy, prone to accidents, physical symptoms (e.g., headaches, gastrointestinal complaints), and concerns or worries about sleep; which can lead to a reduced quality of life.15 Of all these daytime symptoms, fatigue appears to be the most prevalent daytime complaint, as evidenced by both its emphasis in the scientific literature,4 and in clinical settings.11 Indeed, self-reported fatigue is higher in people experiencing insomnia compared to other sleep disorders (e.g., obstructive sleep apnea), yet comparable to that of cancer and chronic fatigue syndrome.16,17 Fatigue associated with insomnia can be considered as subjective feelings of tiredness, weariness and exhaustion,18 as opposed to the likelihood to doze or fall asleep, which is often associated with daytime sleepiness.19 To date, the most popular measures of fatigue used in insomnia research are the Fatigue Severity Scale (FSS)20 and the Multidimensional Fatigue Inventory (MFI).21
The FSS is a self-report questionnaire, designed to measure the level of a person's fatigue in a variety of situations (e.g., “I am easily fatigued,” “Exercise brings on my fatigue”).20 It is a relatively brief measure, containing only 9 items that provide a global measure of fatigue, and was originally developed on clinical samples (e.g., multiple sclerosis, lupus). In contrast, the MFI contains 20 items divided into 5 subscales: general fatigue (e.g., “I feel tired”), physical fatigue (e.g., “Physically I feel only able to do a little”), reduction in activities (e.g., “I feel very active”), reduction in motivation (e.g., “I dread having to do things”), and mental fatigue (e.g., “My thoughts easily wander”),21 though no global fatigue score is provided. The MFI was primarily developed to measure fatigue in cancer patients. Both measures possess good internal consistency (e.g., FSS α = 0.80; MFI α = 0.84), characteristics essential for a valid daytime impairment measure in treatment outcome studies.11 One criticism of the FSS, however, is the scale's questionable validity (i.e., it includes “fatigue” in each item without providing an initial description of the term).4
Not only do self-reported measures require strong psychometric properties, but they also need to be sensitive to treatment.11 Unfortunately, recent treatment outcome studies using these scales have not delivered promising results. Lichstein and colleagues employed the FSS, among other measures, to assess daytime functioning when comparing various treatments of insomnia in older adults.22 Although sleep diaries demonstrated treatment gains over time for the habitual sleep period (e.g., sleep latency), improvements were not reflected in their associated daytime fatigue. As a result, Lichstein and colleagues encouraged “further exploration of the role of fatigue and other dimensions of daytime impairment in isolating insomnia subtypes” (p. 238). Similarly, Quesnel and colleagues used the MFI in a study investigating the efficacy of a cognitive-behavioral intervention for insomnia in women being treated for breast cancer.23 Their intervention proved partially successful, with 2 of the 5 subscales (general fatigue and physical fatigue) showing decreases at posttreatment. However, the other 3 subscales (mental fatigue, motivation, and activities) remained unchanged, despite significant improvements of sleep demonstrated with sleep diaries and polysomnography. A more recent study though by Savard and colleagues did find significant improvements in global fatigue using the French-Canadian short-form of the MFI (15 items).24
Currently, the FSS and MFI are recommended as standard measures.4 Although these fatigue measures show acceptable reliability properties, they either lack clinical sensitivity (i.e., the FSS), or brevity (i.e., the MFI). According to Morin, treatment outcome measures should be reliable and valid, clinically sensitive to detect meaningful changes, and for practical purposes be brief to reduce the burden on patients.11 Finally, there exists no scale that has validated fatigue associated with insomnia. To address these current concerns, we have developed a new daytime fatigue measure based on our clinical experience in the treatment of insomnia. The aim of the present paper is to present the psychometric properties and clinical sensitivity of this scale for an insomnia sample: the Flinders Fatigue Scale. This was done by using the Flinders Fatigue Scale in two separate studies. Study 1 was a validation study of the Flinders Fatigue Scale, with Study 2 investigating the clinical sensitivity of the Flinders Fatigue Scale in response to a cognitive-behavior therapy program for insomnia (CBT-I). A second aim of this paper is to assess the relationship between fatigue and daytime sleepiness associated with insomnia. Even though these concepts have been used interchangeably in the literature and colloquially, recent studies have demonstrated a lack of correspondence.25 The present paper will therefore validate fatigue against sleepiness by investigating their relationship at assessment and across the course of therapy for the insomnia patients in Study 2.
METHODS
Study 1
Participants and Procedure
Participants from Study 1 consisted of 1093 volunteers (mean [SD] age = 38.6 [14.7] y, 349 males, 744 females), of whom 626 were identified as poor sleepers. Participants completed both the Flinders Fatigue Scale and the Pittsburgh Sleep Quality Index (PSQI).26 The PSQI is a reliable self-report measure of sleep disturbance that consists of seven factors, which when summed yield a global PSQI score. Higher PSQI scores indicate greater sleep disturbance. The PSQI has good psychometric properties, with a sensitivity of 90% and specificity of 87% of distinguishing between poor and good sleepers,26 and a sensitivity of 99% and specificity of 84% in discriminating primary insomniacs versus healthy good sleepers.27 Subjects in Study 1 were classified as poor sleepers based on the standard criterion of a global PSQI score >5.26 Subjects were recruited via a snowball sampling method,28 and completed on-line versions of the Flinders Fatigue Scale and PSQI. The study was approved by the Flinders University Social and Behavioural Ethics Committee.
Study 2
Participants and Procedure
One hundred and sixty-seven insomnia patients consecutively enrolled in a 5-week cognitive-behavioral therapy for insomnia (CBT-I) program at the Repatriation General Hospital in Adelaide, South Australia participated in Study 2. Participants were excluded from analyses if diagnosed with insomnia secondary to a circadian rhythm disorder (N=32), insomnia comorbid with sleep disordered breathing (N=17; respiratory disturbance index ≥30), restless leg syndrome (N=2), periodic limb movements of sleep (N=2), or suspected narcolepsy (N=1). This left a final sample of 113 insomnia disorder patients (mean [SD] age = 48.3 [15.0] y, (38 male, 75 female). Insomnia disorder patients were diagnosed according to DSM-IV criteria.1 Diagnoses included primary insomnia (N=85), insomnia due to a mental disorder (N=18), insomnia due to a medical condition (N=9), and insomnia due to a substance (N=1). Approximately 56% of insomnia disorder patients were taking prescribed sleep medication at pretreatment. Diagnoses were made on the basis of a clinical interview, information from a 7-day sleep diary, and overnight polysomnography. The CBT-I program consisted of sleep education (e.g., teaching aspects of sleep architecture), behavioural techniques (e.g., bedtime restriction, stimulus control therapy, sleep hygiene), and cognitive techniques (e.g., cognitive restructuring, sleep perception). CBT-I was administered individually by LL, MG, and JH. The FFS was administered as part of a questionnaire battery at pretreatment, posttreatment, and at a 2-month follow-up. This battery included 7-day sleep diaries and the Epworth Sleepiness Scale (ESS).19 The sleep diaries provided subjective estimates of insomnia patients' sleep parameters (i.e., sleep onset latency [SOL]; wake after sleep onset [WASO]; total sleep time [TST]; and sleep efficiency [SE]). The ESS provided a measure of daytime sleepiness. This scale was included as daytime sleepiness is considered distinct from daytime fatigue.4,25 The ESS requires respondents to indicate how likely they would fall asleep or doze (i.e., 0–would never doze, to 3–high chance at dozing) in 8 scenarios (e.g., sitting and reading). ESS scores range from 0–24, with higher scores indicating greater daytime sleepiness. ESS scores higher than 10 indicate excessive daytime sleepiness.19 The ESS is considered a reliable and valid measure.29 The study was approved by the Flinders University Social and Behavioural Ethics Committee and the Repatriation General Hospital Ethics Committee.
Flinders Fatigue Scale
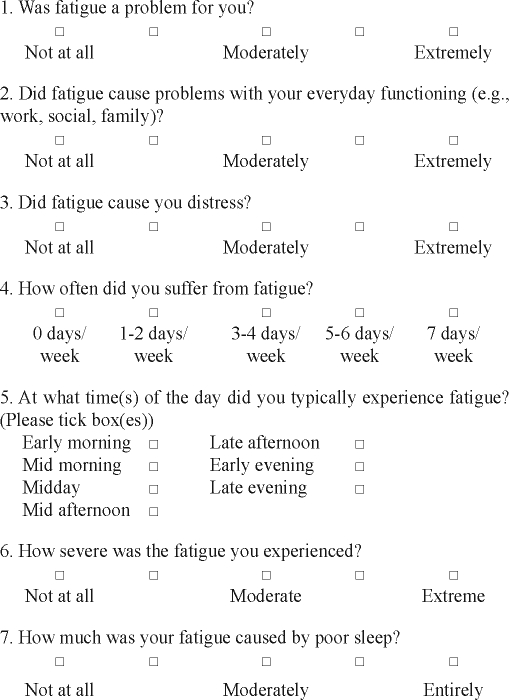
Seven items were developed for the Flinders Fatigue Scale based on reports from insomnia patients in our clinics. The scale (including the 7 items) were reviewed by an external insomnia therapist/researcher. These items were pilot tested on a small sample of insomnia patients (N=28) for readability, brevity/time efficiency, and psychometric properties. All patients completed all items, and all found the scale understandable and easy to use. Initial analyses demonstrated promising psychometric properties (e.g., very good internal consistency) and clinical sensitivity (i.e., significant decrease in fatigue scores in response to CBT-I). Thus, all 7 items, and the scale as a whole, were retained and further tested on the samples presented in this paper.
The Flinders Fatigue Scale is a 7-item scale that measures various characteristics of fatigue (e.g., frequency, severity) experienced over the past 2 weeks (see Appendix). The items tap into commonly reported themes of how problematic fatigue is, the consequences of fatigue, frequency, severity, and insomnia patients' perception of fatigue's association with sleep. Six items are presented in Likert format, with responses ranging from 0 (not at all) to 4 (extremely). Item 5 measures the time of day when fatigue is experienced and uses a multiple-item checklist. Respondents can indicate more than one response for item 5, and it is scored as the sum of all times of the day indicated by the respondent. One item explicitly asks for respondents' impression of whether they attribute their fatigue to their sleep. Total fatigue is calculated as the sum of all individual items. Total fatigue scores range from 0 to 31, with higher scores indicating greater fatigue. A clear description of the term “fatigue” is provided in the initial instructions to the scale.
Statistical Analyses
To assess the Flinders Fatigue Scale's internal reliability, a Cronbach alpha coefficient was calculated from the samples from both studies. To assess the factor structure, a principle components factor analysis with a varimax rotation and a criterion of eigen values greater than 1.0 was used. As factor analysis typically requires a relatively large sample size,30 the factor analysis was performed on the fatigue data from Study 1 (N=1093). Pearson product-moment correlations were calculated between the Flinders Fatigue Scale with the ESS administered at pretreatment to the insomnia group in Study 2. The correlations were used to assess the discriminant validity of the Flinders Fatigue Scale with the ESS.
Independent samples t-tests were performed between good sleepers and poor sleepers on their fatigue scores from Study 1. To determine the scales sensitivity to treatment (in this case cognitive-behavior therapy for insomnia, CBT-I), a one-way repeated measures ANOVA was performed on the daytime fatigue data, with the repeated factor “time” (i.e., pretreatment, posttreatment, follow-up). If a significant main effect for “time” was found, planned comparisons were conducted between pretreatment and posttreatment, and posttreatment and follow-up. One-way repeated measures ANOVAs were also performed on sleep diary (SOL, WASO, TST, and SE), and the ESS data to illustrate changes in sleep and daytime sleepiness co-occurring with changes in fatigue. All ANOVAs were performed on data for participants who completed the measures at all three time points (i.e., pretreatment, posttreatment, 2-month follow-up). Where Flinders Fatigue Scale item data were missing (<7%), these data were replaced by the scale mean for that participant.
RESULTS
Internal Consistency, Factor Structure, and Discriminant Validity
For the two samples, the internal consistency of the Flinders Fatigue Scale was 0.91 (Study 1) and 0.86 (pretreatment data from Study 2), respectively. The exploratory factor analysis of the fatigue data from Study 1 revealed a single factor that explained 67.1% of the variance for the Flinders Fatigue Scale. Table 1 presents the factor loadings for each item from the Flinders Fatigue Scale.
Table 1.
Factor loadings for the seven items from the Flinders Fatigue Scale.
| Item | Factor loading |
|---|---|
| Item 1. Was fatigue a problem for you? | 0.83 |
| Item 2. Did fatigue cause problems with your everyday … | 0.77 |
| Item 3. Did fatigue cause you distress? | 0.71 |
| Item 4. How often did you suffer from fatigue? | 0.80 |
| Item 5. At what time(s) of the day did you typically … | 0.67 |
| Item 6. How severe was the fatigue you experienced? | 0.54 |
| Item 7. How much was your fatigue caused by poor sleep? | 0.38 |
Note: Complete statements for each item of the Flinders Fatigue Scale can be found in the Appendix.
No significant correlation was found between the pretreatment Flinders Fatigue Scale and ESS from Study 2, r(110) = −0.06, p = 0.54, suggesting good discriminant validity between these 2 measures.
Poor and Good Sleeper Differences
The mean±SD fatigue value for poor sleepers (11.67±6.77) was significantly greater than the value for the good sleepers (6.22±4.55), t1091 = 15.07, p < 0.0001. Table 2 presents the pretreatment means and standard deviations of fatigue scores for the various insomnia disorders from Study 2. There were no significant differences between these groups on their mean fatigue scores, F2,110 = 2.27, p = 0.09.
Table 2.
Mean (SD) Fatigue Scores for the Insomnia Participants from Study 2.
| Insomnia Subtype | n | Mean | SD |
|---|---|---|---|
| Primary insomnia | 85 | 17.74 | 5.77 |
| Insomnia due to a mental disorder | 18 | 21.33 | 5.71 |
| Insomnia due to a medical condition | 9 | 20.44 | 6.23 |
| Insomnia due to a substance | 1 | 18.00 | NA |
Treatment Outcome Sensitivity
Table 3 presents the mean (SD) fatigue scores, as well as ESS and sleep diary parameters for the insomnia patients in Study 2 at pretreatment, posttreatment, and follow-up.
Table 3.
Mean (SD) and of Daytime Fatigue, Sleepiness, and Sleep Parameters for the Insomnia Patients at Pretreatment, Posttreatment, and 2-Month Follow-Up
| Pretreatment | Posttreatment | Follow-up | |
|---|---|---|---|
| FFS | 17.74 (5.15) | 12.45 (5.18) | 9.21 (5.60) |
| ESS | 4.19 (3.40) | 5.46 (3.87) | 4.21 (2.63) |
| SOL (min) | 62.38 (49.76) | 28.26 (21.86) | 25.49 (17.86) |
| WASO (min) | 119.40 (76.92) | 42.54 (27.09) | 50.89 (39.16) |
| TST (hr) | 5.21 (1.59) | 5.64 (1.49) | 5.99 (1.38) |
| SE (%) | 59.88 (18.54) | 80.63 (10.95) | 79.95 (12.31) |
Note: FFS = Flinders Fatigue Scale; ESS = Epworth Sleepiness Scale; SOL = sleep onset latency; WASO = wake after sleep onset; TST = total sleep time; SE = sleep efficiency (calculated as total sleep time ÷ time in bed × 100).
For the insomnia patients undergoing the 5-week CBT-I treatment, a significant main effect for time was found for the Flinders Fatigue Scale, F2,84 = 39.45, p < 0.001, with a large effect size of 1.10 (Cohen's d). Specifically, there was a significant decrease from pretreatment to posttreatment, F1,42 = 28.41, p < 0.001, that continued to decline at the 2-month follow-up, F1,42 = 15.90, p<0.0001. These decreases in daytime fatigue scores were coincident with decreases in sleep onset latency and wake after sleep onset, SOL, F1.38,96.29 = 35.00, p < 0.001, Cohen's d = 0.64; WASO, F1.29,84.38 = 68.59, p < 0.001, Cohen's d = 0.96; and significant increases in total sleep time and sleep efficiency, TST, F2,140 = 14.82, p < 0.001, Cohen's d = 0.55; SE, F1.70,112.02 = 86.68, p < 0.0001, Cohen's d = 1.17. Of interest, there was also a significant main effect for time for the ESS scores, F2,124 = 2.80, p = 0.007, incorporating a significant increase at posttreatment, F1,62 = 6.56, p=0.013, and a subsequent significant decrease at the 2-month follow-up, F1,62 = 8.68, p=0.005.
As a more direct test of the association between sleep improvements and daytime functioning, correlations between these measures during treatment were performed. The decreases in fatigue from pretreatment to posttreatment were significantly associated with decreases in SOL, r(77) = 0.34, p = 0.003, and WASO, r(76) = 0.25, p = 0.03, and increases in TST, r(76) = −0.26, p = 0.03, and SE, r(75) = −0.29, p = 0.01. However, the decreases in fatigue were not correlated with the increases in sleepiness, r(79) = 0.09, p > 0.05.
DISCUSSION
In this paper the Flinders Fatigue Scale has demonstrated strong psychometric properties, including very good internal consistency and a single factor accounting for a large proportion of the variance. However, such strong psychometric properties of fatigue scales are not new. More importantly, the Flinders Fatigue Scale has been shown to be a very useful clinical tool to be used in insomnia treatment research, due to its ease of use, its ability to be distinguished from sleepiness, its response to cognitive-behaviour therapy, and its relationship to changes in sleep parameters.
The Flinders Fatigue Scale includes fewer items (7 items) than both the FSS (9 items) and the MFI (20 items). Further, the Flinders Fatigue Scale was developed to tap into characteristics that many insomnia patients report in a clinical setting (i.e., how much fatigue is related to their sleep, the frequency of fatigue during the week, and during different times across the day). Unlike the FSS, the Flinders Fatigue Scale is prefaced by a description of what is meant by the term fatigue, in order for respondents to identify and be familiar with the concept of fatigue prior to responding. This also helps to improve the Flinders Fatigue Scale's construct validity. Thus, the Flinders Fatigue Scale presents with good face validity for measuring fatigue in insomnia, and is briefer than other measures—a characteristic highlighted in guidelines for clinical trials of insomnia treatment.11
The Flinders Fatigue Scale also possesses other strong psychometric properties. For instance, in two separate samples it was shown to have good internal consistency (Cronbach alpha) of between 0.86 and 0.91—figures comparable to those found in the FSS and MFI.4 The larger Cronbach alpha of 0.91 could have been partly due to the large sample size in Study 1,31 whereas future studies that employ the Flinders Fatigue Scale are more likely to use a sample size equivalent to that used in Study 2 (N=113), which still demonstrated good internal consistency. Further, the exploratory factor analysis showed the Flinders Fatigue Scale is composed of a single factor that explains 67% of the variance. Other studies in non–sleep disordered samples have found that the FSS is also comprised of a single factor.32,33 On the other hand, the factor analysis applied to the 20-item MFI by the original authors found it consisted of multiple subscales (e.g., general fatigue, physical fatigue, mental fatigue, motivation, and activities).21 Since the original factor analytical study, further studies have found different results. For instance, the French version of the MFI was found to have 4 factors (all but the “physical fatigue” factor).34 Moreover, a recent investigation of the factor structure of 9 common fatigue measures (including the MFI) found that all items from these measures simply load onto 2 factors: “distressed-fatigue” or “vigour,” with the MFI mainly loading on the vigour factor.35 These authors concluded that unidimensional measures of fatigue are as effective in measuring “fatigue” as multidimensional measures.
The various MFI subscales may be useful when investigating the daytime consequences of insomnia in clinical settings. We note that the items from the Flinders Fatigue Scale also provide useful clinical information (i.e., fatigue due to sleep, when fatigue is experienced during the day). We suggest that the nature of the single factor in the Flinders Fatigue Scale could be considered “daytime fatigue”—a term similar to one of the subscales of the MFI (i.e., “general fatigue”), and somewhat concordant with the notion of distressed-fatigue.35 However, unlike the MFI and FSS, the daytime fatigue factor from the Flinders Fatigue Scale was found to be sensitive to treatment.
In the present study it was found that the Flinders Fatigue Scale was responsive to cognitive-behavior therapy for insomnia (CBT-I). Specifically, significant reductions in fatigue were found from pretreatment to posttreatment, and further reductions were found from posttreatment to a 2-month follow-up. These changes coincided with and were directly related to improvements over time in all sleep parameters (sleep onset latency, wake after sleep onset, total sleep time, and sleep efficiency). As Morin states, in order “to be considered clinically meaningful, sleep improvements should also lead to a reduction of daytime fatigue” (p. 264).11 It would appear that the Flinders Fatigue Scale has been able to detect such clinically meaningful changes for insomnia patients where previous measures have not. Thus, the Flinders Fatigue Scale is the first fatigue measure to be validated for insomnia patients undergoing treatment. Furthermore, these results also suggest that the measurement of fatigue in Study 2 was intrinsically related to sleep. Various studies have demonstrated that people experiencing insomnia are more hyperaroused, as indicated by a higher metabolic rate36 or core body temperature.37,38 Moreover, elevated metabolism in various arousal- and emotion-regulating brain regions has been linked to greater subjective fatigue in people with insomnia.39 Thus, the present study provides further evidence of the link between sleeplessness and fatigue.
An interesting finding was the lack of correspondence between daytime fatigue and daytime sleepiness. No significant correlation was found between these measures at pretreatment. Furthermore, the significant improvements in daytime fatigue and sleep parameters were not reflected in changes of daytime sleepiness. Of note is that the Flinders Fatigue Scale is prefaced by the description of what fatigue is, and what it is not (namely sleepiness; see Appendix). Therefore, the lack of correlation between fatigue and sleepiness in Study 2 could partly result from these instructions. Nevertheless, daytime fatigue and daytime sleepiness have been considered as separate constructs.4,25,40 Interestingly, 2 of the 3 synonyms used to describe fatigue (i.e., tired, weary, exhausted) in the Flinders Fatigue Scale have recently been included in the broad definition of fatigue in the sleep medicine literature.41 Furthermore, previous research has found little overlap between objective measures of sleepiness (i.e., the multiple sleep latency test) and subjective fatigue,16 which suggests fatigue and sleepiness are conceptually different for people experiencing insomnia, regardless of whether sleepiness is measured objectively or subjectively.
Generally, the Flinders Fatigue Scale was able to discriminate between good and poor sleepers, with poor sleepers scoring significantly higher on this measure. No significant differences were found, though, in fatigue scores between the various insomnia disorder patients. Interestingly, the mean values of the insomnia patients were higher than that of the poor sleepers in Study 1. This may be due to a number of reasons. It may be that people with insomnia not only report greater daytime fatigue than people with other sleep disorders,16 but those who seek treatment report greater daytime fatigue. This would be consistent with the finding that fatigue is one of the main reasons people with insomnia will seek treatment.11
Although there are a number of positive outcomes from this preliminary investigation of the Flinders Fatigue Scale, a number of limitations must also be acknowledged. First, the test-retest reliability of the Flinders Fatigue Scale was not tested in the present paper. The stability of a scale over time is an important psychometric property and currently remains unknown. Likewise, convergent validity is another important psychometric property, and this was also not tested. Future work is needed to test the Flinders Fatigue Scale's convergent validity with other subjective (e.g., Fatigue Severity Scale) and objective (e.g., cardiopulmonary stress test) measures of fatigue. With regard to important clinical parameters, future research is needed to validate the Flinders Fatigue Scale across sleep disorders, other medical and psychiatric conditions, as well as healthy normal subjects. Such future investigations could help to produce meaningful clinical cut-off scores which will aid in the assessment and evaluation of insomnia treatment in both clinical and research settings. At this stage we would caution readers attempting to derive clinical cut-off scores with the available data in Study 1 (i.e., 1 SD above the mean), as it was not representative of insomnia in the population. That is, not only was the PSQI used (which can differentiate good sleepers from poor sleepers, as opposed to identifying clinical levels of insomnia), but the proportion of poor sleepers (in this case >50%) was much higher than that found from insomnia prevalence data (15%–20%).1 Future studies should develop normative data using more representative sampling methods. For example, normative data of good sleepers would provide clinically meaningful data for clinicians to assess whether their patients' Flinders Fatigue Scale scores after treatment are within the range of good sleepers. Finally, although all items from the Flinders Fatigue Scale loaded onto a single factor, the factor loading of item 7 was the lowest. This could indicate this item is of a different nature than the other 6 items. Further work is required to determine whether or not item 7 should be included as an item of the scale or as an appendage for clinical use.
Summary
The Flinders Fatigue Scale represents the first brief, and reliable measure validated with insomnia patients. Its single “daytime fatigue” factor, which explains 67% of the variance, appears not only sensitive to the effects of cognitive-behaviour therapy and is also related to improvements in sleep, but is also distinguishable from daytime sleepiness. Future research would help to further assess the scale's convergent and discriminant validity, test-retest reliability, as well as provide normative data and potentially useful clinical cut-off scores.
ACKNOWLEDGMENTS
The authors wish to thank the Faculty of Social Sciences, Flinders University for their financial assistance, Corey Durward for his assistance with the on-line administration of the scales, Prof. Doug McEvoy for his continued support of the Insomnia Treatment Program at the Adelaide Institute for Sleep Health, Ms. Debbie Gilmour for her administrative assistance with the Insomnia Treatment Program at the Adelaide Institute for Sleep Health, the editor and two anonymous reviewers for their mentoring advice on previous versions of this paper, and all the participants who volunteered for the study.
Financial support: Faculty of Social Sciences, Flinders University.
APPENDIX
Flinders Fatigue Scale
We are interested in the extent that you have felt fatigued (tired, weary, exhausted) over the last two weeks. We do not mean feelings of sleepiness (the likelihood of falling asleep). Please circle the appropriate response in accordance with your average feelings over this two-week period.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 3.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 5.Lineberger MD, Carney CE, Edinger JD, Means MK. Defining insomnia: Quantitative criteria for insomnia severity and frequency. Sleep. 2006;29:479–85. doi: 10.1093/sleep/29.4.479. [DOI] [PubMed] [Google Scholar]
- 6.Brooks JO, 3rd, Friedman L, Bliwise DL, Yesavage JA. Use of the wrist actigraph to study insomnia in older adults. Sleep. 1993;16:151–5. doi: 10.1093/sleep/16.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 8.Edinger JD, Hoelscher TJ, Webb MD, Marsh GR, Radtke RA, Erwin CW. Polysomnographic assessment of DIMS: Empirical evaluation of its diagnostic value. Sleep. 1989;12:315–22. [PubMed] [Google Scholar]
- 9.Reite M, Buysse DJ, Reynolds C, Mendelson W. The use of polysomnography in the evaluation of insomnia. Sleep. 1995;18:58–70. doi: 10.1093/sleep/18.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Chesson A, Hartse K, Anderson WM, et al. Practice parameters for the evaluation of chronic insomnia. Sleep. 2000;23:1–5. [PubMed] [Google Scholar]
- 11.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;7:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 12.Stepanski EJ, Koshorek G, Zorick F, Glinn M, Roehrs TA, Roth T. Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics. 1989;30:421–7. doi: 10.1016/S0033-3182(89)72248-9. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health. NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens Sci Statements. 2005;22:1–30. [PubMed] [Google Scholar]
- 14.Colten HR, Altevogt B, editors. Washington, DC: The National Academies Press; 2006. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. [PubMed] [Google Scholar]
- 15.Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF-36: Evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med. 2001;63:49–55. doi: 10.1097/00006842-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lichstein KL, Means MK, Noe SL, Aguillard RN. Fatigue and sleep disorders. Behav Res Ther. 1997;35:733–40. doi: 10.1016/s0005-7967(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 17.Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A'Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79:1479–86. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro CM, Flanigan M, Fleming JA, et al. Development of an adjective checklist to measure five FACES of fatigue and sleepiness: data from a national survey of insomniacs. J Psychosom Res. 2002;52:467–473. doi: 10.1016/s0022-3999(02)00407-5. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Krupp LB, LaRocca NG, Muir-Nash J, Steinburg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 21.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI): Psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 22.Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aquillard RN. Relaxation and sleep compression for late-life insomnia: A placebo-controlled trial. J Consult Clin Psychol. 2001;69:227–39. doi: 10.1037//0022-006x.69.2.227. [DOI] [PubMed] [Google Scholar]
- 23.Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol. 2003;71:189–200. [PubMed] [Google Scholar]
- 24.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of a cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 25.Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM. Subjective fatigue and sleepiness: Two independent consequences of sleep disorders? J Sleep Res. 2005;14:245–53. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert N. Researching social life. London: Sage; 1993. [Google Scholar]
- 29.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 30.Guadagnoli E, Velicer WF. Relations of sample size to the stability of component patterns. Psychol Bull. 1988;103:265–75. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- 31.Shevlin M, Miles JNV, Davies MNO, Walker S. Coefficient alpha: A useful indicator of reliability? Pers Individ Dif. 2000;28:229–37. [Google Scholar]
- 32.Kleinman L, Zodet MW, Hakim Z, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res. 2000;9:499–508. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AH. Validity of cancer-related fatigue instruments. Pharmacotherapy. 2002;22:1433–41. doi: 10.1592/phco.22.16.1433.33690. [DOI] [PubMed] [Google Scholar]
- 34.Gentile S, Delaroziere JC, Favre F, Sambuc R, San Marco JL. Validation of the French ‘multidimensional fatigue inventory’ (MFI 20) Eur J Cancer Care. 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 35.Christiansen L, Piper-Terry M. Comparison of psychometric measures of fatigue. Soc Behav Pers. 2004;32:225–31. [Google Scholar]
- 36.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 37.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23:1–7. [PubMed] [Google Scholar]
- 38.Gradisar M, Lack L, Wright H, et al. Do chronic primary insomniacs have impaired heat loss when attempting sleep? Am J Physiol Regul Integr Comp Physiol. 2006;290:R1115–21. doi: 10.1152/ajpregu.00266.2005. [DOI] [PubMed] [Google Scholar]
- 39.Nofzinger EA, Buysse DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–9. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 40.Merkelbach S, Schulz H. What have sleepiness and fatigue in common? J Sleep Res. 2006;15:105–6. doi: 10.1111/j.1365-2869.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness from fatigue: Focus on definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]