Abstract
Tobacco use continues to be a major cause of cancer in the developed world and, despite significant progress in this country in tobacco control which is driving a decrease in cancer mortality, there are still over one billion smokers in the world. This perspective discusses some selected issues in tobacco carcinogenesis focusing on progress during the 20 years of publication of Chemical Research in Toxicology. The topics covered include metabolism and DNA modification by tobacco-specific nitrosamines, tobacco carcinogen biomarkers, an unidentified DNA ethylating agent in cigarette smoke, mutations in the K-RAS and p53 gene in tobacco-induced lung cancer and their possible relationship to specific carcinogens, secondhand smoke and lung cancer, emerging issues in smokeless tobacco use, and a conceptual model for understanding tobacco carcinogenesis. It is hoped that a better understanding of mechanisms of tobacco-induced cancer will lead to new and useful approaches for prevention of lung cancer and other cancers caused by tobacco use.
Keywords: tobacco specific nitrosamines, secondhand smoke, smokeless tobacco, tobacco carcinogen biomarkers
Introduction
While the use of tobacco products continues to be an immense public health problem, and arguably the largest voluntary source of human exposure to carcinogens in the world, remarkable progress has been achieved in the past 20 years, both in our understanding of mechanisms of tobacco carcinogenesis, and in tobacco control. The clean indoor air which we now take for granted in bars, restaurants, and other public places in many countries would have been unimaginable in the mid 1980s. Public disapproval of smoking and tobacco marketing has risen to new highs. Nevertheless, there are still approximately 1.3 billion smokers in the world and hundreds of millions of smokeless tobacco users (1). Cigarette smoking causes 30% of all cancer mortality in developed countries (2), and smokeless tobacco use is an important cause of cancer, particularly in southern Asia (3,4). The goal of our research is to understand mechanisms of tobacco carcinogenesis and apply this knowledge to the prevention of tobacco-induced cancer.
I congratulate the editors of Chemical Research in Toxicology for establishing the premier journal in chemical aspects of toxicology, which has proven to be particularly timely for chemists interested in carcinogenesis. This perspective will cover only a few selected topics in tobacco carcinogenesis, a broad field encompassing studies of individual agents as well as complex mixtures, and which, if reviewed comprehensively, would probably fill this entire issue. Only certain individual agents are discussed here while others, equally important, are virtually ignored, along with inhalation studies of cigarette smoke, which have been discussed in a recent perspective (5).
Tobacco-specific nitrosamines: metabolism, DNA adduct formation and repair
Tobacco-specific nitrosamines, first characterized with respect to their presence in tobacco products and carcinogenicity in the 1970s, have emerged clearly as one of the most important groups of carcinogens in tobacco products (3,6). Seven tobacco-specific nitrosamines have been identified in tobacco products, but two of these- 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N′-nitrosonornicotine (NNN)- are the most important because of their carcinogenic activities and their consistent presence in both unburned tobacco and its smoke, frequently in relatively considerable amounts (7). NNK selectively induces mainly lung tumors in all species tested and is particularly potent in the rat (8). NNK also causes tumors of the pancreas, nasal mucosa, and liver (8). NNN produces esophageal and nasal cavity tumors in rats and respiratory tract tumors in mice and hamsters (8). NNK and NNN are considered carcinogenic to humans by the International Agency for Research on Cancer (3).
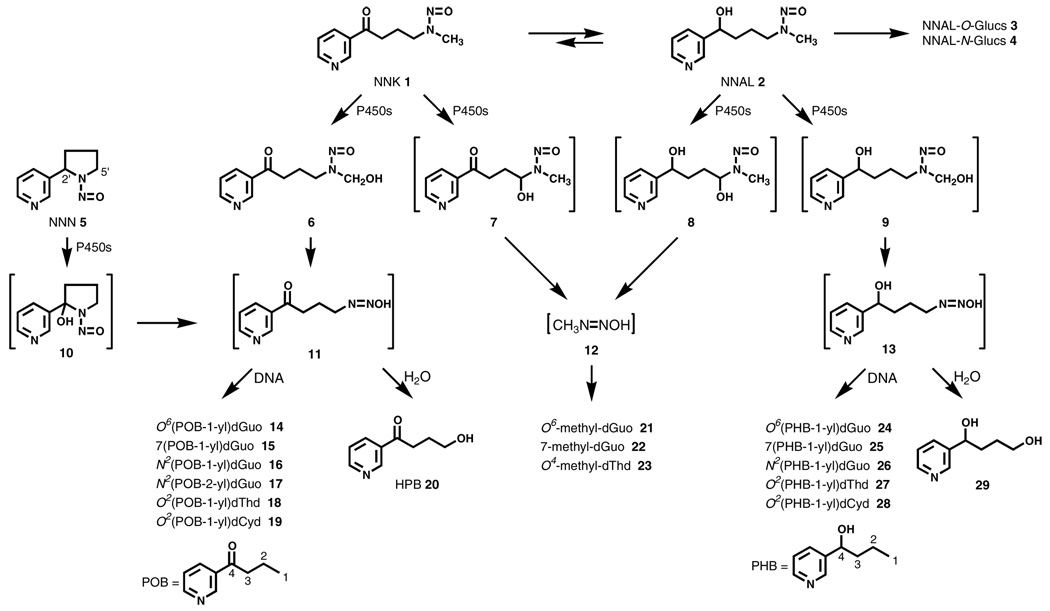
It is well established that dialkylnitrosamines such as NNK require metabolic activation by cytochrome P450 catalyzed α-hydroxylation to exert their carcinogenic properties (9). Scheme 1 illustrates the α-hydroxylation pathways for NNK (1) and its major metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, 2), as well as the 2′-α-hydroxylation pathway for NNN (5) (8). α-Hydroxylation of the NNK methyl group leads to intermediate 6 which spontaneously loses formaldehyde yielding 4-(3-pyridyl)-4-oxobutanediazohydroxide (11). The same intermediate is formed by 2′-hydroxylation of NNN. α-Hydroxylation at the methylene group of NNK produces intermediate 7 which spontaneously decomposes to methanediazohydroxide (12). Similar intermediates are produced by α-hydroxylation of NNAL. Methanediazohydroxide (12), formed in these reactions from NNK and NNAL, reacts with DNA to produce the well known DNA adducts –, O6-methyl-dGuo (21), 7-methyl-dGuo (22), and O4-methyl-dThd (23), that are common to many methylating carcinogens. But DNA adduct formation by intermediates 11 and 13 was unknown in 1986. Subsequently, it was found that neutral thermal or acid hydrolysis of DNA from NNK, NNN or NNAL-treated animals produced 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB, 20), confirming this pathway of DNA alkylation resulting in pyridyloxobutyl (POB)-DNA adducts (8,10–12). Released HPB could be quantified by GC-MS and its presence was established in the lung DNA of smokers, as well as in NNK-treated rodents (8,13,14). The structures of the adducts formed by this pathway remained elusive until the late 1990s when Peterson and co-workers characterized O6(POB-1-yl)dGuo (14)(15), a highly mutagenic DNA adduct (16), and 2003-4 when our group identified adducts 15–19 and 24–28 as summarized in Scheme 1 (17–19).
Scheme 1.
DNA adduct formation from NNK, NNN, and NNAL.
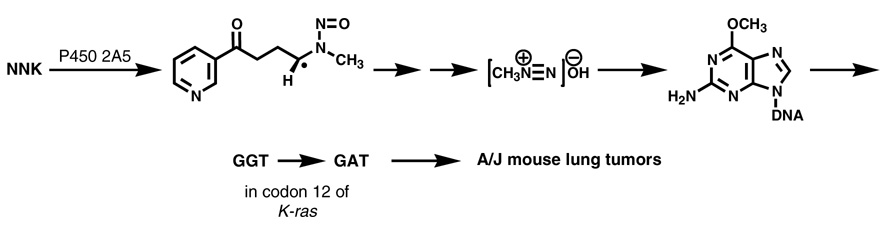
There is no doubt that cytochrome P450 catalyzed α-hydroxylation of NNK is critical for its carcinogenicity. A review published in 1988 mentions the importance of characterizing which P450s are involved in NNK metabolism, but no data were available at that time (6). Presently, steady state kinetic parameters for P450 catalyzed NNK metabolism have been reported for 8 human enzymes, 2 rabbit enzymes, 5 rat enzymes, and 2 mouse enzymes (20). Human P450s 2A13 and 2B6, rat P450 2A3, and mouse P450 2A5 may be the most important catalysts of NNK metabolic activation in these species, based on their kinetic parameters. The critical role of mouse P450 2A5 has been clearly shown by deuterium labeling studies which demonstrated the stereoselective abstraction of the 4(R)- hydrogen of NNK, leading to a cascade of events resulting in lung tumor induction in A/J mice (21)(Figure 1). Thus, 4(R)-[4-2H1]NNK was significantly less tumorigenic than 4(S)-[4-2H1]NNK due to a demonstrated deuterium isotope effect in the metabolism of this compound by P450 2A5; levels of O6-methyl-dGuo were also reduced in the lungs of mice treated with 4(R)-[4-2H1]NNK compared to NNK or 4(S)-[4-2H1]NNK (21). Earlier, we demonstrated a high correlation between persistent O6-methyl-dGuo in this model and the number of lung tumors per mouse, as well as the presence of G → A transition mutations in the k-ras gene isolated from the lung tumors (22,23). This mutation, shown by others to rapidly cause lung cancer in mice (24), is a known consequence of miscoding due to O6-methyl-dGuo (25). Collectively, these data provide powerful support for the events summarized in Figure 1 and demonstrate the critical nature of the initial P450-catalyzed hydrogen abstraction, without which tumor induction would not occur.
Figure 1.
Abstraction of the prochiral rear 4-hydrogen of NNK, catalyzed by mouse lung P450 2A5 (and possibly other P450s), is the requisite step which initiates a cascade of events leading to lung tumor formation in the A/J mouse. A single dose of 10 µmol of NNK induces about 10 lung tumors per mouse after 16 weeks in this model without the need for any exogenous tumor promoter or genetic manipulation (136). Deuterium labeling studies demonstrate that levels of O6-methyl-dGuo in DNA and lung tumor multiplicity are significantly decreased in animals treated with 4(R)-[4-2H1]NNK compared to those treated with unlabelled or 4(S)-[4-2H1]NNK (21). The initially formed intermediate shown here is converted to α-methylene-hydroxyNNK (7, Scheme 1) which spontaneously yields methanediazohydroxide and the methyl diazonium ion (shown). The latter reacts with DNA to produce the adduct O6-methyl-dGuo. Lung tumor multiplicity is highly correlated with levels of persistent O6-methyl-dGuo in DNA (22). This DNA adduct causes G → A mutations in codon 12 of the k-ras oncogene leading to lung tumor formation (23,24).
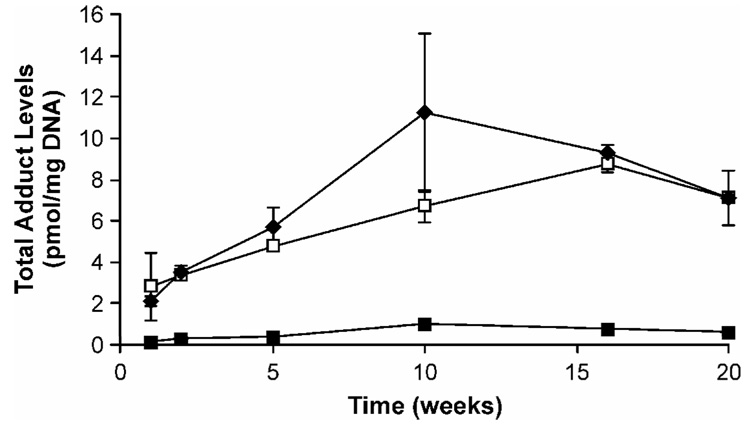
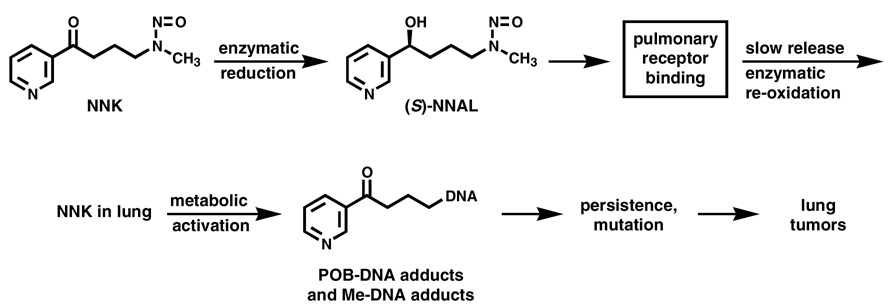
Evidence from deuterium labeling studies and the formation and persistence of O6-methyl-dGuo and POB-DNA adducts in Clara cells and type II cells of the rat lung demonstrate that both types of adducts are likely crucial in the induction of rat lung tumors by NNK (8). These studies were also bolstered by a correlation between the inhibitory effects of 2-phenethyl isothiocyanate (PEITC) on rat lung NNK carcinogenicity and POB-DNA adduct formation in type II cells of the lung (26). PEITC exerts its protective effects mainly through the inhibition of rat lung P450s, and is also a potent inhibitor of human P450 2A13 (Ki = 30 nM) (27). The formation and persistence of individual POB-DNA adducts has recently been characterized in the lung of rats treated chronically with NNK or the enantiomers of NNAL (28). O2(POB-1-yl)dThd, 7(POB-1-yl)Gua, O2(POB-1-yl)Cyt, and O6(POB-1-yl)dGuo, listed in order of their concentrations, were detected at each time point. Adduct levels in lung were similar in rats treated with NNK or (S)-NNAL, and far less in rats treated with (R)-NNAL (Figure 2). These results are consistent with studies that showed the accumulation of (S)-NNAL in rat lung, possibly at a receptor site (29). The current model for the organoselective carcinogenicity of NNK in the rat lung is illustrated in Figure 3. Upon administration to the rat, NNK is extensively reduced mainly to (S)-NNAL which binds to an as yet unknown receptor, while (R)-NNAL is rapidly glucuronidated (29) and eliminated from the body. This receptor binding leads to accumulation of (S)-NNAL in the lung. It is gradually released and re-oxidized to NNK, which then undergoes α-hydroxylation producing persistent O6-methyl-dGuo and POB-DNA adducts, resulting in mutations and lung tumor induction.
Figure 2.
Total POB-DNA adduct levels in the lungs of rats treated with NNK (closed diamonds), (S)-NNAL (open squares) or (R)-NNAL (closed squares). Rats were treated with 10 ppm of each compound added to their drinking water and were killed at the time intervals shown. Individual POB-DNA adducts were analyzed by LC-ESI-MS/MS-SRM as described (28).
Figure 3.
Current model for the selective induction of induce lung tumors in rats treated with NNK. Upon administration to rats, NNK is enzymatically reduced to NNAL, with (S)-NNAL predominating (34). (S)-NNAL is hypothesized to bind to an as yet uncharacterized pulmonary receptor causing its accumulation and persistence in the lung (29). It is presumed to be slowly released from this receptor site and enzymatically reconverted to NNK, at least in part by P450s (20). This NNK then undergoes metabolic activation by P450 2A3 and other P450s in the lung, resulting in POB-DNA adducts (shown) and Me-DNA adducts (see also Scheme 1) (8,20). The persistence of these adducts in Type II cells and Clara cells of the rat lung is believed to result in mutations and lung tumor induction.
Mouse P450 2A5 is an excellent catalyst of 5′-hydroxylation of both (R)- and (S)-NNN, and several other P450 2A enzymes including rat 2A3, mouse 2A4, human 2A6, and human 2A13 are also reasonably efficient (30). Lower activity was found for 2′-hydroxylation of (R)-NNN, and none for 2′-hydroxylation of (S)-NNN. Tissue specific 2′-hydroxylation of NNN by a high affinity P450 is responsible for its carcinogenicity in the rat esophagus, but this enzyme, which is clearly not P450 2A3, has not been identified (8,31). As shown in Scheme 1, 2′-hydroxylation by the unidentified esophageal P450 leads to the same POB-DNA adducts as produced by methyl hydroxylation of NNK, and these have been recently quantified in the esophagus of rats treated chronically with (S)-NNN or (R)-NNN, with the former producing higher adduct levels (32). Adducts formed by 5′-hydroxylation of NNN have also been characterized recently (33). There is no doubt that tissue specific α-hydroxylation of NNN is responsible for its carcinogenicity in the rat esophagus.
There is considerable evidence that elements of the pathway illustrated in Figure 3 are also present in human lung. NNK is readily reduced to (S)-NNAL by all human tissues tested including lung (34). While specific accumulation of (S)-NNAL in human lung has not been reported, this enantiomer is more slowly excreted in human urine than is (R)-NNAL, and the elimination half-life of NNAL (40–45 days) in smokers is unusually long for a small water soluble molecule (35,36). P450 2A13 is expressed in human lung, as are other P450s which metabolize NNAL and NNK, and POB-DNA adducts have been detected in human lung, with levels higher in smokers than in non-smokers (13,20,37,38). Robust metabolism of NNK in human fetal nasal microsomes, catalyzed by P450 2A13, has also been demonstrated (39). While the human lung metabolism and DNA binding of NNK require further characterization, the results obtained to date support parallel human and rat mechanisms.
Impressive advances have occurred in our understanding of the repair of DNA adducts of NNK. Both O6-methyl-dGuo and O6(POB-1-yl)dGuo are repaired by O6-alkylguanine-DNA alkyltransferase (AGT) (40,41). POB-DNA adducts are repaired by AGT in a reaction that results in pyridyloxobutyl transfer to the active site cysteine, similar to O6-methyl-dGuo. Human AGT variants differ in their ability to repair O6-methyl-dGuo and O6(POB-1-yl)dGuo. The AGT mediated repair of O6(POB-1-yl)dGuo was also affected by sequence context, more so than the repair of O6-methyl-dGuo. These effects undoubtedly contribute to the risk of tobacco-related cancer upon exposure to NNK and NNN.
While NNAL (2, Scheme 1) was first identified as a metabolite of NNK in 1980 (42), NNAL O-glucuronidation was not established for another 10 years (43). The extensive formation of this metabolite in the patas monkey suggested that it could be a biomarker for human exposure to NNK (44). This possibility was realized with the development of an analytical method, first reported in 1993 (45). As discussed below, urinary total NNAL has emerged as a powerful biomarker for assessing human exposure to NNK. Pyridine-N-glucuronides of NNAL are also formed metabolically in humans (46).
In summary, significant progress has been achieved in the past 20 years in our understanding of tobacco-specific nitrosamine metabolism, DNA interactions and repair. These studies provide definitive mechanistic insights pertinent to the organoselectivity of these compounds for induction of lung and esophageal tumors in rodents, and possibly in humans. This work has led to the development of exposure biomarkers for both NNK and NNN, and application of these has impacted tobacco control as described below. While quantitative estimates of tobacco-specific nitrosamine exposure are now available from this biomarker work, definition of the corresponding situation in DNA of human tissues, particularly the lung, remains sketchy. This is a critical area for future research involving translation of our deep mechanistic understanding in laboratory animals to the human setting. Ultimately, this could lead to a better understanding of mechanisms and susceptibility to tobacco-induced cancer, and possibly cancer in general.
Tobacco carcinogen biomarkers
Tobacco carcinogen biomarkers are substances measurable in human body fluids or tissues. These biomarkers are specifically related to tobacco carcinogens. The “Hoffmann list” of over 60 carcinogens in cigarette smoke is considered the definitive catalogue of its major cancer causing agents and includes polycyclic aromatic hydrocarbons (PAH), nitrosamines, aromatic amines, aldehydes, volatile organic compounds, metals, and others (47,48). Examples of tobacco carcinogen biomarkers include tobacco carcinogens or their metabolites in breath, blood, or urine; tobacco carcinogen-DNA adducts; and tobacco carcinogen-protein adducts. The International Agency for Research on Cancer (IARC) monograph entitled “Tobacco Smoking”, published in 1986, did not contain any references describing tobacco carcinogen biomarkers (49). In contrast, there were over 350 citations on this topic in the 2004 IARC monograph entitled “Tobacco Smoke and Involuntary Smoking”, a clear demonstration that this critical area of tobacco carcinogenesis has evolved remarkably in the past 20 years (50). Applications of tobacco carcinogen biomarkers include determining carcinogen dose in people who use tobacco products and in non-smokers exposed to secondhand smoke, identifying inter-individual differences in the uptake, metabolic activation, and detoxification of tobacco carcinogens, and ultimately predicting which tobacco user is susceptible to cancer.
Measurement of carcinogen-DNA adducts potentially can provide the most direct link between cellular exposure and cancer, as DNA adducts are critical in the carcinogenic process. But DNA adducts are challenging to quantify because their levels are extremely low, frequently ranging from 1 per 106 to 1 per 108 normal bases in humans (51), and the tissue samples containing them are often available in only small quantities. In recent years, the sensitivity of mass spectrometers has improved dramatically, and the routine detection of amol levels of underivatized DNA adducts is now feasible (52). Although there are still relatively few examples of quantitation of specific DNA adducts in tissues of smokers using mass spectrometry, HPLC-fluorescence, HPLC with electrochemical detection, or postlabelling techniques, this literature is expanding rapidly and includes quantitation of DNA adducts of benzo[a]pyrene (BaP), tobacco-specific nitrosamines (e.g. HPB releasing adducts of NNK or NNN), alkylating agents, aldehydes and other lipid peroxidation products, and products of oxidative damage such as 8-oxo-dGuo (53–58). A much larger body of work has emerged from studies which have used the highly sensitive, but relatively non-specific 32P-postlabelling and immunoassay methods for detection of DNA adducts. The advantages and disadvantages of 32P-postlabelling and immunoassay have been discussed (59–62). In summary, major advantages include high sensitivity allowing analysis of small amounts, generally micrograms, of DNA, relative simplicity of analysis, and no requirements for expensive equipment. Disadvantages include lack of chemical specificity, particularly in 32P-postlabelling analyses, and difficulty in quantitation. Although the adducts detected using this method are often referred to in the literature as “aromatic DNA adducts”, there is strong evidence that they are not related to PAH (63). The application of these methods to tissues obtained from smokers has been extensively reviewed (61). Adduct levels are generally higher in lung tissues of smokers than non-smokers while studies using blood DNA have produced mixed results. Adducts have also been detected in many other tissues and fluids from smokers including larynx, oral and nasal mucosa, bladder, cervix, breast, pancreas, stomach, placenta, fetal tissue, cardiovascular tissues, sputum, and sperm. These studies have been comprehensively reviewed (7,61). A meta-analysis of the relationship of DNA adduct levels in smokers to cancer, as determined by 32P-postlabelling, was carried out using case control studies of lung cancer (5 studies) oral cancer (1) and bladder cancer (1). Six studies measured adducts in white blood cells and one in normal lung tissue. Current smokers showed a significant difference between cases and controls, with cases having higher adduct levels than controls (64).
Protein adduct biomarkers have also been developed. Carcinogen-hemoglobin (Hb) adducts levels have been used as surrogates for DNA adduct measurements (65,66). Serum albumin adducts could also be used in this way. Although these proteins are not considered as targets for carcinogenesis, all carcinogens that react with DNA will also react with protein to some extent. Advantages of Hb adducts as surrogates include the ready availability of relatively large amounts of Hb from blood and the relatively long lifetime of the erythrocyte in humans – 120 days – which provides an opportunity for adducts to accumulate. Studies on protein adducts in smokers have been comprehensively reviewed (7,61).
Hb adducts of aromatic amines have emerged as a highly informative type of carcinogen biomarker, with levels which are consistently higher in smokers than in non-smokers, particularly for 3-aminobiphenyl and 4-aminobiphenyl-Hb adducts. Hb binds aromatic amines efficiently because the heme accelerates the rate of nitrosoarene formation from the hydroxylamine, which is produced metabolically from the aromatic amine by P450 1A2 (67). Binding of the nitrosoarene occurs at the β-93 cysteine residue of human Hb; the adduct is hydrolyzed releasing the free amine which is quantified by GC-MS (67). Adduct levels decrease upon smoking cessation and are related to numbers of cigarettes smoked (67–69). Adducts which form with the amino terminal valine of Hb are also informative. Important examples include those derived from ethylene oxide, acrylonitrile, and acrylamide (70–72). Ethylated N-terminal valine of Hb is also higher in smokers than in non-smokers (72).
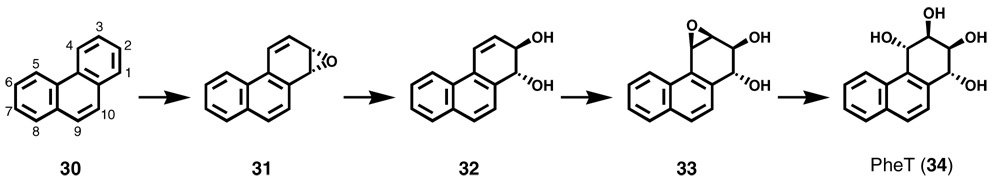
Probably the most practical biomarkers are urinary metabolites of tobacco carcinogens (73). Advantages include the ready availability of samples and the higher concentration of urinary metabolites than of most adducts. Metabolites of PAH and tobacco-specific nitrosamines, and mercapturic acids derived from benzene, acrolein, and 1,3-butadiene are among the most commonly used urinary biomarkers. Among PAH metabolites, 1-hydroxypyrene (1-HOP) has probably been used more extensively than any other biomarker. Pyrene, a non-carcinogen, is a component of all PAH mixtures, and 1-HOP, a metabolite of pyrene which can be readily measured by HPLC with fluorescence detection, has been used in many studies to assess PAH uptake, which is generally 2-3 times higher in smokers than in non-smokers (73,74). We have introduced r-1,t-2,3-c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (PheT, 34, Scheme 2) as a biomarker of PAH uptake plus metabolic activation, as the metabolic pathway from phenanthrene (30), a non-carcinogenic PAH, to phenanthrene diol epoxide (33) and ultimately to PheT is highly analogous to that involved in the metabolic activation of BaP to its diol epoxide metabolite (75,76). PheT measurements will hopefully identify those smokers who are particularly able to catalyze this deleterious pathway.
Scheme 2.
Metabolism of phenanthrene (30) to a bay region diol epoxide (33) and PheT (34).
Total NNAL, the sum of NNAL and its glucuronides, has emerged as a very useful biomarker of NNK exposure (73,77,78). The tobacco-specificity of NNK, and therefore total NNAL, is a key feature of this biomarker because studies in which it is applied are not confounded by environmental or dietary exposures. Total NNAL has been used in many studies to estimate lung carcinogen uptake in smokers. In one, smokers reduced their number of cigarettes smoked per day, but there was not a corresponding decrease in NNK uptake due to smokers’ compensation (79,80). In another, NNK and PAH uptake were compared in smokers of regular, light, and ultra-light cigarettes, and found not to differ, consistent with epidemiologic studies which demonstrate no protection against lung cancer in smokers of light compared to regular cigarettes (81). Other studies evaluated NNK uptake in smokers who switched from their current cigarette brand to products purported to be less hazardous, but the results generally did not support the claims of the product manufacturers (82–84). The most useful application of total NNAL has perhaps been in studies of lung carcinogen uptake by non-smokers exposed to secondhand cigarette smoke (78). The sensitivity and specificity of this biomarker are ideal for such studies, and it is the only tobacco carcinogen biomarker until now consistently elevated in non-smokers exposed to secondhand smoke. The results of these studies demonstrating NNK exposure throughout life in non-smokers exposed to secondhand smoke are summarized in Table 1 (85). These results have had a significant impact on legislation prohibiting indoor smoking because non-smokers are highly adverse to the idea of having a lung carcinogen in their urine.
Table 1.
Non-smokers' exposure to NNK throughout life by measurement of urinary total NNAL
| Exposed Group | Type of Exposure | Total NNAL (fmol/mL urine) | % of Amount in Smokers' Urinea | Reference |
|---|---|---|---|---|
| Fetus | Transplacental | 25 ± 29 (amniotic fluid) | 1.3 | (128) |
| Newborns | Transplacental | 130 ± 150 | 6.5 | (129) |
| Infants (<1 year old) | Air | 83 ± 20 | 4.2 | (85) |
| Elementary School Children | ||||
| Minneapolis | Air | 56 ± 76 | 2.8 | (130) |
| Moldova | Air | 90 ± 77 | 4.5 | (131) |
| Women Living with Smokers | Air | 50 ± 68 | 2.5 | (132) |
| Hospital Workers | Air | 59 ± 28 | 3.0 | (133) |
| Casino Patrons | Air | 18 ± 15 | 0.9 | (134) |
| Restaurant and Bar Workers | Air | 33 ± 34 | 1.7 | (135) |
based on 2 pmol/mL total NNAL in smokers’ urine
S-Phenylmercapturic acid, formed as a result of glutathione conjugation of benzene oxide, a metabolite of the human leukemogen benzene, is a highly practical and specific biomarker of benzene uptake (86,87). Similarly, 3-hydroxypropylmercapturic acid, formed from acrolein, can be readily quantified in human urine (88). Both of these mercapturic acids occur in significantly higher amounts in smokers than in non-smokers and decrease upon smoking cessation.
In summary, urinary biomarkers of tobacco carcinogen exposure are readily measured and have been applied in many studies evaluating toxicant uptake by tobacco users. These biomarkers in particular are likely to play a major role in evaluating potential tobacco related harm in the coming era of tobacco product regulation.
A DNA ethylating agent in cigarette smoke
Recent evidence indicates that there is a DNA ethylating agent present in cigarette smoke, which could not logically be derived from any of the compounds on the “Hoffmann list” of tobacco carcinogens. Two reports showed increased levels of 3-ethyladenine in smokers' urine. In one study, the amount of urinary 3-ethyladenine was more than 5 times greater on days when individuals smoked than on days when they didn't (89). Urinary 3-ethyladenine was also 5–8 times higher in smokers than in non-smokers in that investigation. In a second study, urinary 3-ethyladenine increased in some smokers, on days when they smoked, and a correlation was seen between cigarettes per day and 3-ethyladenine excretion (90). 3-Ethyladenine in urine most likely results from reaction of an ethylating agent with adenine in nucleic acids followed by depurination or repair. Consistent with this, Singh and Farmer demonstrated that cigarette smoke ethylates DNA in vitro, as determined by quantitation of 7-ethyl-Gua (91). Other studies in humans are consistent with this finding as smokers have higher levels of 7-ethyl-Gua in urine (89,90,92), O4-ethyl-dThd in lung (93), and ethylvaline in Hb than do non-smokers (72). The origin of this DNA ethylating agent in cigarette smoke is unknown, but we have suggested ethyl nitrite and endogenous nitrosation of ethyl amine as two possibilities (72). This requires further research because an ethylating agent can produce DNA adducts with miscoding properties, thus potentially contributing significantly to tobacco carcinogenesis.
Mutations in K-RAS and p53 in lung cancer
The first studies demonstrating the common occurrence of mutations in the K-RAS oncogene and the p53 tumor suppressor gene in lung cancer appeared during the 20 year lifetime of Chemical Research in Toxicology. Mutations in K-RAS have been found in approximately 30 to 40 percent of adenocarcinomas of the lung, but infrequently in other lung tumor types or in lung tumors from non-smokers (94–97). All mutations have been found in codons 12, 13, and 61, with mutations in codon 12 being by far the most common. Most of the codon 12 mutations are G→T transversions, GGT → TGT or GGT → GTT. A similar pattern of mutations is found in mouse lung tumors induced by PAH, providing support for their role as causes of lung cancer, although it should be noted that other tobacco smoke carcinogens can produce the same mutations (98). While it may not be possible to ascribe such changes to a particular tobacco smoke carcinogen, their detection in tobacco carcinogen induced lung tumors as well as lung tumors from smokers certainly supports the general hypothesis that these mutations result from DNA adducts of metabolically activated tobacco carcinogens.
The p53 gene is the most commonly mutated tumor suppressor gene in lung cancer. p53 mutations occur in about 40% of human lung cancers, and are generally more common in smokers than in non-smokers (53,99). Significantly higher occurrences of G → T transversion mutations have been observed in the p53 gene in lung cancer in smokers than in non-smokers (53). p53 mutations in lung cancer are observed at “hotspots” within the DNA binding domain of the p53 protein. The major lung cancer mutation hotspots are found at codons 157, 158, 245, 248, 249, and 273 (53). A remarkable concordance was observed between the occurrence of these mutational hotspots and the pattern of DNA adduct formation by PAH diol epoxides in reactions with the p53 gene, as determined by ligation-mediated polymerase chain reaction or by mass spectrometric techniques (53,100–102). Adduct formation is enhanced by the presence of 5-methylcytosine in these reactive CpG dinucleotide sequences. Collectively, these data have been cited as strong evidence for the role of cigarette smoke PAH as causes of lung cancer. However, other smoke constituents can produce a similar spectrum of DNA damage. Notable among these is acrolein, which was recently shown to damage the p53 gene in the same way as PAH diol epoxides (103). Acrolein is far more abundant in cigarette smoke than are PAHs, and acrolein-DNA adducts have been detected in human lung (55). However, acrolein is weakly or non-carcinogenic (104). While further research is necessary to determine the respective role of specific tobacco smoke carcinogens as causes of mutations in the p53 tumor suppressor gene, the results obtained to date in aggregate support the concept that tobacco smoke constituents or their metabolically activated products directly damage the p53 tumor suppressor gene leading to mutations and loss of normal cellular growth control mechanisms.
Secondhand tobacco smoke and lung cancer
Major reviews covering the numerous epidemiologic studies carried out on secondhand cigarette smoke and cancer in the past 20 years have recently been published. The U.S. Surgeon General’s Report on the Health Consequences of Involuntary Exposure to Tobacco Smoke concluded that “the evidence is sufficient to infer a causal relationship between secondhand smoke exposure and lung cancer among lifetime non-smokers” and “the pooled evidence indicates a 20 to 30 percent increase in the risk of lung cancer from secondhand smoke exposure associated with living with a smoker” (105). The IARC Monograph on Involuntary Smoking concluded that “there is sufficient evidence that involuntary smoking causes lung cancer in humans” and that “involuntary smoking is carcinogenic to humans” (106). These groups did not conclude that involuntary smoking causes breast cancer. Many other health effects of involuntary smoking were described in the Surgeon General’s Report.
These conclusions were reached based on strong epidemiologic evidence bolstered by biomarker studies such as those described, and are biologically plausible because secondhand smoke contains all the same carcinogens that are present in the smoke inhaled by a smoker, but the dose is less. The data in Table 1 demonstrate that levels of total NNAL in the urine of non-smokers exposed to secondhand smoke are about 1–7% as great as those in smokers.
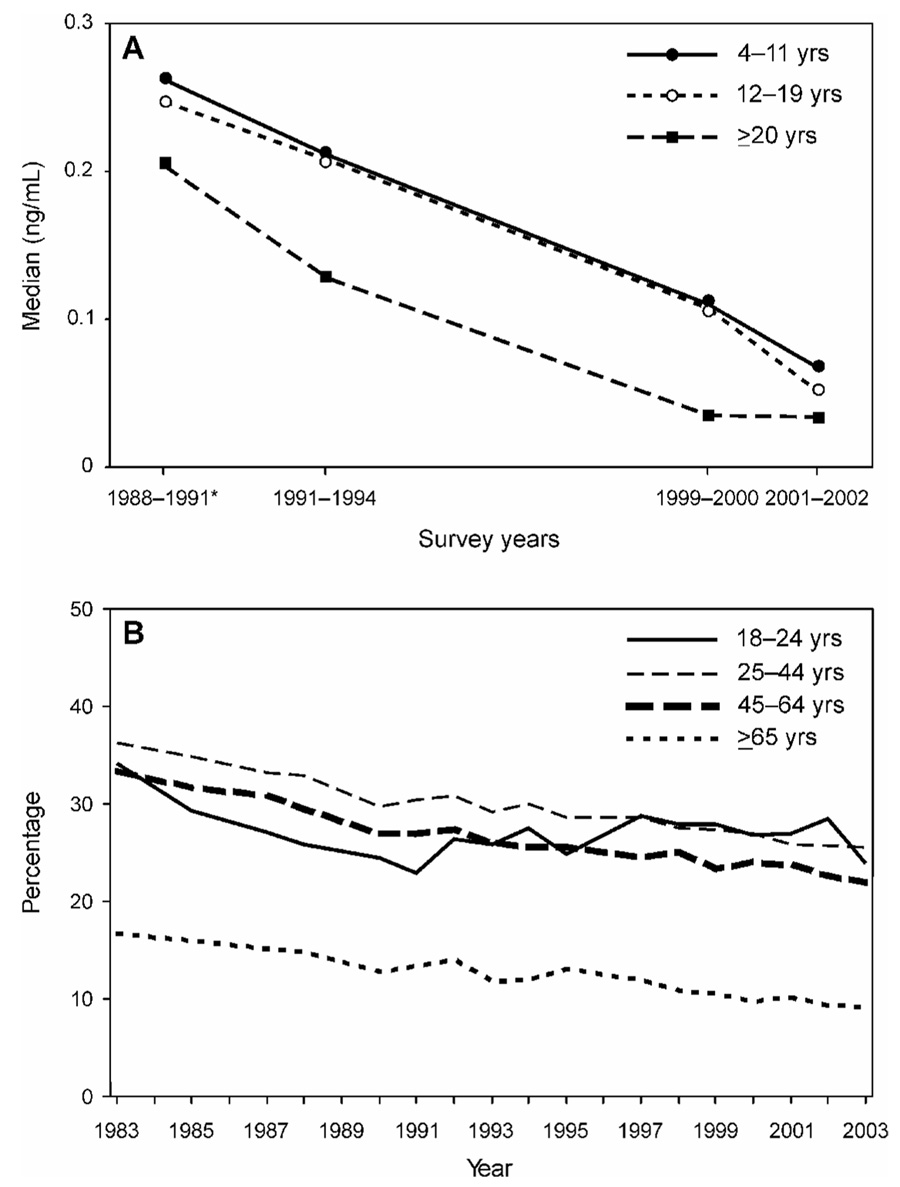
The biomarker and epidemiologic studies which demonstrate that secondhand smoke causes lung cancer have had a major impact on tobacco control. While smoking is considered a voluntary act, and many have little sympathy for the adverse health effects which smokers bring upon themselves, secondhand smoke is a different issue because non-smokers are being affected by the smoke of others. This non-voluntary health harm has spurred legislation in many states to protect non-smokers in workplaces, bars and restaurants. Currently, 20 states and several countries have laws prohibiting smoking in bars and restaurants, legislation that would have been considered improbable 20 years ago. Regulation of indoor smoking reduces cues for smoking, reduces the amount smoked, and ultimately will change social norms. Thus, regulation of indoor smoking has become one of the pillars of tobacco control, along with aggressive counter-advertising and taxation, shown to be effective in decreasing smoking prevalence (107). That such approaches are having an impact is demonstrated in Figure 4A, from the Centers for Disease Control NHANES study, showing a consistent decrease in median serum concentrations of the major nicotine metabolite cotinine, an accepted biomarker of tobacco smoke exposure, in U.S. non-smokers in the period 1988-2002. This is genuine progress which parallels a consistent decline in smoking prevalence in the U.S. in approximately the same period (Figure 4B).
Figure 4.
A. Median serum cotinine levels in non-smokers, by age group, 1988–2002, according to data from the Centers for Disease Control and Prevention NHANES study, (137). B. Sustained decline of smoking prevalence in the United States, from 1983–2003 (138).
The re-reemergence of smokeless tobacco
In 1986, the author co-authored a paper entitled “The Reemergence of Smokeless Tobacco” which described the rise in smokeless tobacco use in the U.S., mostly as oral moist snuff, and particularly among young males (108). Smokeless tobacco, an accepted cause of oral cancer, is contaminated with ppm quantities of carcinogenic tobacco-specific nitrosamines, levels generally 1000 times greater than those of nitrosamines in any other consumer product. That year, the U.S. Congress enacted a law requiring health warning labels on packages of smokeless tobacco and a ban on electronic advertising. Sales of moist snuff have continued to increase in this country. In the past several years, a new concept has emerged. Responsible members of the tobacco control community support the idea of using “low nitrosamine” moist snuff as a substitute for cigarette smoking (109,110). The rationale for this is that moist snuff is demonstrably less carcinogenic in humans, and less toxic in other ways, than cigarette smoking, because it lacks the combustion products. This concept has been backed strongly by the Swedish Match company, manufacturer of Swedish moist snuff (known as “snus”) which is widely used by men in that country, in which the prevalence of cigarette smoking and the incidence of lung cancer among males are the lowest in Europe (111). The problem with this concept is that all commercially available moist snuff products, including snus, remain contaminated with significant levels of carcinogenic tobacco-specific nitrosamines in addition to carcinogenic aldehydes, metals, and sometimes PAH. According to IARC, smokeless tobacco is a human carcinogen, causing oral and pancreatic cancer (3).
Table 2 summarizes levels of tobacco-specific nitrosamines in the most popular American smokeless tobacco brands – Copenhagen, Skoal, and Kodiak – and in Swedish products (112). Levels of NNN and NNK in Swedish products are lower than those in brands sold in the U.S., but the total amounts are still about 1-2 ppm, approximately 1,000 times higher than levels of carcinogenic nitrosamines found in cured meats or beer, which are the consumer products most commonly contaminated with such compounds. We have recently completed a study in which we compared levels of total NNAL in the urine of 182 American smokeless tobacco users and 420 smokers (113). The results demonstrated that total NNAL levels were similar in the urine of smokeless tobacco users and smokers. Other studies demonstrated that users of Swedish snus did experience a reduction in total NNAL, compared to when they used American smokeless brands, but the levels were still significantly higher than when these subjects abstained from use with the aid of nicotine replacement therapy (84). The results of our studies do not support the concept that smokers should switch to smokeless tobacco. Advocating for the use of smokeless tobacco as a substitute for smoking may have the unintended consequence of increasing the use and sales of smokeless tobacco products which lead to similar uptake of tobacco-specific carcinogens as cigarettes. Long term use of nicotine replacement therapy may be a better option.
Table 2.
Tobacco-specific nitrosamines in smokeless tobacco productsa
| Product | µg/g product (wet weight) | ||||
|---|---|---|---|---|---|
| NNNb | NNK | NAT | NAB | Total | |
| Copenhagen | |||||
| Snuff | 2.2 | 0.75 | 1.8 | 0.12 | 4.8 |
| Long cut | 3.9 | 1.6 | 1.9 | 0.13 | 7.5 |
| Skoal | |||||
| Long cut straight | 4.5 | 0.47 | 4.1 | 0.22 | 9.2 |
| Bandits | 0.9 | 0.17 | 0.24 | 0.014 | 1.3 |
| Kodiak | |||||
| Ice | 2.0 | 0.29 | 0.72 | 0.063 | 3.1 |
| Wintergreen | 2.2 | 0.41 | 1.8 | 0.15 | 4.5 |
| Swedish products | |||||
| General | 0.98 | 0.18 | 0.79 | 0.06 | 2.0 |
| Exalt | 2.3 | 0.27 | 0.98 | 0.13 | 3.7 |
reference (112)
Abbreviations: NNN, N′-nitrosonornicotine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NAT, N′-nitrosanatabine; NAB, N′-nitrosoanabasine
A conceptual model for understanding mechanisms of tobacco carcinogenesis
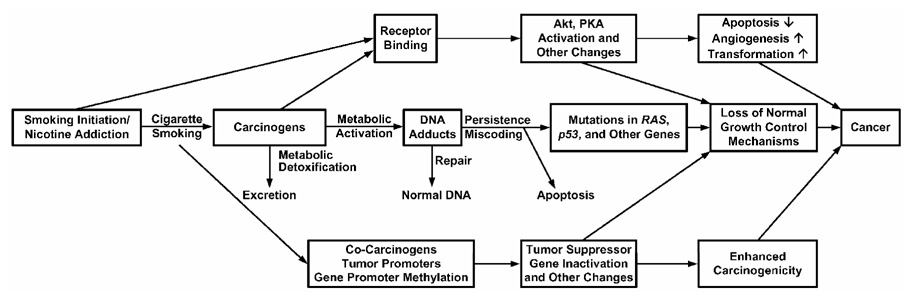
Figure 5 presents our conceptual model for understanding mechanisms of tobacco induced cancer (114,115). The central track of this figure depicts the major established pathways of cancer causation by cigarette smoke. People start smoking for all the wrong reasons, usually as teen-agers. They become addicted to nicotine and cannot stop. Nicotine is not a carcinogen, but each puff of each cigarette contains a mixture of over 60 established carcinogens, many of which require metabolic activation, usually by cytochrome P450 enzymes, to be converted to electrophiles that react with DNA producing adducts (48,116). Cytochrome P450s 1A1 and 1B1, which are inducible by cigarette smoke via interactions with the Ah receptor, are particularly important in the metabolic activation of PAH, and the induction of these enzymes may be a critical aspect of cancer susceptibility in smokers (117). Cytochrome P450s 1A2, 2A6, 2A13, and 2E1 are also important in the activation of cigarette smoke carcinogens. Competing with activation is metabolic detoxification, resulting in excretion of carcinogen metabolites: GSTs and UGTs are particularly important in this respect. It is widely hypothesized that individuals with a higher activation and lower detoxification capacity are at highest risk for tobacco induced cancer. This reasonable hypothesis requires further investigation.
Figure 5.
Conceptual model for understanding mechanisms of tobacco carcinogenesis. The central track involving carcinogen-DNA adduct formation and consequent mutations in critical genes is the major accepted pathway. The top and bottom tracks also contribute but their roles are less well defined. Molecular pathways involved in the box labeled “Loss of Normal Growth Control Mechanisms” have been described by Weinberg (139).
DNA adducts are absolutely central to the carcinogenic process, and there is considerable evidence that these are higher in smokers than in non-smokers (61). Cellular repair systems can remove DNA adducts and maintain a normal DNA structure (118). These systems include direct base repair by alkyltransferases, excision of DNA damage by base and nucleotide excision repair, mismatch repair, and double-strand break repair. If DNA adducts persist unrepaired, they can cause miscoding during replication when DNA polymerase enzymes process them incorrectly (119). There is considerable specificity between specific DNA adducts caused by tobacco smoke carcinogens and the types of observed mutations in genes such as K-RAS and p53, as discussed above. These mutations can cause the loss of normal cellular growth control functions, ultimately resulting in cellular proliferation and cancer. Apoptosis protects the organism by removing cells with DNA damage. The balance between mechanisms leading to apoptosis and those suppressing apoptosis has a major impact on tumor growth (120).
The top and bottom tracks of Figure 5 indicate that other pathways may also contribute to tobacco carcinogenesis. Nicotine and tobacco-specific nitrosamines bind to nicotinic and other cellular receptors (121–124). This binding leads to activation of Akt, protein kinase A, and other pathways. Cigarette smoke activates the epidermal growth factor receptor and cyclooxygenase-2 (125), and causes down-regulation and loss of the Fhit tumor suppressor gene (126). Furthermore, the occurrence of cocarcinogens and tumor promoters in cigarette smoke is well established (49). Another important epigenetic pathway is the enzymatic methylation of promoter regions of genes, which can result in gene silencing or increased mutations (127). These aspects of the process have been insufficiently studied and their contribution to the overall pathway of carcinogenesis and potential importance with respect to the established central track of Figure 5 require further research.
Conclusions
This brief perspective focused on several current topics in tobacco carcinogenesis, emphasizing progress in the past 20 years and identifying some current questions and controversies. There is no doubt that impressive progress has been made since the first issue of Chemical Research in Toxicology appeared – both in basic research and in tobacco control. Further, it is evident that advances in basic research have had an impact on tobacco control, partly through the development and application of tobacco carcinogen biomarkers. But research in tobacco carcinogenesis presents an ever changing and challenging landscape because an economically powerful industry constantly re-invents itself and operates on a global scale. Additional resources should be devoted to research in this area, because tobacco products cause at least 30% of all cancer mortality in the developed world. The currently declining cancer mortality rates in the U.S. are driven in part by prevention of tobacco-induced cancer, and we must do everything possible to ensure that this positive trend continues.
Acknowledgements
The author’s research on tobacco and cancer is supported by NIH grants CA-81301, CA-92025, and DA-13333, and American Cancer Society grant RP-00-138.
References
- 1.World Health Organization. The World Health Report 2003: Shaping the Future. 2003:91–94.
- 2.International Union Against Cancer. 2007 www.deathsfromsmoking.net.
- 3.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines. in press. [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 85. Lyon, FR: IARC; 2004. Betel-quid and areca-nut chewing and some arece-nut-derived nitrosamines; pp. 41–300. [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–1492. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 35–102. [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 9.Preussmann R, Stewart BW. N-Nitroso Carcinogens. In: Searle CE, editor. Chemical Carcinogens, Second Edition, ACS Monograph 182, vol. 2. Washington, DC: American Chemical Society; 1984. pp. 643–828. [Google Scholar]
- 10.Trushin N, Rivenson A, Hecht SS. Evidence supporting the role of DNA pyridyloxobutylation in rat nasal carcinogenesis by tobacco specific nitrosamines. Cancer Res. 1994;54:1205–1211. [PubMed] [Google Scholar]
- 11.Hecht SS, Spratt TE, Trushin N. Evidence for 4-(3-pyridyl)-4-oxobutylation of DNA in F344 rats treated with the tobacco specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N'-nitrosonornicotine. Carcinogenesis. 1988;9:161–165. doi: 10.1093/carcin/9.1.161. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SE, Palomino A, Hecht SS, Hoffmann D. Dose-response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res. 1990;50:5446–5452. [PubMed] [Google Scholar]
- 13.Foiles PG, Akerkar SA, Carmella SG, Kagan M, Stoner GD, Resau JH, Hecht SS. Mass spectrometric analysis of tobacco-specific nitrosamine-DNA adducts in smokers and nonsmokers. Chem. Res. Toxicol. 1991;4:364–368. doi: 10.1021/tx00021a017. [DOI] [PubMed] [Google Scholar]
- 14.Hölzle D, Schlöbe D, Tricker AR, Richter E. Mass spectrometric analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human lung. Toxicology. 2007;232:277–285. doi: 10.1016/j.tox.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Spratt TE, Liu XK, Hecht SS, Pegg AE, Peterson LA. Pyridyloxobutyl adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine is present in 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 1997;10:562–567. doi: 10.1021/tx9602067. [DOI] [PubMed] [Google Scholar]
- 16.Pauly GT, Peterson LA, Moschel RC. Mutagenesis by O6-[4-oxo-4-(3-pyridyl)butyl]guanine in Escherichia coli and human cells. Chem. Res. Toxicol. 2002;15:165–169. doi: 10.1021/tx0101245. [DOI] [PubMed] [Google Scholar]
- 17.Upadhyaya P, Sturla S, Tretyakova N, Ziegel R, Villalta PW, Wang M, Hecht SS. Identification of adducts produced by the reaction of 4-(acetoxymethynitrosamino)-1-(3-pyridyl)-1-butanol with deoxyguanosine and DNA. Chem. Res. Toxicol. 2003;16:180–190. doi: 10.1021/tx0256376. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Cheng G, Sturla SJ, Shi Y, McIntee EJ, Villalta PW, Upadhyaya P, Hecht SS. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco-specific carcinogens. Chem. Res. Toxicol. 2003;16:616–626. doi: 10.1021/tx034003b. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SS, Villalta PW, Sturla SJ, Cheng G, Yu N, Upadhyaya P, Wang M. Identification of O2-substituted pyrimidine adducts formed in reactions of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-1butanone and 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanol with DNA. Chem. Res. Toxicol. 2004;17:588–597. doi: 10.1021/tx034263t. [DOI] [PubMed] [Google Scholar]
- 20.Jalas J, Hecht SS, Murphy SE. Cytochrome P450 2A enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific carcinogen. Chem. Res. Toxicol. 2005;18:95–110. doi: 10.1021/tx049847p. [DOI] [PubMed] [Google Scholar]
- 21.Jalas JR, McIntee EJ, Kenney PMJ, Upadhyaya P, Peterson LA, Hecht SS. Stereospecific deuterium substitution attenuates the tumorigenicity and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Chem. Res. Toxicol. 2003;16:794–806. doi: 10.1021/tx034022l. [DOI] [PubMed] [Google Scholar]
- 22.Peterson LA, Hecht SS. O6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 23.Ronai Z, Gradia S, Peterson LA, Hecht SS. G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis. 1993;14:2419–2422. doi: 10.1093/carcin/14.11.2419. [DOI] [PubMed] [Google Scholar]
- 24.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 25.Singer B, Essigmann JM. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991;12:949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- 26.Staretz ME, Foiles PG, Miglietta LM, Hecht SS. Evidence for an important role of DNA pyridyloxobutylation in rat lung carcinogensis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: effects of dose and phenethyl isothiocyanate. Cancer Res. 1997;57:259–266. [PubMed] [Google Scholar]
- 27.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–790. doi: 10.1093/carcin/bgi301. [DOI] [PubMed] [Google Scholar]
- 28.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2007;20:235–245. doi: 10.1021/tx060207r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman CL, Wu Z, Upadhyaya P, Hecht SS. Stereoselective metabolism and tissue retention in rats of the individual enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), metabolites of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 2004;25:1237–1242. doi: 10.1093/carcin/bgh120. [DOI] [PubMed] [Google Scholar]
- 30.Wong HL, Murphy SE, Hecht SS. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N′-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol. 2004;18:61–69. doi: 10.1021/tx0497696. [DOI] [PubMed] [Google Scholar]
- 31.Murphy SE, Spina DA. Evidence for a high affinity enzyme in rat esophageal microsomes which alpha-hydroxylates N'-nitrosonornicotine. Carcinogenesis. 1994;15:2709–2713. doi: 10.1093/carcin/15.12.2709. [DOI] [PubMed] [Google Scholar]
- 32.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N′-nitrosonornicotine. Chem. Res. Toxicol. 2007;20:246–256. doi: 10.1021/tx060208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upadhyaya P, McIntee EJ, Villalta PW, Hecht SS. Identification of adducts formed in the reaction of 5'-acetoxy-N′-nitrosonornicotine with deoxyguanosine and DNA. Chem. Res. Toxicol. 2006;19:426–435. doi: 10.1021/tx050323e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upadhyaya P, Carmella SG, Guengerich FP, Hecht SS. Formation and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers in vitro in mouse, rat and human tissues. Carcinogenesis. 2000;21:1233–1238. [PubMed] [Google Scholar]
- 35.Carmella SG, Ye M, Upadhyaya P, Hecht SS. Stereochemistry of metabolites of a tobacco-specific lung carcinogen in smokers' urine. Cancer Res. 1999;59:3602–3605. [PubMed] [Google Scholar]
- 36.Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 37.Zhu LR, Thomas PE, Lu G, Reuhl KR, Yang GY, Wang LD, Wang SL, Yang CS, He XY, Hong JY. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab. Dispos. 2006;34:1672–1676. doi: 10.1124/dmd.106.011049. [DOI] [PubMed] [Google Scholar]
- 38.Holzle D, Schlobe D, Tricker AR, Richter E. Mass spectrometric analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human lung. Toxicology. 2007;232:277–285. doi: 10.1016/j.tox.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Wong HL, Murphy SE, Hecht SS. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem. Res. Toxicol. 2005;18:913–918. doi: 10.1021/tx0500777. [DOI] [PubMed] [Google Scholar]
- 40.Mijal RS, Thomson NM, Fleischer NL, Pauly GT, Moschel RC, Kanugula S, Fang Q, Pegg AE, Peterson LA. The repair of the tobacco specific nitrosamine derived adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine by O6-alkylguanine-DNA alkyltransferase variants. Chem. Res. Toxicol. 2004;17:424–434. doi: 10.1021/tx0342417. [DOI] [PubMed] [Google Scholar]
- 41.Mijal RS, Kanugula S, Vu CC, Fang Q, Pegg AE, Peterson LA. DNA sequence context affects repair of the tobacco-specific adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine by human O6-alkylguanine-DNA alkyltransferases. Cancer Res. 2006;66:4968–4974. doi: 10.1158/0008-5472.CAN-05-3803. [DOI] [PubMed] [Google Scholar]
- 42.Hecht SS, Young R, Chen CB. Metabolism in the F344 rat of 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Cancer Res. 1980;40:4144–4150. [PubMed] [Google Scholar]
- 43.Morse MA, Eklind KI, Toussaint M, Amin SG, Chung FL. Characterization of a glucuronide metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its dose-dependent excretion in the urine of mice and rats. Carcinogenesis. 1990;11:1819–1823. doi: 10.1093/carcin/11.10.1819. [DOI] [PubMed] [Google Scholar]
- 44.Hecht SS, Trushin N, Reid-Quinn CA, Burak ES, Jones AB, Southers JL, Gombar CT, Carmella SG, Anderson LM, Rice JM. Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the Patas monkey: pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis. 1993;14:229–236. doi: 10.1093/carcin/14.2.229. [DOI] [PubMed] [Google Scholar]
- 45.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers' urine. Cancer Res. 1993;53:721–724. [PubMed] [Google Scholar]
- 46.Carmella SG, Le K, Upadhyaya P, Hecht SS. Analysis of N- and O-glucuronides of 4-(methylnitrosamino)-1-(3- pyridyl)-1-butanol (NNAL) in human urine. Chem. Res. Toxicol. 2002;15:545–550. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann D, Hecht SS. Advances in tobacco carcinogenesis. In: Cooper CS, Grover PL, editors. Handbook of Experimental Pharmacology. Heidelberg: Springer-Verlag; 1990. pp. 63–102. [Google Scholar]
- 48.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 53–119. [PMC free article] [PubMed] [Google Scholar]
- 49.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 38. Lyon, FR: IARC; 1986. Tobacco Smoking; pp. 37–375. [PubMed] [Google Scholar]
- 50.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 1012–1065. [PMC free article] [PubMed] [Google Scholar]
- 51.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 52.Singh R, Farmer PB. Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 2006;27:178–196. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- 53.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 54.Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, Schoket B, Gyorffy E, Minarovits J, Poirier MC, Bowman ED, Weston A, Doerge DR. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem. Res. Toxicol. 2005;18:1306–1315. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography-electrsopray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Wang M, Villalta PW, Luo X, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chem. Res. Toxicol. 2007;20:108–113. doi: 10.1021/tx060232x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holzle D, Schlobe D, Tricker AR, Richter E. Mass spectrometric analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human lung. Toxicology. 2007;232:277–285. doi: 10.1016/j.tox.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- 60.Kriek E, Rojas M, Alexandrov K, Bartsch H. Polycyclic aromatic hydrocarbon-DNA adducts in humans: relevance as biomarkers for exposure and cancer risk. Mutat. Res. 1998;400:215–231. doi: 10.1016/s0027-5107(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 61.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 62.Wild CP, Pisani P. Carcinogen DNA and protein adducts as biomarkers of human exposure in environmental cancer epidemiology. Cancer Detection and Prevention. 1998;22:273–283. doi: 10.1046/j.1525-1500.1998.cdoa38.x. [DOI] [PubMed] [Google Scholar]
- 63.Arif JM, Dresler C, Clapper ML, Gairola CG, Srinivasan C, Lubet RA, Gupta RC. Lung DNA adducts detected in human smokers are unrelated to typical polyaromatic carcinogens. Chem. Res. Toxicol. 2006;19:295–299. doi: 10.1021/tx0502443. [DOI] [PubMed] [Google Scholar]
- 64.Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol. Biomarkers & Prev. 2003;12:157–160. [PubMed] [Google Scholar]
- 65.Golkar SO, Ehrenberg L, Segerback D, Hallstrom I. Evaluation of genetic risks of alkyating agents II. Haemoglobin as a dose monitor. Mutat. Res. 1976;34:1–10. doi: 10.1016/0027-5107(76)90256-6. [DOI] [PubMed] [Google Scholar]
- 66.Ehrenberg L, Osterman-Golkar S. Alkylation of macromolecules for detecting mutagenic agents. Teratogenesis Carcinog. Mutagen. 1980;1:105–127. doi: 10.1002/tcm.1770010111. [DOI] [PubMed] [Google Scholar]
- 67.Skipper PL, Tannenbaum SR. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990;11:507–518. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- 68.Castelao JE, Yuan JM, Skipper PL, Tannenbaum SR, Gago-Dominguez M, Crowder JS, Ross RK, Yu MC. Gender- and smoking-related bladder cancer risk. J. Natl. Cancer Inst. 2001;93:538–545. doi: 10.1093/jnci/93.7.538. [DOI] [PubMed] [Google Scholar]
- 69.Maclure M, Bryant MS, Skipper PL, Tannenbaum SR. Decline of the hemoglobin adduct of 4-minobiphenyl during withdrawal from smoking. Cancer Res. 1990;50:181–184. [PubMed] [Google Scholar]
- 70.Bergmark E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997;10:78–84. doi: 10.1021/tx960113p. [DOI] [PubMed] [Google Scholar]
- 71.Fennell TR, MacNeela JP, Morris RW, Watson M, Thompson CL, Bell DA. Hemoglobin adducts from acrylonitrile and ethylene oxide in cigarette smokers: effects of glutathione S-transferase T1-null and M1-null genotypes. Cancer Epidemiol. Biomarkers & Prev. 2000;9:705–712. [PubMed] [Google Scholar]
- 72.Carmella SG, Chen M, Villalta PW, Gurney JG, Hatsukami DK, Hecht SS. Ethylation and methylation of hemoglobin in smokers and non-smokers. Carcinogenesis. 2002;23:1903–1910. doi: 10.1093/carcin/23.11.1903. [DOI] [PubMed] [Google Scholar]
- 73.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 74.Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann. Occup. Hyg. 2001;45:3–13. [PubMed] [Google Scholar]
- 75.Hecht SS, Carmella SG, Yoder A, Chen M, Li Z, Le C, Jensen J, Hatsukami DK. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiol. Biomarkers & Prev. 2006;15:1805–1811. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- 76.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol. Biomarkers & Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 77.Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine and Tob. Res. 2006;8:600–622. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob. Control. 2003;13 Suppl 1:i48–i56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatsukami DK, Le CT, Zhang Y, Joseph AM, Mooney ME, Carmella SG, Hecht SS. Toxicant exposure in cigarette reducers vs. light smokers. Cancer Epidemiol. Biomarkers & Prev. 2006;15:2355–2358. doi: 10.1158/1055-9965.EPI-06-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, Roe MR, Puumala SS, Li YS, Le C, Jensen J, Hatsukami D. Effects of reduced cigarette smoking on uptake of a tobacco-specific lung carcinogen. J. Natl. Cancer Inst. 2004;96:107–115. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- 81.Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, Joseph AM, Hatsukami DK. Similar uptake of lung carcinogens by smokers of regular, light, and ultra-light cigarettes. Cancer Epidemiol. Biomarkers & Prev. 2005;14:693–698. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 82.Mendoza-Baumgart MI, Tulunay OE, Hecht SS, Zhang Y, Murphy SE, Le CT, Jensen J, Hatsukami DK. Low nitrosamine oral non-combustible tobacco products compared to medicinal nicotine: toxicant exposure and behavioral preferences. Nic. Tob. Res. 2006 doi: 10.1080/14622200701704228. submitted. [DOI] [PubMed] [Google Scholar]
- 83.Hatsukami DK, Ebbert JO, Anderson A, Lin H, Le C, Hecht SS. Smokeless tobacco brand switching: a means to reduce toxicant exposure? Drug Alcohol Depend. 2006;87:217–224. doi: 10.1016/j.drugalcdep.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used "reduced exposure" tobacco products. J. Natl. Cancer Inst. 2004;96:844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 85.Hecht SS, Carmella SG, Le K, Murphy SE, Boettcher AJ, Le C, Koopmeiners J, An L, Hennrikus DJ. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol. Biomarkers & Prev. 2006;15:988–992. doi: 10.1158/1055-9965.EPI-05-0596. [DOI] [PubMed] [Google Scholar]
- 86.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regulatory Toxicol. and Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Scherer G, Meger M, Meger-Kossien I, Pachinger A. Biological monitoring of the tobacco-smoke related exposure to benzene. Proc. Am. Assn. Cancer Res. 2001;42:150. [Google Scholar]
- 88.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived 3-hydroxypropylmercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry: effects of cigarette smoking. Chem. Res. Toxicol. 2007 doi: 10.1021/tx700075y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kopplin A, Eberle-Adamkiewicz G, Glüsenkamp KH, Nehls P, Kirstein U. Urinary excretion of 3-methyladenine and 3-ethyladenine after controlled exposure to tobacco smoke. Carcinogenesis. 1995;16:2637–2641. doi: 10.1093/carcin/16.11.2637. [DOI] [PubMed] [Google Scholar]
- 90.Prevost V, Shuker DEG. Cigarette smoking and urinary 3-alkyladenine excretion in man. Chem. Res. Toxicol. 1996;9:439–444. doi: 10.1021/tx9501041. [DOI] [PubMed] [Google Scholar]
- 91.Singh R, Kaur B, Farmer PB. Detection of DNA damage derived from a direct acting ethylating agent present in cigarette smoke by use of liquid chromatography-tandem mass spectrometry. Chem. Res. Toxicol. 2005;18:249–256. doi: 10.1021/tx049793j. [DOI] [PubMed] [Google Scholar]
- 92.Chao MR, Wang CJ, Chang LW, Hu CW. Quantitative determination of urinary N7-ethylguanine in smokers and non-smokers using an isotope dilution liquid chromatography/tandem mass spectrometry with on-line analyte enrichment. Carcinogenesis. 2006;27:146–151. doi: 10.1093/carcin/bgi177. [DOI] [PubMed] [Google Scholar]
- 93.Godschalk R, Nair J, van Schooten FJ, Risch A, Drings P, Kayser K, Dienemann H, Bartsch H. Comparison of multiple DNA adduct types in tumor adjacent human lung tissue: effect of cigarette smoking. Carcinogenesis. 2002;23:2081–2086. doi: 10.1093/carcin/23.12.2081. [DOI] [PubMed] [Google Scholar]
- 94.Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure MJ, Sidransky D. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 95.Westra WH, Slebos RJC, Offerhaus CJA, Goodman SN, Evers SG, Kensler TW, Askin FB, Rodenhuis S, Hruban RH. K-ras oncogene activation in lung adenocarcinomas from former smokers. Cancer. 1993;72:432–438. doi: 10.1002/1097-0142(19930715)72:2<432::aid-cncr2820720219>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 96.Rodenhuis S, Slebos RJC, Boot AJM, Evers SG, Mooi WJ, Wagenaar SS, van Bodegom PC, Bos JL. Incidence and possible clinical signifinance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 97.Mills NE, Fishman CL, Scholes J, Anderson SE, Rom WN, Jacobson DR. Detection of K-ras oncogene mutations in bronchoalveolar lavage fluid for lung cancer diagnosis. J. Natl. Cancer Inst. 1995;87:1056–1060. doi: 10.1093/jnci/87.14.1056. [DOI] [PubMed] [Google Scholar]
- 98.Ross JA, Nesnow S. Polycyclic aromatic hydrocarbons: correlations between DNA adducts and ras oncogene mutations. Mutat. Res. 1999;424:155–166. doi: 10.1016/s0027-5107(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 99.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 100.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hot spots in P53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 101.Tretyakova N, Matter B, Jones R, Shallop A. Formation of benzo[a]pyrene diol epoxide-DNA adducts at specific guanines within K-ras and p53 gene sequences: stable isotope-labeling mass spectrometry approach. Biochemistry. 2002;41:9535–9544. doi: 10.1021/bi025540i. [DOI] [PubMed] [Google Scholar]
- 102.Matter B, Wang G, Jones R, Tretyakova N. Formation of diastereomeric benzo[a]pyrene diol epoxide-guanine adducts in p53 gene-derived DNA sequences. Chem. Res. Toxicol. 2004;17:731–741. doi: 10.1021/tx049974l. [DOI] [PubMed] [Google Scholar]
- 103.Feng Z, Hu W, Hu Y, Tang M-S. Acrolein is a major cigarette-related lung cancer agent. Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 63. Lyon, FR: IARC; 1995. Acrolein; pp. 337–372. [PMC free article] [PubMed] [Google Scholar]
- 105.United States Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, D.C: U.S. Govt. Printing Office; 2004. [Google Scholar]
- 106.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 1191–1413. [PMC free article] [PubMed] [Google Scholar]
- 107.National Institutes of Health. NIH State-of-the-Science Conference Statement on Tobacco Use: Prevention, Cessation, and Control. NIH Consensus and State-of the-Science Statements. 2006;23:1–26. [PubMed]
- 108.Connolly GN, Winn DM, Hecht SS, Henningfield JE, Walker B, Jr, Hoffmann D. The reemergence of smokeless tobacco. N. Engl. J. Med. 1986;314:1020–1027. doi: 10.1056/NEJM198604173141605. [DOI] [PubMed] [Google Scholar]
- 109.Levy DT, Mumford EA, Cummings KM, Gilpin EA, Giovino G, Hyland A, Sweanor D, Warner KE. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiol. Biomarkers & Prev. 2004;13:2035–2042. [PubMed] [Google Scholar]
- 110.Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. Am. J. Public. Health. 2006;96:1934–1939. doi: 10.2105/AJPH.2005.075499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob. Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob. Res. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 113.Hecht SS, Carmella SG, Murphy SE, Riley W, Le C, Luo X, Mooney M, Hatsukami DK. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol. Biomarkers & Prev. 2007 doi: 10.1158/1055-9965.EPI-07-0227. in press. [DOI] [PubMed] [Google Scholar]
- 114.Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 115.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 116.Miller JA. Research in chemical carcinogenesis with Elizabeth Miller - a trail of discovery with our associates. Drug Metab. Dispos. 1994;26:1–36. doi: 10.3109/03602539409029782. [DOI] [PubMed] [Google Scholar]
- 117.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 118.Christmann M, Tomicic MT, Roos WP, Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 119.Lehmann AR. Translesion synthesis in mammalian cells. Exp. Cell Res. 2006;312:2673–2676. doi: 10.1016/j.yexcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Bode AM, Dong Z. Signal transduction pathways in cancer development and as targets for cancer prevention. Prog. Nucleic Acid. Res. Mol. Biol. 2005;79:237–297. doi: 10.1016/S0079-6603(04)79005-4. [DOI] [PubMed] [Google Scholar]
- 121.Dennis PA, Van Waes C, Gutkind JS, Kellar KJ, Vinson C, Mukhin AG, Spitz MR, Bailey-Wilson JE, Yeh GC, Anderson LM, Wiest JS. The biology of tobacco and nicotine: bench to bedside. Cancer Epidemiol. Biomarkers & Prev. 2005;14:764–767. doi: 10.1158/1055-9965.EPI-04-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 124.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat. Rev. Cancer. 2002;2:455–463. doi: 10.1038/nrc824. [DOI] [PubMed] [Google Scholar]
- 125.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, Carew JF, Altorki NK, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65:664–670. [PubMed] [Google Scholar]
- 126.D'Agostini F, Izzotti A, Balansky R, Zanesi N, Croce CM, De Flora S. Early loss of Fhit in the respiratory tract of rodents exposed to environmental cigarette smoke. Cancer Res. 2006;66:3936–3941. doi: 10.1158/0008-5472.CAN-05-3666. [DOI] [PubMed] [Google Scholar]
- 127.Belinsky SA. Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer. Carcinogenesis. 2005;26:1481–1487. doi: 10.1093/carcin/bgi020. [DOI] [PubMed] [Google Scholar]
- 128.Milunsky A, Carmella SG, Ye M, Hecht SS. A tobacco-specific carcinogen in the fetus. Prenat. Diagn. 2000;20:307–310. doi: 10.1002/(sici)1097-0223(200004)20:4<307::aid-pd797>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 129.Lackmann GM, Salzberger U, Tollner U, Chen M, Carmella SG, Hecht SS. Metabolites of a tobacco-specific carcinogen in the urine of newborns. J. Natl. Cancer Inst. 1999;91:459–465. doi: 10.1093/jnci/91.5.459. [DOI] [PubMed] [Google Scholar]
- 130.Hecht SS, Ye M, Carmella SG, Fredrickson A, Adgate JL, Greaves IA, Church TR. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol. Biomarkers & Prev. 2001;10:1109–1116. [PubMed] [Google Scholar]
- 131.Stepanov I, Hecht SS, Duca G, Mardari I. Uptake of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by Moldovan children. Cancer Epidemiol. Biomarkers & Prev. 2006;15:7–11. doi: 10.1158/1055-9965.EPI-05-0293. [DOI] [PubMed] [Google Scholar]
- 132.Anderson KE, Carmella SG, Ye M, Bliss R, Le C, Murphy L, Hecht SS. Metabolites of a tobacco-specific lung carcinogen in the urine of nonsmoking women exposed to environmental tobacco smoke in their homes. J. Natl. Cancer Inst. 2001;93:378–381. doi: 10.1093/jnci/93.5.378. [DOI] [PubMed] [Google Scholar]
- 133.Parsons WD, Carmella SG, Akerkar S, Bonilla LE, Hecht SS. A metabolite of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in the urine of hospital workers exposed to environmental tobacco smoke. Cancer Epidemiol. Biomarkers & Prev. 1998;7:257–260. [PubMed] [Google Scholar]
- 134.Anderson KE, Kliris J, Murphy L, Carmella SG, Han S, Link C, Bliss RL, Murphy SE, Hecht SS. Metabolites of a tobacco-specific lung carcinogen in nonsmoking casino patrons. Cancer Epidemiol. Biomarkers & Prev. 2003;12:1544–1546. [PubMed] [Google Scholar]
- 135.Tulunay O, Hecht SS, Carmella SG, Zhang Y, Lemmonds C, Murphy SE, Hatsukami DK. Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol. Biomarkers & Prev. 2005;14:1283–1286. doi: 10.1158/1055-9965.EPI-04-0570. [DOI] [PubMed] [Google Scholar]
- 136.Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi CI, Chung FL. Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis. 1989;10:1901–1904. doi: 10.1093/carcin/10.10.1901. [DOI] [PubMed] [Google Scholar]
- 137.Centers for Disease Control and Prevention. Quickstats: Median serum cotinine levels in nonsmokers, by age group - national health and nutrition examination survey (NHANES), United States,1988–1991 through 2001–2002. MMWR. 2006;55:1130. [Google Scholar]
- 138.Centers for Disease Control and Prevention. Cigarette smoking among adults--United States, 2003. MMWR. 2005;54:509–513. [PubMed] [Google Scholar]
- 139.Weinberg RA. The Biology of Cancer. New York: Garland Science; 2007. [Google Scholar]