Abstract
We have previously used MALDI mass spectrometry to highlight ammonium- or guanidinium-aromatic interactions via cation-π bonding and ammonium- or guanidinium-phosphate interactions through salt bridge formation. In the present work, the gas-phase stability and dissociation pathways of the interaction between phosphorylated peptides and compounds containing quaternary amines are demonstrated using electrospray ionization mass spectrometry. The presence of one quaternary amine in a compound is enough to form a noncovalent complex with a phosphorylated residue. However, if two quaternary amines are present in one molecule, the electrostatic interactions of the quaternary amines with the phosphate results in a “covalent-like” stability, and these bonds can withstand fragmentation by collision-induced dissociation at energies similar to those that fragment covalent bonds. Such interactions are important in accounting for physiological, pathophysiological, and pharmacological effects of many therapeutic compounds and small molecules containing quaternary amines or phosphates.
Introduction
We have previously shown using Ion-Mobility (IM) MALDI that formation of phosphate-quaternary amine complexes enhances detection of hard-to-detect phosphorylated peptides, since the mobility drift time of the noncovalent complex (NCX) of an ionized quaternary amine-phosphorylated peptide is reduced enough to distinguish the NCX ion by both its drift time and its mass.1 We have also demonstrated that stable NCXs are formed between phospholipids and quaternary amines2 and a phosphorylated epitope of the nicotinic receptor and small organic compounds such as chlorisondamine and its analogs.3,4 These results proved promising and led to the present analysis of ionized NCX formed between small molecules containing one or two quaternary amines and each of three phosphorylated peptides (AAApXAAA-NH2) where pX denotes the only variant, the phosphorylated residue (Ser, Thr, or Tyr). The purpose of our study is to better understand the role the phosphorylated residue plays in NCX formation and its contribution to the interaction. We believe that salt-bridge formation enhances the ionization probability, thus improving the detection of phosphorylated peptides.5
A fast perusal of The Physician's Desk Reference (PDR)6 or The Pharmacological Basis of Therapeutics7 reveals that an inordinate number of therapeutic compounds contain quaternary amines, phosphates, or aromatic rings, giving them the needed chemical groups to interact with the targeted biomolecules, which therefore obviates the importance of understanding the chemistry and stability of such interactions. The positive charge on the nitrogen of the quaternary amines interacts with the pair of negative delocalized charges on the phosphate, forming NCX. In the present work, in addition to acetylcholine (ACH) which contains one quaternary amine, we used molecules containing two quaternary amines to find out if and how the presence of an additional charge could make a significant difference in NCX formation. These molecules (Figure 1) are important as they are still used as therapeutic agents. Succinyl choline is a depolarizing skeletal muscle relaxant which acts at the cholinergic receptors of the motor end plate to produce depolarization. Hexamethonium is an acetylcholine receptor antagonist that acts only at neuronal nicotinic receptors at preganglionic sites in both the sympathetic and parasympathetic nervous system, which are both regulated preganglionically by nicotinic acetylcholine receptors. Decamethonium is both a depolarizing muscle relaxant and neuromuscular blocking agent acting as an agonist of the nicotinic acetycholine receptors in the motor end plate causing depolarization.7
Figure 1.
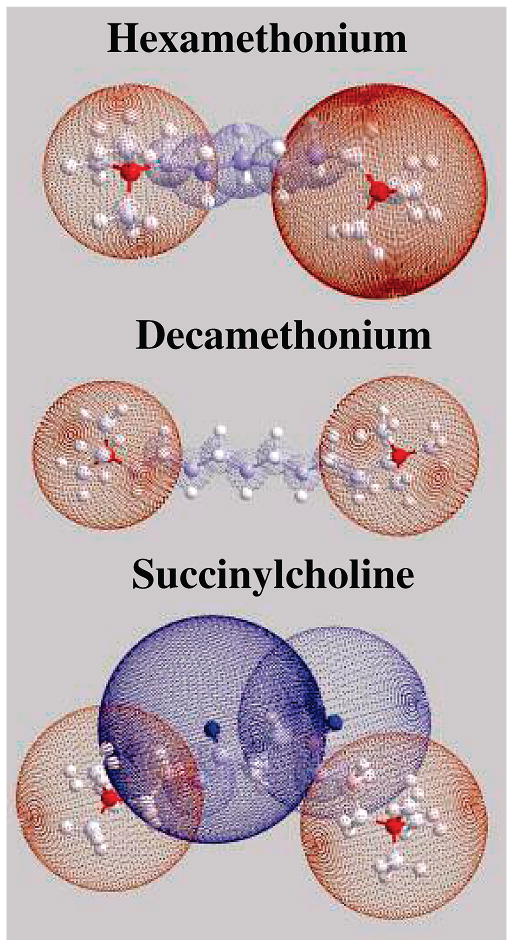
Chem 3D molecular models of hexamethonium, decamethonium, and succinylcholine.
Materials and Methods
Peptides
Three amidated phosphopeptides, AAApSAAA (MW = 610.25 Da), AAApTAAA (MW = 624.26 Da), and AAApYAAA (MW = 686.28 Da), where p denotes a phosphorylated residue, were synthesized at the John Hopkins School of Medicine Peptide Synthesis Core Facility (Baltimore, MD). Stock solutions were prepared in water at a concentration of 1 nmol/μL (Table 1).
Table 1.
Phosphopeptides
| peptide | formula | [M + H]+ | [M + Na]+ | [M + K] |
|---|---|---|---|---|
| A3pYA3 | C27H43N8O11P | 687.29 | 709.27 | 725.24 |
| A3pSA3 | C21H39N8O11P | 611.25 | 633.24 | 649.21 |
| A3pTA3 | C22H41N8O11P | 625.27 | 647.25 | 663.23 |
Quaternary Amines
Hexamethonium (HXM, MW = 202.24) chloride, decamethonium (DCM, MW = 258.48) bromide, and succinylcholine (SCH, MW = 290.98) chloride containing each two quaternary amines and Acetylcholine(ACH, MW = 146.23) chloride, a neurotransmitter containing one quaternary amine, were purchased from Sigma (St. Louis, MO). Stock solutions were prepared in water at a concentration of 1 nmol/uL.
Sample Preparation
Sample mixtures of each of the quaternaryamines at a final concentration of 100 pmol/μL and the three phosphopeptides at a final concentration of 10 pmol/μL in 20% ethanol were used for mass analysis. Also, each phosphopeptide (10 pmol/μL) was added to a mixture of 100 pmol each DCM, HXM, and SCH).
Instrument
A Q-TOF Global Ultima mass spectrometer (Waters, Milford, MA) was used for electrospray analysis. A flow rate of 5 μL/min was used to introduce the sample into the mass spectrometer. The mass spectrometer was operated in positive ion mode with a capillary voltage of 3.25 kV, a sampling cone voltage of 50 V, a source temperature of 100 °C, a desolvation temperature of 300 °C, a desolvation gas flow rate of 500 L/Hr, and a cone gas flow of 100 L/Hr. For MS/MS analysis, a selection mass window of ∼4 m/z with a collision gas (Argon) pressure of 13 psi was employed. Collision energies between 3–9 V were used in the collision cell for ion fragmentation. Mass spectra are the sum of 50 consecutive 1 s scans. The average intensity values reported in this study are the result of three replicate sample runs.
Molecular Modeling
The quaternary amines (HXM, DCM, SCH) were modeled by Chem 3D Pro Version 10 (Cambridge-Soft, Cambridge, MA). The models were energy minimized using the MM2 force field with a minimum rms gradient of 0.100. The structures were color coded by elements as follows: carbon (gray), nitrogen (red), oxygen (blue).
Results and Discussion
Acetylcholine (ACH)
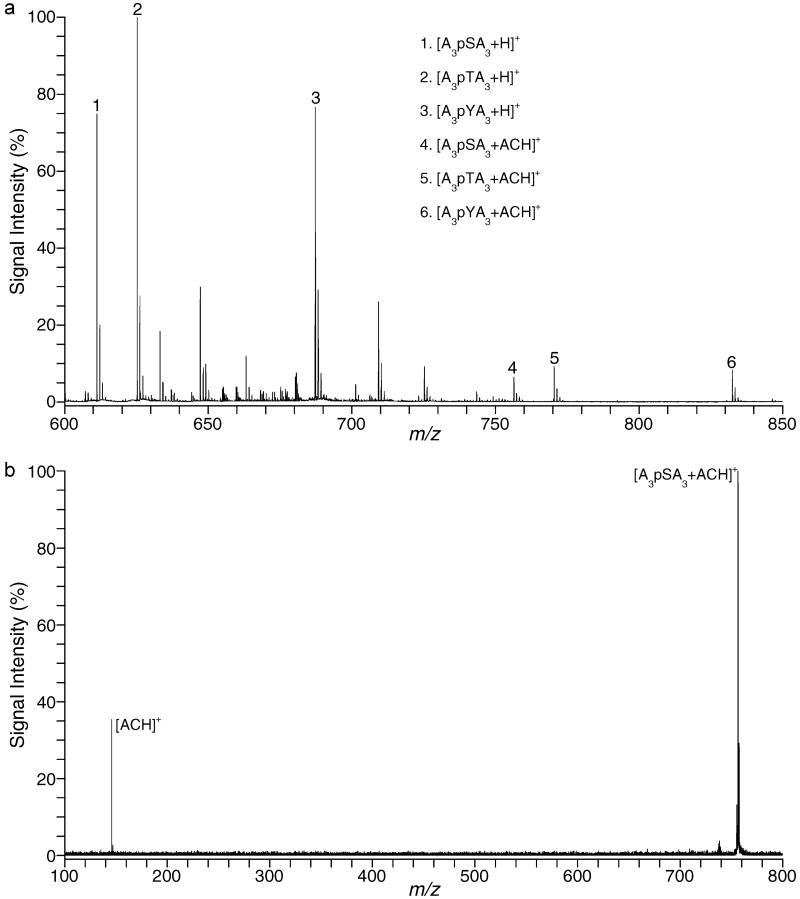
Formation of NCX between each of the quaternary amines and the phosphorylated residues was monitored by ESI. Figure 2a shows MH+ resulting from the formation of NCX between each of the phosphorylated peptides and ACH. The relative abundance of the complexes (peaks 4, 5, and 6) seems to be evenly distributed (Table 2). Collision induced dissociation (CID) of each of the NCX molecular ions at collision energy (CE) of 15 V resulted in dissociation of ACH from the peptide. No peptide fragmentation was detected and the MH+ of the negatively charged peptide was suppressed. Figure 2b shows the CID of the ACH-AAApSAAA (peak 4) complex. Almost identical spectra were obtained with the other two NCX (peaks 5 and 6).
Figure 2.
(a) ESI spectrum of the NCX formed between ACH and each A3pXA3 peptide. (b) MSMS of the NCX of ACH-A3pSA3 at m/z 756, CE = 15 V.
Table 2.
NCXs with ACH
| peptide | formula | [M + H]+ | % RA |
|---|---|---|---|
| A3pYA3 | C34H58N9O13P | 832.40 | 8 |
| A3pSA3 | C28H54N9O13P | 756.36 | 6 |
| A3pTA3 | C29H56N9O13P | 770.38 | 9 |
Decamethonium (DCM)
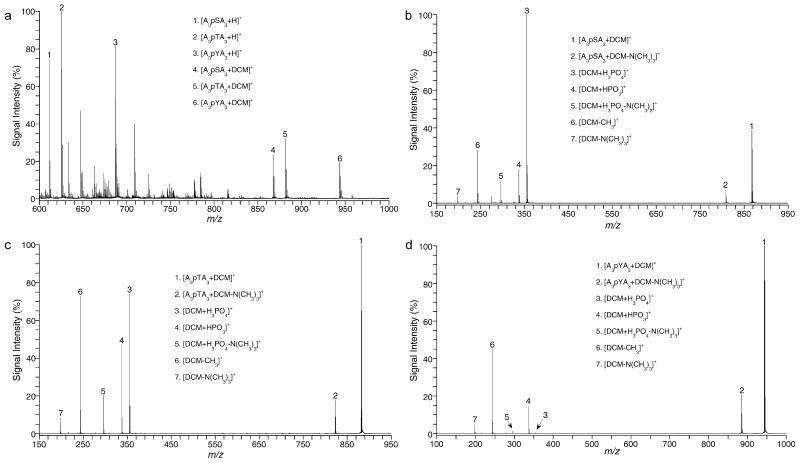
Contains two quaternary amines separated by a chain of 10 methyl groups. Figure 3a shows MH+ resulting from the formation of NCX between each of the phosphorylated peptides and DCM (Table 3). The relative abundances of the complexes (peaks 4, 5, and 6) are 23.0, 31.4, and 18.6%, respectively, for AAApSAAAA-DCM, AAApTAAA-DCM, and AAApYAAA-DCM. In Figures 3b and c, the CID of each of the NCX MH+ of AAApSAAA-DCM and AAApTAAA-DCM at a CE of 30 V resulted in three dissociation pathways. The first pathway shows the disruption of the Coulombic interaction between the quaternary amines and the phosphorylated peptide. In the second pathway, the complex is dissociated along the covalent bond between the oxygen from Ser/Thr and the phosphorus of the phosphate (DCM + HPO3, RA = 17.8 and 47.3%, respectively). In the third and dominant pathway, the covalent bond break occurs between the Ser/Thr carbon and the Ser/Thr oxygen, which remains with the phosphate (DCM + H3PO4, RA = 100 and 75%, respectively). The base peaks in Figure 3b is that of the DCM + H3PO4 ion, whereas in Figure 3c it is that of the NCX ion.
Figure 3.
(a) ESI spectrum of the NCX formed between DCM and each A3pXA3 peptide. (b) MSMS of the NCX of DCM-A3pSA3 at m/z 868, CE = 30 V. (c) MSMS of the NCX of DCM-A3pTA3 at m/z 882, CE = 30 V. (d) MSMS of the NCX of DCM-A3pYA3 at m/z 944, CE = 30 V.
Table 3.
NCXs with DCM
| peptide | formula | [M + H]+ | % RA |
|---|---|---|---|
| A3pYA3 | C43H79N10O11P | 943.57 | 18.6 |
| A3pSA3 | C37H75N10O11P | 867.54 | 23.0 |
| A3pTA3 | C38H77N10O11P | 881.56 | 31.4 |
However, in the case of AAApYAAA (Figure 3d), we mainly see dissociation pathways one and two (DCM + HPO3, RA = 14.6%), whereas the third dissociation is almost nonexistent (DCM + H3PO4, RA = 1.5%) and is most likely due to sequential losses of HPO3 from pY followed by a loss of H2O from elsewhere in the peptide8,9 and the base peak is the MH+ of the NCX. In addition, the relative abundance of the second dissociation pathway for pY is less than those of the same pathways involving pS or pT. The dominant pathway seems to be the one involving dissociation of the NCX. This is probably due to the resonance stabilization of the bond between the ring and the oxygen, which makes the breakage of the covalent bond between the tyrosine ring carbon and the oxygen much less thermodynamically favorable. In all cases, we see the loss of one quaternary amine (N(CH3)3) from the complex (peak 2, Figure 3d), the loss of a methyl from DCM (peak 6, Figure 3d) and the loss of a quaternary amine (N(CH3)3)+ from DCM (peak 7, Figure 3d).
Although we have seen dissociation pathways similar to the first and the second between phosphorylated residues and the guanidinium groups of two or more adjacent Arginine residues (with their delocalized positive charge),10–15 the attraction exerted by the DCM's with its two localized and distant positive charges, seem to be even more powerful than that exerted by the Arg's guanidinium, although the charged entities (the(N(CH3)3)+ are separated by a carbon chain (CH2)10, implying that quaternary amines charges can coordinate and exert their charge effect over a distance.
Hexamethonium (HXM)
Contains two quaternary amines separated by a chain of six methyl groups. The spectrum recording (data not shown), shows MH+ resulting from the formation of NCX between each of the phosphorylated peptides and HXM (Table 4). The relative abundances of the complexes are 24, 30 and 17%, respectively, for AAApSAAAA-HXM, AAApTAAA-HXM, and AAApYAAA-HXM. The CID of each of the NCX molecular ions of AAApSAAA-HXM and AAApTAAA-HXM at a CE of 30 V resulted in the same three dissociation pathways observed with DCM as described above. The first being the disruption of the salt bridge linking the quaternary amines and the phosphorylated peptide. In the second the dissociation is again along the covalent bond between the oxygen from Ser/Thr and the phosphorus of the phosphate (HXM + HPO3, RA = 9.6 and 21.9%, respectively). In the third and dominant pathway, the covalent bond break also occurs between the Ser/Thr carbon and the Ser/Thr oxygen, where the oxygen remains with the phosphate (HXM + H3PO4, RA = 100 and 67.1%, respectively). The base peaks were again HXM + H3PO4 and NCX, respectively.
Table 4.
NCXs with HXM
| peptide | formula | [M + H]+ | % RA |
|---|---|---|---|
| A3pYA3 | C39H71N10O11P | 887.51 | 17.0 |
| A3pSA3 | C33H67N10O11P | 811.48 | 24.0 |
| A3pTA3 | C34H69N10O11P | 825.50 | 30.0 |
In the case of AAApYAAA, we mainly see the first dissociation pathways and a very weak (HXM+HPO3, RA=14.6%), while the third dissociation pathway is non existent and the base peak is the MH+ of the NCX. In all cases we see the loss of one quaternary amine (N(CH3)3) from the complex. In the case of the pS and pT, we observe the losses of a methyl from HXM and of a (N(CH3)3)+ from (HXM + H3PO4).
Succinylcholine (SCH)
The structure of SCH looks like ACH and its mirror image connected through their acetyl. In addition to two quaternary amines it has at each end two esters that are hydrogen-bond acceptors, each with a delocalized pair of electrons and separated by two methyls. The MH+ resulting from the formation of NCX between each of the phosphorylated peptides and SCH (Table 5) has the following relative abundances 28.6, 38.6, and 23.0% for AAApSAAAA-SCH, AAApTAAA-SCH, and AAApYAAA-SCH, respectively. The CID of each of the NCX molecular ions of AAApSAAA-SCH and AAApTAAA-SCH at a CE of 30 V resulted in the same three dissociation pathways described above. The first being the disruption of the salt bridge linking the quaternary amines and the phosphorylated peptide. In the second, the dissociation is again along the covalent bond between the oxygen from Ser/Thr and the phosphorus of the phosphate (SCH + HPO3, RA = 10.0 and 12.9%, respectively). In the third and dominant pathway, the covalent bond break also occurs between the Ser/Thr carbon and the Ser/Thr oxygen which remains with the phosphate (SCH + H3PO4, RA = 68.6 and 21.4%, respectively), the base peaks were again SCH-[N(CH3)3]. The loss of a methyl group, the loss of a (N(CH3)3)+ from (SCH + H3PO4) and from SCH are also seen.
Table 5.
NCXs with SCH
| peptide | formula | [M + H]+ | % RA |
|---|---|---|---|
| A3pYA3 | C41H71N10O15P | 975.49 | 23.0 |
| A3pSA3 | C35H67N10O15P | 899.46 | 28.6 |
| A3pTA3 | C35H67N10O15P | 913.48 | 38.6 |
In the case of AAApYAAA, the second dissociation pathway is the dominant one and again the (SCH + HPO3, RA = 3%) is rather weak, whereas the third dissociation pathway is nonexistent and the base peak is the MH+ of SCH-CH3. Again the tyrosine resonance involving the bond between the ring and the oxygen is preventing the breakage of the covalent bond between the ring's carbon and the oxygen. Here again, we see the loss of one quaternary amine (N(CH3)3) from the complex, and from SCH and the dominant MH+ is that of (SCH-CH3).
Quaternary Amines Mixtures
With AAApSAAA, the NCX RA were as follow for the singly charged MH+: 18.8% with HXM, 8.3% with DCM, and 19.4% with SCH. With AAApTAAA the NCX were 25.0% with HXM, 9.7% with DCM, and 25.0% with SCH. In the case of AAApYAAA the NCX were 71.4% with HXM, 34.3% with DCM, and 51.4% with SCH.
With all three peptides, HXM and SCH formed almost an equal amount of NCX, DCM competed less favorably. This is probably due to the fact that the distance between the quaternary amines in DCM is greater than in HXM and SCH.
In conclusion, we have demonstrated that a pair of quaternary amines located at each end of a carbon chain can generate stable NCX with phosphorylated residues through salt bridge formation. When submitted to CID, the NCX formed by the peptides and quaternary amines dissociate. However, two other unexpected pathways are seen.
In one, the covalent bond between the amino acid oxygen and the phosphate phosphorus break resulting in ions corresponding to a dephosphorylated peptide ion (AAApXAAA-HPO3) and a diquaternary amine + HPO3 noncovalent complex. In the other pathway, the covalent bond break occurs between the Ser/Thr carbon and the phosphate oxygen resulting in ions corresponding to a dephosphorylated peptide ion (AAApS/pTAAA-H3PO4) and a diquaternary amine + H3PO4 noncovalent complex. To our knowledge, CID fragmentation showing the cleavage of a phosphate group covalently attached to a peptide while the noncovalent interaction between a phosphate and quaternary amines group is preserved has not been previously demonstrated. Although the data obtained show what happen in the gas-phase, it still is an indicator of the stability of such interactions in a biological milieu.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH. We thank the Office of National Drug Control Policy (ONDCP) for instrumentation funding, without which this and other projects could not have been accomplished.
References
- 1.Woods AS, Fuhrer K, Gonin M, Egan T, Ugarov M, Gillig KJ, Schultz JA. AngiotensinII/Acetylcholine Non-Covalent Complexes Analyzed with MALDI-Ion Mobility-TOFMS. J Biomol Tech. 2003;14:1–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Woods AS. The Mighty Arginine, the Stable Quaternary Amines, the Powerful Aromatics and the Aggressive Phosphate: Their Role in the Noncovalent Minuet. J Proteome Res. 2004;3:478–484. doi: 10.1021/pr034091l. [DOI] [PubMed] [Google Scholar]
- 3.Woods AS, Moyer SC, Wang HY, Wise RA. Interaction of Chlorisondamine with the Neuronal Nicotinic Acetylcholine Receptor. J of Proteome Research. 2003;2:207–212. doi: 10.1021/pr025578h. [DOI] [PubMed] [Google Scholar]
- 4.Wang HYJ, Taggi AE, Meinwald J, Wise RA, Woods AS. A Study of the Interaction of Chlorisondamine and Chlorisondamine analogs with An Epitope of the α−2 Neuronal Acetylcholine Nicotinic Receptor Subunit. J Proteome Res. 2005;4:532–539. doi: 10.1021/pr049786g. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LN, Lewis RJ. Structural basis for control by phosphorylation. Chem Rev. 2001;101:2209–2242. doi: 10.1021/cr000225s. [DOI] [PubMed] [Google Scholar]
- 6.The Physician Desk Reference (PDR) 62nd. Thomson; Montvale, NJ: 2008. [Google Scholar]
- 7.Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th. McGraw-Hill; New York: 2001. [Google Scholar]
- 8.Moyer SC, Cotter RJ, Woods AS. Fragmentation of phosphopeptides by atmospheric pressure MALDI and ESI/ion trap mass spectrometry. J Am Soc Mass Spectrom. 2002;13:274–283. doi: 10.1016/S1044-0305(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 9.Moyer SC, VonSeggern CE, Cotter RJ. Fragmentation of phosphotyrosine containing peptides by atmospheric pressure MALDI/ion trap mass spectrometry. J Am Soc Mass Spectrom. 2003;14:581–592. doi: 10.1016/S1044-0305(03)00142-9. [DOI] [PubMed] [Google Scholar]
- 10.Woods AS, Ferre S. The Amazing Stability of the Arginine-Phosphate Electrostatic Interaction. J Proteome Res. 2005;4:1397–1402. doi: 10.1021/pr050077s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson SN, Wang HYJ, Yergey A, Woods AS. Phosphate stabilization of intermolecular interactions. J Proteome Res. 2006;5:122–126. doi: 10.1021/pr0503578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson SN, Wang HYJ, Woods AS. A study of the Fragmentation Patterns of the Phosphate-Arginine Noncovalent Bond. J Proteome Res. 2005;4:2360–2363. doi: 10.1021/pr050261d. [DOI] [PubMed] [Google Scholar]
- 13.Woods AS, Ciruela F, Fuxe K, Agnati LF, Lluis C, zprintx, Franco R, Ferré S. The role of electrostatic interaction in receptor-receptor heteromerization. J Mol Neurosci. 2005;6:125–132. doi: 10.1385/JMN:26:2-3:125. [DOI] [PubMed] [Google Scholar]
- 14.Schug KA, Lindner W. Noncovalent binding between guanidinium and anionic groups: focus on biological- and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues. Chem Rev. 2005;105:67–114. doi: 10.1021/cr040603j. [DOI] [PubMed] [Google Scholar]
- 15.Alves S, Fournier I, Alfonso C, Wind F, Tabet J. Gas-phase ionization/desolvation processes and their effect on protein charge state distribution under matrix-assisted laser desorption/ionization conditions. Eur J Mass Spectrom. 2006;12:369–383. doi: 10.1255/ejms.822. [DOI] [PubMed] [Google Scholar]