Abstract
This study was conducted to compare quantifiable measures of vascularity obtained from contrast-enhanced color flow images of breast lesions to pathologic vascularity measurements. Nineteen patients with solid breast masses received Levovist® Injection (10 ml at 300 mg/ml; Berlex Laboratories, Montville, NJ, USA). Color flow images of the mass pre and post contrast were obtained using an HDI 3000 scanner (Philips Medical Systems, Bothell, WA, USA) optimized for clinical scanning on an individual basis. After surgical removal specimens were sectioned in the same planes as the ultrasound images and stained with an endothelial cell marker (CD31). Microvessel area (MVA) and intratumoral microvessel density (MVD) were determined for vessels 10–19μm, 20–29μm, 30–39μm, 40–49μm, and >50μm in diameter using a microscope and image processing software. From the ultrasound images the number of color pixels before and after contrast administration relative to the total area of the breast mass was calculated as a first order measure of fractional tumor vascularity. Vascularity measures were compared using reverse stepwise multiple linear regression analysis. In total, 58 pathology slides (with 8106 frames) and 185 ultrasound images were analyzed. There was a significant increase in flow visualization pre to post Levovist injection (p=0.001), but no differences were found between the 11 benign and the 8 malignant lesions (p>0.35). Ultrasound vascularity measurements post contrast correlated significantly with pathology (0.15≤r2≤0.46; p<0.03). The 30–39μm vessel range contributed most significantly to the MVD relationship (p<0.001), while the MVA was mainly influenced by vessels 20–29μm (p<0.004). Pre contrast ultrasound only correlated with pathology for relative MVA (r2=0.16; p=0.01). In conclusion, contrast-enhanced color flow imaging provides a noninvasive measure of breast tumor neovascularity corresponding mainly to vessels 20–39μm in diameter, when used in a typical clinical setting.
Keywords: Ultrasound contrast agent, Color flow imaging, Microvessel density, Breast cancer, Tumor vascularity
Introduction
Mammography is the imaging mode of choice for screening, detecting and diagnosing breast lesions (Kopans 1998; Tabár & Dean 2003). Nonetheless, the vast majority of breast biopsies performed in clinical practice (between 65 % and 90 %) are found to be benign when assessed histopathologically (Kopans 1998; Zonderland et al. 1999). Hence, a reliable and quantifiable technique for improved characterization of malignant and benign breast masses is needed.
Ultrasound imaging is an important adjunct to mammography that quite easily differentiates between cystic and solid lesions (Stavros et al. 1995; Taylor et al. 2002; Zonderland et al. 1999). It can also provide real time guidance of breast biopsies. Moreover, ultrasound imaging of anatomical features has been shown to improve the characterization of solid breast lesions as benign or malignant (Stavros et al. 1995; Taylor et al. 2002; Zonderland et al. 1999). Another important and independent predictor of malignancy is the angiogenic vascular morphology associated with breast tumors (de Jong et al. 2000; Gasparini & Harris 1995; Weidner et al. 1991; 1992). Tumor angiogenesis is the development of a new vascular network out of preexisting vessels and is considered essential for the progression of solid tumors (Carmeliet & Jain 2000; Folkman 1990; Li 2000). However, breast lesion characterization based on Doppler ultrasound flow measurements have produced mixed results, due to overlap between flow measurements in benign and malignant tumors (Adler et al. 1990; Bohm-Velez & Mendelson 1989; Taylor et al. 2002). One problem may be the lack of sensitivity to detecting flow in small tumor vessels using standard ultrasound techniques (Ferrara et al. 2000).
Microbubble based ultrasound contrast agents produce 15 to 25 dB increases in the echo intensities of blood flow signals (especially when combined with power Doppler imaging), thus, improving the sensitivity of ultrasonic flow imaging markedly (Ferrara et al. 2000; Forsberg et al. 1998; Goldberg et al. 2001). Consequently, we hypothesized that quantitative measures of breast lesion vascularity could be obtained noninvasively from contrast-enhanced color flow images and this was confirmed in our small, preliminary evaluation of 10 patients (Chaudhari et al. 2000). Hence, the purpose of our current study was to prospectively compare noninvasive, quantitative measures of vascularity obtained from contrast-enhanced ultrasound color flow imaging to invasive pathological vascularity measurements in a larger patient group. As a secondary objective, this project attempted to establish the size of the vessels that contribute most to the ultrasound results obtained with real-time color flow imaging.
Materials and Methods
Nineteen patients were enrolled in this prospective study between 1997 and 2000. These were women over 21 years of age scheduled for an excisional biopsy of a breast mass. All patients were referred after palpation and/or x-ray mammography identified a solid mass. Subjects had to be medically stable, while pregnant or nursing women were excluded. The study was approved by the university’s Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. All patients gave written informed consent prior to enrollment in the study. It should be noted, that while this project was sponsored in part by Berlex Laboratories, the authors of this article had sole control of the data generated.
Acquisition of Ultrasound and Pathology Data
First, baseline color flow images, which identified the mass or abnormality seen by x-ray mammography, were obtained by an experienced sonographer (DAM) using an HDI 3000 scanner (Philips Medical Systems, Bothell, WA, USA) and a broad bandwidth linear array transducer (5 – 10 MHz). Image parameters such as output power, gain, and pulse repetition frequency (range 700 – 1500 Hz) were adjusted for each individual patient to optimize flow visualization and then kept constant in accordance with the typical clinical use of ultrasound imaging. This resulted in frame-rates of 10 to 16 Hz. Transaxial scans of the mass were performed at 5 levels, each encompassing 20 % of the cranio-caudal dimension. Flow images were recorded on S-VHS videotape for each level.
Next, an ultrasound contrast agent was administered intravenously via a peripheral vein, preferably the antecubital vein. The agent used was Levovist injection (SH U 508A; Berlex Laboratories, Montville, NJ, USA) in a 10 ml dose (concentration: 300 mg/ml). Levovist consists of air-filled microbubbles between 2 and 8 μm in diameter with 97 % being smaller than 6 μm (Goldberg et al. 2001). It is a non-toxic, neutral pH, biodegradable contrast agent made from galactose and 0.1 % palmitic acid as an additive. Levovist is approved for use in general radiology applications in the European Union, but not by the Food and Drug Administration (FDA) and it was, therefore, employed in this study under a physician sponsored IND (Investigational New Drug). The breast lesion was scanned with the gain and other Doppler flow settings unaltered from the pre contrast settings (except that the gain could be lowered if excessive color blooming occurred due to the contrast agent; Forsberg et al. 1994). At the point of maximal enhancement flow images were recorded on S-VHS tape for each of the 5 levels.
After the ultrasound study was completed, all patients underwent surgery to remove the breast lesion. At the time of surgery, the transaxial (i.e., imaging) plane of the specimen were marked as front, back, right, and left using different sutures. Specimens were fixed in 10 % neutral buffered formalin for 12 to 24 hours, dehydrated in graded alcohols, cleaned in xylene, and embedded in paraffin using standard methods. Careful attention was paid to the labeling of each specimen to ensure that it was sectioned in the same plane as the one the ultrasound images were obtained in. As part of the patients clinical care 2 to 6 sections of 5 μm thickness (on average 3) were prepared and a histopathological diagnosis of benign or malignant was rendered (by JPP). Since the process of angiogenesis involves the activation, migration, and proliferation of endothelial cells (Carmeliet & Jain 2000; Folkman 1990; Li 2000), immunohistochemical staining of each section, using a monoclonal antibody (JC70A; Dako Denmark A/S, Glostrup, Denmark) against the platelet endothelial cell adhesion molecule (PECAM or CD31), was performed next. This molecule is a potent endothelial cell marker since its expression is restricted to vascular system platelets and endothelium (Newman 1997). Finally, the sections were mounted onto glass slides for analysis.
Image Analysis
A semi-automated histomorphometry system based on a DXC-970MD color CCD camera (Sony Corporation, Tokyo, Japan) connected to an SMZ-10A microscope (Nikon, Melville, NJ, USA) was used to analyze both ultrasound and pathology data with the operators blinded to the final diagnosis. Moreover, ultrasound and pathology data from the same patient were analyzed on separate occasions at least 6 weeks apart to eliminate any operator bias. The histomorphometry system employed a 10x objective and 10x ocular magnification (total magnification: 100x) to provide a digital view of each histological slide on a desktop computer (Chaudhari et al. 2000). A motorized stage, controlled by ImagePro Plus software (Media Cybernetics, Silver Spring, MD), was used to move the specimen until Red Green Blue (RGB) images (each 640 pixels by 480 pixels or 1.27 mm2) of the entire tumor area were acquired. The RGB images were converted to Hue Saturation Intensity (HSI) images to allow vessel (saturation image) and tissue (blue image) enhanced images to be extracted (Barbareschi et al. 1995; Chaudhari et al. 2000). Mathematical morphometry was used to obtain an image on which automated count and measurements of microvessels was performed. For each slide the total microvessel area (total MVA) and count (total MVC) were determined. Moreover, these values were scaled by the tumor area to determine the relative vessel area (rMVA) and the relative microvessel density (rMVD), respectively. Finally, rMVA and rMVD were divided into five categories based on the range of vessel diameters involved a) 10–19 μm, b) 20–29 μm, c) 30–39 μm, d) 40–49 μm, and e) 50 μm and above (these parameters were designated as MVA and MVD, respectively). Notice, that all of these parameters were derived from the entire tumor specimen, unlike most studies on prognostic indicators of breast cancer (e.g., de Jong et al. 2000; Gasparini & Harris 1995; Weidner et al. 1991; 1992), which typically rely on data from a few select regions of high vessel concentration (so called “hot spots”).
Erroneous diameter measurements may occur if a microvessel appears in an oblique angle in a specimen (due to vessel orientation and cutting angle). To counteract this error both the minimum and average diameter of the vessel were determined and compared. The minimum diameter was selected as the vessel’s actual diameter if it was less than a third of the average diameter (assuming this indicated that the vessel had been cut in an oblique angle) - otherwise the average diameter was chosen as the vessels actual diameter. The MVA was calculated from the actual vessel diameter assuming a circular vessel. Finally, the measurements were also corrected for 10 % shrinkage due to the slide preparation.
The histomorphometry system was also used to digitize video images of the ultrasound scans as RGB color images from the 5 levels before and after contrast injection. The breast lesion was manually outlined on the ultrasound image and the RGB channels used to automatically segment and count the number of color pixels as well as the total number of pixels the within the lesion (Bell et al. 1995; Chaudhari et al. 2000). Fractional breast tumor vascularity (FV) was calculated (in percent) as the number of color pixels (ci) relative to the total number of pixels (ci + xi) within the breast mass (Chaudhari et al. 2000; Fleischer 2000; Forsberg et al. 2002):
| (1) |
The FV was determined for each of the 5 levels before and after administration of Levovist.
Statistical Analysis
Since this was a pilot study, no statistical power analysis could be performed a priori. In order to compare the ultrasound and the pathology data sets, an equal number of data points shold be available from both methods. Ten ultrasonic data points (5 pre and 5 post contrast) were consistently obtained regardless of the size of the mass, since scans at 5 levels of each lesion could always be acquired (albeit, sometimes with overlap between the levels). However, depending on the size of the breast lesions studied, it was not always posssible to produce 5 pathological sections from each specimen. Consequently, linear interpolation was used to reduce the ultrasound data until an equal number of points were available in both data sets (Chaudhari et al. 2000).
Comparisons of ultrasonic flow data pre to post contrast as well as relative to the histopathological diagnosis of benign or malignant were conducted with two-way student’s t-tests using Stata 9.0 (Stata Corporation, College Station, TX, USA) with p-values less than 0.05 considered an indication of significance. The two-way t-test was also used to compare the pathological measures of vascularity relative to the lesion diagnosis. The correlation coefficients (r2) between ultrasonic and pathological data sets (benign and malignant) were determined using single and multiple variable linear regression techniques (Rosner 1990). Finally, a reverse stepwise multiple regression analysis was employed to establish which parameters (in this case, which vessel range) contributed most significantly to the overall correlation coefficient.
Results
The 19 subjects enrolled in this study were predominantly Caucasian (11 women or 58 %), 5 subjects (26 %) were African-American, one participant (5 %) was Asian and two women requested to be listed as “other”. The mean age was 54 years with a range from 24 to 81 years. There were 8 cancers and 11 benign lesions in this population (i.e., 8/19 or 42 % of the breast masses were malignant) with average diameters of 14 and 12 mm, respectively, as measured by ultrasound (p = 0.55). A statistically significant difference in age was found between patients with benign and malignant lesions (44 ± 12 vs. 67 ± 11 years; p = 0.0005). The majority of cancers were invasive (6 out of 8), but there was also 2 cases of ductal carcinoma in situ. The histopathological classification of the 11 benign lesions included 6 fibroadenomas and 2 cases of fibrocystic changes. The remaining masses were classified in 2 cases as stromal fibrosis and in one case as schlerosing adenosis.
An example of power Doppler imaging of a fibroadenoma before and after contrast administration is presented in Figure 1. In total, 58 pathology slides (with 8106 frames) and 185 ultrasound images were analyzed. One pre-contrast ultrasound image was excluded from analysis, because of excessive color blooming due to patient motion. Moreover, in one patient ultrasound data was only available from 3 levels, due to a technical failure. Hence, there were 58 pairs available in the final data set. There was a significant increase (p = 0.001) in the flow visualized pre to post Levovist injection (FV: 2.6 ± 5.44 % vs. 6.5 ± 10.87 %). This was also the case when benign and malignant lesions were analyzed separately (p < 0.03). However, there was no difference between flow (i.e., FV) in the 11 benign and the 8 malignant lesions (p > 0.35). Likewise, there were no differences in rMVD or rMVA relative to lesion diagnosis (p > 0.12). However, both total MVA and MVC were greater in malignant than in benign tumors (on average 0.23 mm2 vs. 0.14 mm2 and 574 vessels vs. 358 vessels; p ≤ 0.034).
Figure 1.
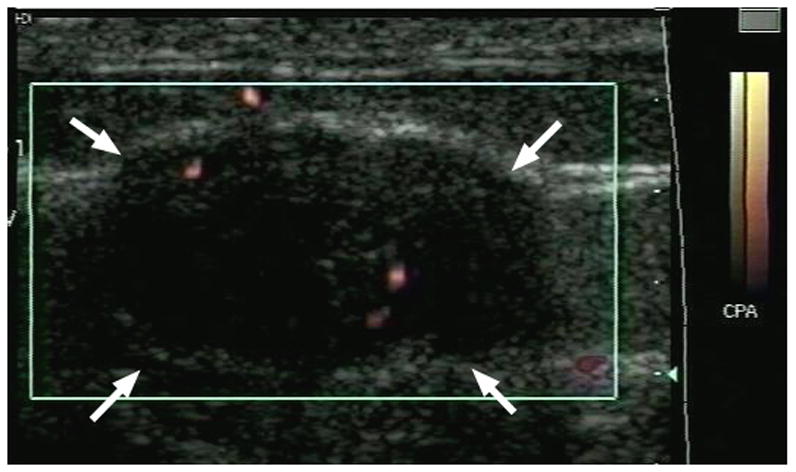
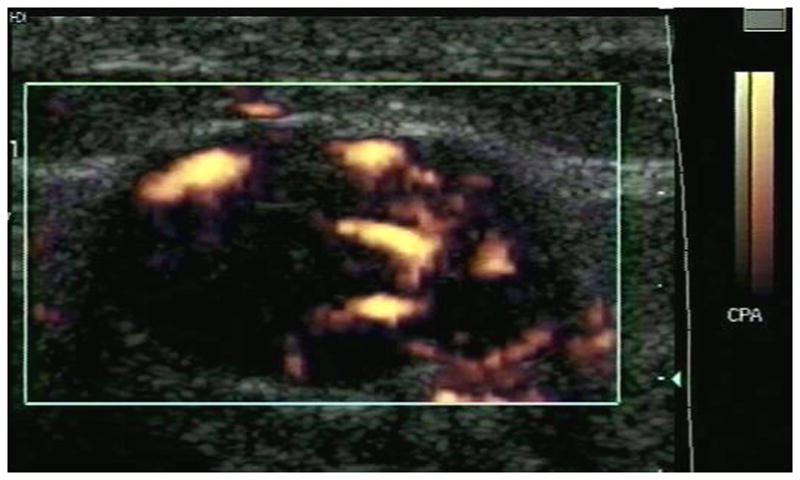
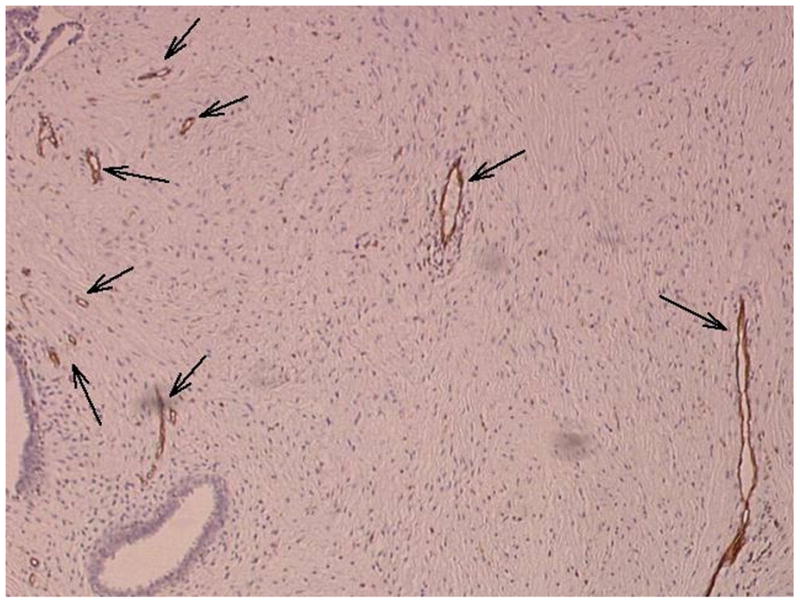
Example of a fibroadenoma (arrows) imaged in power Doppler mode pre (A) and post (B) injection of 10 ml of Levovist. A pathology specimen obtained from the same lesion is presented in (C) with areas stained with CD31 shown in brown (arrows).
Significant correlations were found between the ultrasonic FV obtained post injection and the MVA as well as the MVD in the five vessel ranges (Table 1; r2 ≥ 0.27; p < 0.005), when analyzing the entire data set (i.e., benign and malignant lesions evaluated jointly). The overall microvessel density (rMVD) did not correlate with the ultrasonic FV, whereas the rMVA did (pre as well as post injection). The vessel ranges that contributed significantly to the multiple linear regression results are listed in Table 2. For the MVA vessels 20 to 29 μm in diameter contributed most significantly to the linear relationship with the ultrasonic FV (p = 0.003), while for the MVD it was vessels in the 30 to 39 μm range (p < 0.001).
Table 1.
Linear regression r2 values obtained from all 19 lesions (N = 58).
| Pathological parameter | Pre injection | Post injection |
|---|---|---|
| Total MVA | 0.00 | 0.01 |
| Total MVC | 0.01 | 0.00 |
| rMVA | 0.16 * | 0.15 * |
| rMVD | 0.00 | 0.03 |
| MVA-5 ranges | 0.10 | 0.27 ** |
| MVD-5 ranges | 0.04 | 0.46 ** |
p < 0.03;
p < 0.005
Table 2.
Calculated t-statistic for the significant variables by range based on the data obtained from all 19 lesions (N = 58).
| Pathologic parameter | t–statistic |
|---|---|
| MVA; 20–29 μm vessels | −3.06 * |
| MVA; ≥ 50 μm vessels | −2.36 * |
| MVD; 30–39 μm vessels | 5.33 ** |
| MVD; 40–49 μm vessels | 5.21 ** |
| MVD; 10–19 μm vessels | 5.08 ** |
| MVD; 20–29 μm vessels | 5.01 ** |
| MVD; ≥ 50 μm vessels | 4.81 ** |
p ≤ 0.02;
p ≤ 0.001
The data was also analyzed split by benign and malignant breast lesions (Tables 3–5). In these smaller data sets more pre injection correlations between the ultrasoinc FV and the pathologic measures of vascularity (i.e., rMVA and the MVA as well as the MVD in the five vessel ranges ) were found to be significant (r2 ≥ 0.24; p < 0.02). For the benign lesions (Table 4), the vessel ranges that contributed most significantly to the multiple linear regression results were the same as for the combined data set (i.e., 20 – 29 and 30 – 39 μm for MVA and MVD, respectively). However, in the case of the malignant lesions (Table 5) the most significant contribution to the correlation with MVA came from larger vessels (40 – 49 μm), while the MVD results were still dominated by vessels 30 – 39 μm in diameter.
Table 3.
Linear regression r2 values obtained from the 11 benign and 8 malignant lesions analyzed separately. The benign data set contained 34 data points, while the malignant data set was based on 24 data points.
| BENIGN DATA | MALIGNANT DATA | |||
|---|---|---|---|---|
| Pathological parameter | Pre injection | Post injection | Pre injection | Post injection |
| Total MVA | 0.02 | 0.01 | 0.03 | 0.01 |
| Total MVC | 0.00 | 0.00 | 0.09 | 0.00 |
| rMVA | 0.24 * | 0.24 * | 0.09 | 0.00 |
| rMVD | 0.03 | 0.01 | 0.10 | 0.12 |
| MVA-5 ranges | 0.14 | 0.65 ** | 0.63 ** | 0.50 * |
| MVD-5 ranges | 0.03 | 0.64 ** | 0.51 * | 0.43 |
p < 0.02;
p < 0.002
Table 5.
Calculated t-statistic for the significant variables by range based on the 8 malignant lesions (N = 24).
| Pathological parameter | t-statistic | |
|---|---|---|
| Pre injection | Post injection | |
| MVA; 40–49 μm vessels | 4.44 ** | 4.52 ** |
| MVA; 10–19 μm vessels | - | 2.40 * |
| MVD; 30–39 μm vessels | 2.49 * | - |
| MVD; 10–19 μm vessels | 2.32 * | - |
p ≤ 0.03;
p < 0.001
Table 4.
Calculated t-statistic for the significant variables by range based on the 11 benign lesions post-injection (N = 34).
| Pathologic parameter | t–statistic |
|---|---|
| MVA; 20–29 μm vessels | −5.19 ** |
| MVA; ≥ 50 μm vessels | −4.71 ** |
| MVA; 40–49 μm vessels | −4.61 ** |
| MVA; 10–19 μm vessels | −4.55 ** |
| MVA; 30–39 μm vessels | −4.11 ** |
| MVD; 30–39 μm vessels | 6.34 ** |
| MVD; 40–49 μm vessels | 6.15 ** |
| MVD; 10–19 μm vessels | 6.10 ** |
| MVD; 20–29 μm vessels | 6.04 ** |
| MVD; ≥ 50 μm vessels | 5.91 ** |
p ≤ 0.001
Discussion
In total, 19 patients with 8 cancers and 11 benign lesions were evaluated in this study, which corresponds to 185 ultrasound images and 8106 frames from 58 pathology slides. This is almost double the number of patients and images evaluated in our previous study (Chaudhari et al. 2000), but it is still a relatively small patient population. When analyzing the entire data set (Table 1), a significant, but not very strong, linear relationship was found between the ultrasonic FV obtained post injection and the MVA as well as the MVD in the five vessel ranges with r2 values of 0.27 and 0.46 (p < 0.005). The 30 to 39 μm vessel range contributed most significantly to the MVD relationship (Table 2; p ≤ 0.001), while the MVA was mainly influenced by vessels 20 to 29 μm in diameter (Table 2; p ≤ 0.02). The angiogenic vessels associated with cancers vary widely in size with diameters ranging from 20 to 200 μm (Li 2000). Thus, the vessel range established in this study (20–39 μm) corresponds to the smaller angiogenic vessels (although when the malignant lesions were analyzed separately larger vessels 30–49 μm were dominant).
The overall microvessel density (rMVD) did not correlate with the ultrasonic FV, whereas the rMVA did (pre as well as post injection). Likewise, there was no difference in rMVD relative to lesion diagnosis (p > 0.12). This result is not in agreement with the well-established use of the rMVD as an independent prognostic indicator of breast cancer (Weidner et al. 1991; 1992). However, the methodology employed to determine the rMVD (immunohistochemical stain, location and number of regions assessed, etc.) influences its value as a prognostic indicator (Hlatky et al. 2002). The method of Weidner and colleagues relied on data from a few regions of high vessel concentration (i.e., “hot spots”), while this study determined the rMVD based on all vessels within the lesion and this may be the reason for the discrepancy between the results. Moreover, the rMVD is not a direct measure of the flow and angiogenic dependency of a tumor; rather it reflects the local intercapillary distance and, hence, the metabolic burden of the supported tumor cells (Hlatky et al. 2002), which presumably explains the lack of correlation with the ultrasonic flow measurements observed in this study.
There are relatively few reports in the literature on contrast enhanced ultrasound evaluations of breast masses and most deal with qualitative image assessments and diagnostic accuracy only (e.g., Forsberg et al. 2004; Hochmuth et al. 2002; Kedar et al. 1996; Kim et al. 2002; Madjar et al. 2000; Martinez et al. 2003; Özdemir et al. 2004; Stuhrmann et al. 2000). Three studies on quantitative assessments of breast lesions using contrast enhanced Doppler imaging have been published (Huber et al. 2001; Kettenbach et al. 2005; Yang et al. 2002). Kettenbach et al. (2005) reported a significant difference between flow in benign and malignant lesions (p < 0.03), contrary to our results where no such difference was seen (p > 0.35). In the study by Yang and coworkers (2002), contrast did not improve the correlation between ultrasound and rMVD measurements (the r2 value decreased from a high of 0.22 to 0.12 and below after contrast administration). Similarly, Huber and colleagues (2001) found no correlation between microvessel counts and ultrasonic FV in fibroadenomas (p > 0.13). These results are in agreement with the current study, which did not find any correlation between the ultrasonic FV measurements and rMVD or total MVC (cf., Table 1). However, our study did establish a significant linear relationship between the ultrasonic FV obtained post injection and the MVA as well as the MVD in the five vessel ranges (p < 0.005). Most likely, the differences reported above are due to the relatively small patient populations studied (ranging from 34 to 220 women) and the difference in the methodology used for determining the pathological markers (5 vascular “hot spots” versus complete tumor assessment).
There are a number of limitations to the study, which must be considered. The number of patients studied was relatively small, which limits the statistical power of the study and, therefore, the conclusions which can be made. Moreover, only a limited number a slides per patient (on average 3) were provided for clinical assessments (and, thus, for this study), so even though we evaluated the entire tumor area not all tissue in the lesions were assessed. The ultrasound images were averaged to match the number of pathological specimens available, but the elevation thickness of the images are clearly still orders of magnitude larger than the thickness of the specimen slides indicating that a complete match cannot be achieved. Furthermore, while optimizing imaging parameters on an individual patient basis is the clinical approach and will compensate for attenuation and other biological differences, it will also introduce variability in the imaging. Whether the reduction in biological variability or the increase in imaging variability is the dominant factor in the final result is unknown (but it is clearly another source of error).
We have previously shown that the in vitro and in vivo destruction of ultrasound contrast microbubbles is independent of the Mechanical Index (Forsberg et al. 2005; 2006), but the different frame rates utilized in this study (10–16 Hz) and the use of contrast enhanced color flow imaging will produce some bubble destruction making it difficult to translate our results to other ultrasound examinations. Newer contrast imaging techniques, such as pulse inversion harmonic imaging and intermittent power Doppler, are know to reduce bubble destruction and provide improved depiction of microvascularity compared to color flow imaging (Goldberg et al. 2001), but those imaging modes were not available at the time this study was initiated (and our objective was to assess the typical clinical use of ultrasound imaging). It is, therefore, conceivable that smaller microvessels (< 10 μm) may correlate with the ultrasound results if newer, less destructive imaging modes designed specifically for imaging microvascularity (such as intermittent power Doppler) are employed. However, data from contrast enhanced breast studies using such imaging techniques is, to the best of our knowledge, not yet available and this remains an area for future research.
Finally, a statistical limitation of this study is the multiple comparisons being conducted (6 pathological parameters for pre- and post-injection i.e., 12 in total). To account for the statistically significant results in light of this, a Bonferroni correction may be applied, which assigns the traditional 0.05 p-value divided by the number of comparisons (here 12) to be the p-value of significance (Bland and Altman 1995). On the other hand, there are also problems associated with the Bonferroni correction. By controlling the group-wise error rate, each individual test is held to an unreasonably high standard and makes it likely that legitimately significant results will not be detected (Perneger 1998). Hence, we chose not to adopt Bonferroni correction in this study. However, it should be noted, that even if Bonferroni correction was applied the MVA as well as the MVD in the five vessel ranges would still produce a significant correlation (since 0.05/12 = 0.004 cf., Table 1).
In summary, in this study a quantitative parameter – the fractional tumor vascularity – derived from contrast enhanced color flow images has been shown to provide a noninvasive measure of breast tumor neovascularity similar to those of the invasive pathology markers MVA and MVD; albeit based on a relatively small patient population and somewhat weak correlations. Moreover, the contrast enhanced flow signals obtained with real-time color flow imaging in a typical clinical setting that contribute to this parameter appear to correspond mainly to vessels 20 to 39 μm in diameter (i.e., the smaller angiogenic neovessels).
Acknowledgments
This project was supported by the U.S. Army Medical Research Material Command under DAMD17-97-1-7116, by the National Institute of Health (NIH) through grants NIH CA60854 and NIH CA48010 as well as by Berlex Laboratories, Montville, NJ. We gratefully acknowledge the assistance provided by C. Dascenzo and C. Slotoroff in recruiting patients for this study as well as the efforts of Y. Lee, F.N. Saikali, A. Voodarla, R. Yoon and A. Yu with regards to the image analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. 1990;16:553–559. doi: 10.1016/0301-5629(90)90020-d. [DOI] [PubMed] [Google Scholar]
- Barbareschi M, Weidner N, Gasparini G, Morelli L, Forti S, Eccher C, Fina P, Caffo O, Leonardi E, Mauri F, Bevilacqua P, Palma PD. Microvessel density quantification in breast carcinomas: assessment by manual vs. a computer assisted image analysis system. Appl Immunohistochem. 1995;3:75–84. [Google Scholar]
- Bell DS, Bamber JC, Eckersley RJ. Segmentation and analysis of colour Doppler images of tumour vasculature. Ultrasound Med Biol. 1995;21:635–637. doi: 10.1016/0301-5629(95)00006-d. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. Br Med J. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm-Velez M, Mendelson EB. Computed tomography, duplex Doppler ultrasound and magnetic resonance imaging in evaluating the breast. Semin Ultrasound CT MR. 1989;10:171–176. [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Chaudhari MH, Forsberg F, Voodarla A, Saikali FN, Goonewardene S, Needleman L, Finkel GC, Goldberg BB. Breast tumor vascularity identified by contrast enhanced ultrasound and pathology: initial results. Ultrasonics. 2000;38:105–109. doi: 10.1016/s0041-624x(99)00146-8. [DOI] [PubMed] [Google Scholar]
- de Jong JS, van Diest PJ, Baak JP. Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology. 2000;36:306–312. doi: 10.1046/j.1365-2559.2000.00850.x. [DOI] [PubMed] [Google Scholar]
- Fleicher AC. Sonographic depiction of tumor vascularity and flow from in vivo models to clinical applications. J Ultrasound Med. 2000;19:55–61. doi: 10.7863/jum.2000.19.1.55. [DOI] [PubMed] [Google Scholar]
- Ferrara KF, Merritt CRB, Burns PN, Foster S, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with US: imaging, Doppler and contrast agents. Acad Radiol. 2000;7:824–839. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Canc Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Dicker AP, Thakur ML, Rawool NM, Liu JB, Shi WT, Nazarian LN. Comparing contrast enhanced ultrasound to immunohistochemical markers of angiogenesis in a human melanoma xenograft model: preliminary results. Ultrasound Med Biol. 2002;28:445–451. doi: 10.1016/s0301-5629(02)00482-9. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Goldberg BB, Merritt CRB, Parker L, Maitino AJ, Palazzo JJ, Merton DA, Schultz SM, Needleman L. Diagnosing breast lesions with contrast-enhanced 3-dimensional power Doppler imaging. J Ultrasound Med. 2004;23:173–182. doi: 10.7863/jum.2004.23.2.173. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Liu JB, Burns PN, Merton DA, Goldberg BB. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med. 1994;13:357–365. doi: 10.7863/jum.1994.13.5.357. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Merton DA, Goldberg BB. In vivo destruction of ultrasound contrast microbubbles is independent of the Mechanical Index (letter) J Ultrasound Med. 2006;25:143–144. [PubMed] [Google Scholar]
- Forsberg F, Merton DA, Liu JB, Needleman L, Goldberg BB. Clinical applications of ultrasound contrast agents. Ultrasonics. 1998;36:695–701. doi: 10.1016/s0041-624x(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Shi WT, Merritt CRB, Dai Q, Solcova M, Goldberg BB. On the usefulness of the mechanical index displayed on clinical ultrasound scanners for predicting contrast microbubble destruction. J Ultrasound Med. 2005;24:443–450. doi: 10.7863/jum.2005.24.4.443. [DOI] [PubMed] [Google Scholar]
- Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995;13:765–782. doi: 10.1200/JCO.1995.13.3.765. [DOI] [PubMed] [Google Scholar]
- Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents: Basic Principles and Clinical Applications. 2. London, England: Martin Dunitz Ltd.; 2001. [Google Scholar]
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Canc Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- Hochmuth A, Boehm T, Bitzer C, Fleck M, Schneider A, Kaiser WA. Differentiation of breast masses using 3-D sonographic and echo-enhancer-based evaluation of the vascular pattern: initial experiences. Ultrasound Med Biol. 2002;28:845–851. doi: 10.1016/s0301-5629(02)00533-1. [DOI] [PubMed] [Google Scholar]
- Huber S, Vesely M, Zuna I, Delorme S, Czembirek H. Fibroadenomas: computer-assisted quantitative evaluation of contrast-enhanced power Doppler features and correlation with histopathology. Ultrasound Med Biol. 2001;27:3–11. doi: 10.1016/s0301-5629(00)00282-9. [DOI] [PubMed] [Google Scholar]
- Kedar RP, Cosgrove D, McCready VR, Bamber JC, Carter ER. Microbubbble contrast agent for color Doppler US: Effect on breast masses. Radiology. 1996;198:679–686. doi: 10.1148/radiology.198.3.8628854. [DOI] [PubMed] [Google Scholar]
- Kettenbach J, Helbich TH, Huber S, Zuna I, Dock W. Computer-assisted quantitative assessment of power Doppler US: effects of microbubble contrast agent in the differentiation of breast tumors. Eur J Radiol. 2005;53:238–244. doi: 10.1016/j.ejrad.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Kim SW, Park SS, Ahn SJ, Moon WK, Im JG, Yeo JS, Chung JK, Noh DY. Identification of angiogenesis in primary breast carcinoma according to the image analysis. Breast Cancer Res Treat. 2002;74:121–129. doi: 10.1023/a:1016150213253. [DOI] [PubMed] [Google Scholar]
- Kopans DB. Breast Imaging. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- Li WW. Tumor angiogenesis: molecular pathology, therapeutic targeting, and imaging. Acad Radiol. 2000;7:800–811. doi: 10.1016/s1076-6332(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Madjar H, Prompeler HJ, Del Favero C, Hackeloer BJ, Llull JB. A new Doppler signal enhancing agent for flow assessment in breast lesions. Eur J Ultrasound. 2000;12:123–130. doi: 10.1016/s0929-8266(00)00105-1. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Medina CJ, Bustos C, Hernandez JA. Assessment of breast lesions using Doppler with contrast agents. Eur J Gynaecol Oncol. 2003;24:527–530. [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir A, Kilic K, Özdemir H, Yucel C, Andac S, Colak M. Contrast-enhanced power Doppler sonography in breast lesions: effect on differential diagnosis after mammography and gray scale sonography. J Ultrasound Med. 2004;23:183–195. doi: 10.7863/jum.2004.23.2.183. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. Br Med J. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 3. Boston, MA: PWS-Kent Publishing Co.; 1990. [Google Scholar]
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- Stuhrmann M, Aronius R, Schietzel M. Tumor vascularity of breast lesions: potentials and limits of contrast-enhanced Doppler sonography. Am J Roentgenol. 2000;175:1585–1589. doi: 10.2214/ajr.175.6.1751585. [DOI] [PubMed] [Google Scholar]
- Tabár L, Dean PB. Mammography and breast cancer: the new era. Int J Gynaecol Obstet. 2003;82:319–326. doi: 10.1016/s0020-7292(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Taylor KJ, Merritt C, Piccoli C, Schmidt R, Rouse G, Fornage B, Rubin E, Georgian-Smith D, Winsberg F, Goldberg B, Mendelson E. Ultrasound as a complement to mammography and breast examination to characterize breast masses. Ultrasound Med Biol. 2002;28:19–26. doi: 10.1016/s0301-5629(01)00491-4. [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Yang WT, Tse GMK, Lam PKW, Metreweli C, Chang J. Correlation between color power Doppler sonographic measurement of tumor vasculature and immunohistochemical analysis of microvessel density for the quantitation of angiogenesis. J Ultrasound Med. 2002;21:1227–1235. doi: 10.7863/jum.2002.21.11.1227. [DOI] [PubMed] [Google Scholar]
- Zonderland HM, Coerkamp EG, Hermans J, van de Vijver MJ, van Voorthuisen AE. Diagnosis of breast cancer: contribution of US as an adjunct to mammography. Radiology. 1999;213:413–422. doi: 10.1148/radiology.213.2.r99nv05413. [DOI] [PubMed] [Google Scholar]


