Abstract
We recently found that the inhibitory effect of (−)-epigallocatechin gallate (EGCG) on epidermal growth factor (EGF) binding to the epidermal growth factor receptor (EGFR) is associated with alterations in lipid organization in the plasma membrane of colon cancer cells. Since changes in lipid organizations are thought to play a role in the trafficking of several membrane proteins, in this study we examined the effects of EGCG on cellular localization of the EGFR in SW480 cells. Treatment of the cells for 30 min with as little as 1 μg/ml of EGCG caused a decrease in cell surface-associated EGFRs and this was associated with internalization of EGFRs into endosomal vesicles. Similar effects were seen with a green fluorescent protein (GFP)–EGFR fusion protein. As expected, the EGFR protein was phosphorylated at tyrosine residues, ubiquitinated and partially degraded when the cells were treated with EGF, but treatment with EGCG caused none of these effects. The loss of EGFRs from the cell surface induced by treating the cells with EGF for 30 min persisted for at least 2 h. However, the loss of EGFRs from the cell surface induced by temporary exposure to EGCG was partially restored within 1–2 h. These studies provide the first evidence that EGCG can induce internalization of EGFRs into endosomes, which can recycle back to the cell surface. This sequestrating of inactivated EGFRs into endosomes may explain, at least in part, the ability of EGCG to inhibit activation of the EGFR and thereby exert anticancer effects.
Introduction
Green tea contains several polyphenolic compounds including the catechins (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin, epicatechin-3-gallate and (−)-epicatechin (EC). Among these constituents, EGCG is the major biologically active component. It has been shown to inhibit the growth of several types of cancer cell lines (1–4). This is associated with inhibition of phosphorylation at tyrosine residues (i.e. activation) of the epidermal growth factor receptor (EGFR) and subsequent inhibition of several downstream signaling pathways (5). EGCG can also inhibit activation of other receptor tyrosine kinases (RTKs), including HER2, HER3, HER4, insulin-like growth factor-I receptor (6,7), platelet-derived growth factor receptor (8), fibroblast growth factor (9) and vascular endothelial growth factor receptors (10). Although there is evidence that EGCG may directly interfere with binding of the ligand epidermal growth factor (EGF) to the EGFR (9), the ubiquitous effects of EGCG on several RTKs suggest that it might act on these plasma membrane-associated proteins by a more general mechanism.
It is well known that exposure of cells to EGF results in rapid autophosphorylation, including tyrosine (Tyr) 1045, and activation of EGFR molecules at the cell surface (11,12). The phosphorylation at Tyr 1045 provides a docking site for the ubiquitin ligase c-Cbl, resulting in ubiquitination of the EGFR and removed of the EGFR via endocytosis from the cell surface into an early endosomal compartment (13). G-protein coupled receptors, as well as other RTKs, are also downregulated after ligand activation (14,15). In general, ligand binding causes the respective cell surface receptor complexes to be selectively recruited into clathrin-coated pits (11,13). Following internalization, the ligand–receptor complexes enter endosomes, where the receptors and their ligands are sorted to various intracellular destinations. Thus, some receptors can be recycled back to the cell surface via early endosomes and others are targeted to late endosomes and lysosomes for proteolytic degradation. There is increasing evidence that receptor internalization acts not only to terminate signaling but that internalized endosome-associated receptors are also able to stimulate specific signal transduction pathways (16–18).
Recent studies indicate that several agents in addition to specific ligands can induce internalization of the EGFR (19–21). Thus, the 225 mouse antibody, which is known to block binding of specific ligands to the EGFR, induces internalization and downregulation of the EGFR via a mechanism distinct from that underlying EGF-induced EGFR internalization and downregulation (19). Oxidative stress, in the form of hydrogen peroxide, activates the EGFR but this is associated with negligible phosphorylation at Tyr 1045, the major docking site for the ubiquitin ligase c-Cbl (20). Thus, hydrogen peroxide-activated EGFRs fail to recruit the c-Cbl protein and, therefore, they do not undergo ubiquitination. This results in activated receptors uncoupled from the normal pathway of proteolytic downregulation. This mechanism enhances cell proliferation and may contribute to oxidant-mediated tumorigenesis (20). It was recently reported that tumor necrosis factor-α induces transient, ubiquitin-independent internalization of the EGFR, whereas ultraviolet irradiation causes persistent internalization of the EGFR (21). Thus, several agents, in addition to specific ligands, can induce internalization of the EGFR but they differ in their effects on the fate of the receptors, downstream signaling and cell proliferation.
The EGFR and several other RTKs are associated with detergent-insoluble ordered lipid domains in the plasma membrane, so-called ‘lipid rafts’, and there is evidence that these domains play critical roles in both the activation and the internalization of these receptors (22–24). In addition, studies in cells with the Niemann–Pick type C1 defect indicate that changes in lipid organization can alter lipid- and protein-trafficking pathways (25). We recently reported that EGCG inhibits the binding of EGF to EGFRs on the cell surface of human colon cancer cells, and also the subsequent dimerization and activation of the EGFR, and obtained evidence that these effects of EGCG are associated with an alteration in lipid organization in these cells (26). In the present study, we examined the effects of EGCG on cellular localization and internalization of the EGFR in SW480 colon cancer cells since, as mentioned above, lipid organization is thought to play a role in receptor internalization (24).
Materials and methods
Cell culture and chemicals
Unless indicated otherwise SW480 human colon cancer cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, San Diego, CA), containing 10% fetal bovine serum, as described previously in (26). EGCG, EC and polyphenon E were kindly provided by Dr Yukihiko Hara (Mitsui Norin Co., Shizuoka, Japan). All these compounds were solubilized in 100% dimethyl sulfoxide and used at a final concentration of 0.1% dimethyl sulfoxide. EGF, saponin, cycloheximide and monensin were purchased from Sigma Chemical Co. (St Louis, MO).
Quantification of cell surface EGFR by enzyme-linked immunosorbent assay
SW480 cells were seeded in 96-well plates (7 × 103 cells per well) in complete growth medium, and after 24 h, they were incubated in serum-free medium for an additional 24 h. Then the cells were exposed to the mouse anti-EGFR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) that recognizes the extracellular domain of the EGFR (1:50 dilution), in DMEM containing 1% bovine serum albumin (BSA), for 15 min at 37°C. The cells were then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C (see Figure 1A) and then fixed with 3% paraformaldehyde for 10 min on ice. After blocking with 1% BSA in phosphate-buffered saline for 1 h, the cells were exposed to an anti-mouse IgG, horseradish peroxidase-linked whole antibody (GE healthcare, Piscataway, NJ) for 1 h at room temperature, followed by washing four times with phosphate-buffered saline containing 1% BSA. In Figure 1B, the cells were first treated with EGF (100 ng/ml) or EGCG (20 μg/ml) with or without catalase (30 U/ml) or superoxide dismutase (SOD) (15 U/ml) for 30 min at 37°C. After fixation and blocking as described above, the cells were exposed to the mouse anti-EGFR antibody (1:100 dilution) for 1 h, followed by exposure to an anti-mouse IgG, horseradish peroxidase-linked whole antibody for 1 h at room temperature. Finally, the cells were exposed to 50 μl of 1-step™ Ultra TMB-ELISA reagent (Pierce, Rockford, IL) for 5 min at room temperature. Fifty microliter of 2 M sulfuric acid was then added to each well to stop the reaction. The absorbance of each sample at 450 nm was then measured.
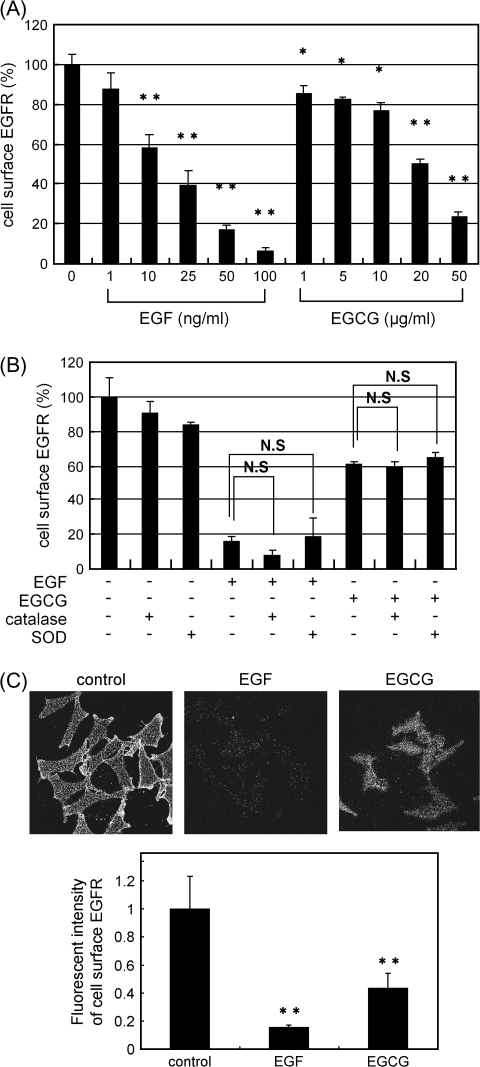
Fig. 1.
Both EGF and EGCG induce decreases in cell surface-associated EGFR. (A) SW480 cells were first labeled for 15 min at 37°C with an anti-EGFR antibody that recognizes the extracellular domain of the EGFR. The cells were treated with EGF or EGCG at the indicated concentration for 30 min at 37°C. The amount of cell surface EGFR was then measured by ELISA (see Materials and Methods). The asterisks indicate a significant decrease (*P < 0.05, **P < 0.01, respectively) with respect to the control (first lane on left). (B) The cells were treated with EGF (100 ng/ml) or EGCG (20 μg/ml) with or without catalase (30 U/ml) or SOD (15 U/ml) for 30 min at 37°C. They were then fixed, exposed to the anti-EGFR antibody and the amount of cell surface-associated EGFR was measured by ELISA as described in (A). NS designates no significant difference between the indicated pairs. (C) The fluorescent intensities of cell surface EGFR was analyzed in untreated cells or cells treated with 100 ng/ml of EGF or 20 μg/ml of EGCG by fluorescence microscopy and the fluorescent intensity of cell surface EGFR was quantitated by the MetaMorph. Representative results from at least three independent experiments are shown. For additional details see Materials and Methods.
Immunofluorescence microscopy studies
The live cells grown on coverslip-bottom dishes were first exposed to the mouse anti-EGFR antibody (1:50 dilution) that recognizes the extracellular domain of EGFR, in DMEM containing 1% BSA for 15 min at 37°C, and then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C, followed by fixation with 3% paraformaldehyde for 10 min on ice. The cells were then exposed to Alexa Fluor 488 goat anti-mouse IgG antibody, with or without 100 μg/ml of saponin to permeabilize the cell membrane, for 1 h at room temperature. Some dishes were exposed to acid-stripping buffer (27) (100 mmol/l glycine, 20 mmol/l magnesium acetate and 50 mmol/l KCl, pH 2.2) for 150 s at room temperature to remove anti-EGFR antibody bound to any remaining EGFR on the cell surface after treating the cells with EGF or EGCG. For colocalization study, after labeling with anti-EGFR antibody, the cells were fixed and exposed to 0.1% TX-100 to permeabilize the cell membrane for 10 min and then treated with anti-early endosome antigen-1 (EEA1) or anti-phosphorylated EGFR (Tyr 1045) antibody (Cell Signaling, Beverly, MA) (1:100 dilution) for 1 h. The cells were then exposed to Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 546 goat anti-mouse IgG antibody (1:500) and then examined by confocal microscopy.
Fluorescence microscopy
Confocal microscopy was performed using an Axiovert 100M inverted microscope equipped with a LSM 510 laser scanning unit and a ×63 1.4 NA plan Apochromat objective (Carl Zeiss, Inc., Boston, MA). Alexa 488-labeled EGFR was excited with an argon laser emitting at 488 nm, and a 505 nm long pass filter was used to monitor emissions. Quantitative image analyses for the amount of cell surface EGFR were performed using MetaMorph (Molecular Devices, Sunnyvale, CA). Wide field fluorescence microscopy images were obtained using a Leica DMIRB inverted microscope. The fluorescence intensity of cell surface EGFR-labeled Alexa 488 was measured using this MetaMorph software. BIOREVO (BZ-9000) (Keyence, Tokyo, Japan) was used for colocalization study. Image analysis for quantification of internalized EGFR was performed using Scion Image (Scion Corporation, Frederick, ML). Cell area was defined manually using differential interference image. Corresponding fluorescence image (8-bits) was thresholded at a gray value of 95, which is the half of averaged gray value of intracellular vesicles in all images. Then, the number of vesicles in the cell area was counted automatically using ‘analyze particle’ function in Scion Image.
Transfection with the GFP-fusion EGFR
The green fluorescent protein (GFP)-fusion EGFR plasmid was kindly provided by Dr W.J.Gullick (Cancer Biology, Department of Biosciences, University of Kent at Canterbury, Canterbury, UK). SW480 cells (1 × 106 cells/100 mmol/L diameter dish) were transfected with 10 μg of plasmid DNA using a Lipofectin reagent (Invitrogen) in opti-MEM 1 medium (Invitrogen) for 24 h. This medium was removed and the cells were then treated for 30 min with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C in serum-free DMEM and then examined by confocal microscopy.
Western blotting and immunoprecipitation assay
The cells were lysed and protein extracts were examined by western blot analysis as described previously (26). The antibodies used in these studies were anti-Ub, anti-EGFR (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phosphotyrosine (BD Biosciences, San Diego, CA) and anti-β-actin (Sigma). Anti-mouse IgG or anti-rabbit IgG antibodies were used as the secondary antibodies. For detection of immunoprecipitated EGFR, cell lysates (500 μg each) were incubated for 3 h at 4°C with an anti-EGFR antibody precoupled to anti-mouse IgG–agarose beads. Protein extracts were then eluted and examined by western blotting using the indicated antibody.
Assay for Alexa 488 EGF binding to the EGFR using flow cytometry
Prior to treatment, the cells were sparsely plated such that they were <50% confluent on the day of analysis. The cells were treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C in serum-free medium as indicated in Figure 4 and then exposed to the Alexa Fluor ® 488 EGF complex (Molecular Probe, Eugene, OR) (100 ng/ml) for 1 h at 4°C to prevent Alexa EGF-induced internalization of the EGFR. The cells were then washed with cold phosphate-buffered saline, harvested by the addition of 100% trypsin and gentle scraping at 4°C, followed by fixation with 3% paraformaldehyde for 10 min. The cells were then analyzed for cell surface-bound Alexa 488 EGF by flow cytometry using a FACS Calibur instrument (Becton Dickinson, Franklin Lakes, NJ). The data were analyzed using the CELL Quest computer program (Becton Dickinson).
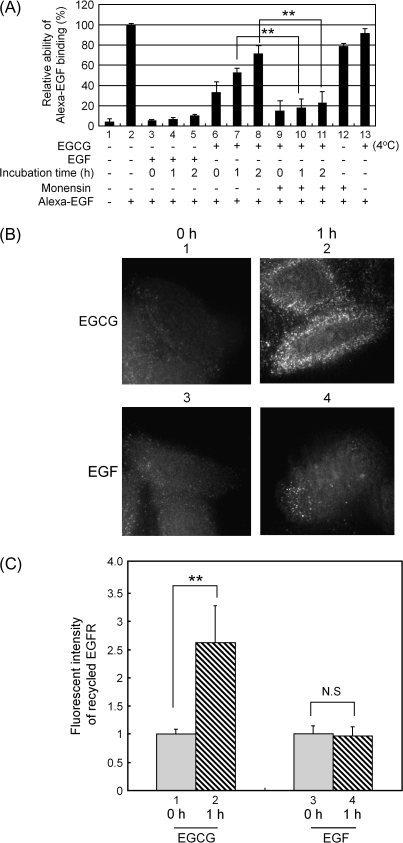
Fig. 4.
The EGFR that is internalized following transient treatment with EGCG can recycle back to the cell surface. (A) Quantification of cell surface EGFR using Alexa–EGF binding. The cells were treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C (lanes 1–12) or as a control they were incubated with EGCG at 4°C (lane 13). The medium was then changed and the cells were incubated in serum-free growth medium lacking EGF or EGCG at 37°C for 0, 1 or 2 h. They were then exposed to Alexa 488-conjugated EGF (100 ng/ml) for 1 h to label cell surface-associated EGFR. This was done at 4°C to prevent Alexa 488 EGF-induced internalization of the EGFR. As indicated in some assays, monensin (100 μM) was added at 15 min prior to the addition of EGF or EGCG to inhibit recycling of internalized vesicles. After the final incubation, the cells were washed with cold phosphate-buffered saline, harvested by the addition of trypsin and gentle scraping at 4°C and fixed with 3% paraformaldehyde for 10 min. They were then analyzed for cell surface-bound Alexa 488 EGF by flow cytometry. (B) Analysis of recycling of the EGFR by fluorescence microscopy. SW480 cells were first labeled for 15 min at 37°C with the anti-EGFR antibody that recognizes the extracellular domain of the EGFR for 15 min. They were then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C. Any antibody remaining on the cell surface was then removed by treatment with the acid-stripping buffer for 150 s (see Materials and Methods). The cells were then not further incubated (panels 1 and 3) or they were incubated (panels 2 and 4) for 1 h at 37°C in serum-free growth medium without the addition of EGF or EGCG. They were then fixed with paraformaldehyde and exposed to the Alexa 488-conjugated anti-mouse secondary antibody in the absence of saponin and then examined by fluorescence microscopy. (C) Quantification of the amount of the EGFR that was recycled back to cell surface based on the fluorescent intensities in (B) which were analyzed by the MetaMorph. **P < 0.01, significant difference obtained by a comparison between indicated pairs. NS designates no significant difference obtained by a comparison between indicated pairs. Representative results from at least three independent experiments are shown.
Results
EGCG causes a decrease in the level of cell surface-associated EGFR
In our initial studies, we examined whether treatment of SW480 cells with EGCG might affect the amount of cell surface-associated EGFR that is available for binding to EGF. Cell surface-associated EGFRs were first tagged with an anti-EGFR antibody that recognizes the extracellular domain of EGFR. Then the cells were treated with increasing concentrations of EGF (0–100 ng/ml) or EGCG (1–50 μg/ml) for 30 min at 37°C, and the residual level of cell surface-associated antibody-tagged EGFR was determined using an enzyme-linked immunosorbent assay (ELISA) (see Materials and Methods). As shown in Figure 1A, with increasing concentrations of EGF, there was a dose-dependent reduction in cell surface-associated EGFR. Treatment with EGCG for only 30 min also caused a dose-dependent reduction in cell surface-associated EGFR. A significant effect was seen with as little as 1 μg/ml (∼2.2 μM) of EGCG. Treatment of the cells with polyphenon E, a mixture of catechins (26), had a similar effect but EC that lacks biologic effects was inactive in this assay and a time course study indicated that 20 μg/ml of EGCG caused a significant reduction of cell surface-associated EGFR within 5 min (data not shown). We also performed a modification of the above ELISA experiment in which the cells were first treated with EGF or EGCG. This was followed by fixation, and the cells were then exposed to the anti-EGFR antibody for 1 h, (Figure 1B). The results were similar to those obtained in Figure 1A. EGCG did not affect the amount of cell surface EGFR when the cells were treated with EGCG at 4°C (data not shown). In subsequent studies, we used 100 ng/ml EGF to maximize its effects on the EGFR. This concentration has been used in previous cell culture studies (28). We used 20 μg/ml of EGCG since this caused ∼50% loss of cell surface EGFR (Figure 1A) and ∼50% growth inhibition of SW480 cells (S. Adachi and I.B. Weinstein, unpublished data). Some of the effects of EGCG, especially in cell culture systems, may be due to the generation of reactive forms of oxygen (29,30). Therefore, we repeated some of these ELISA assays in the presence of either SOD or catalase (Figure 1B). It is apparent that neither SOD nor catalase had an appreciable effect on the ability of either EGF (100 ng/ml) or EGCG (20 μg/ml) to cause a decrease in cell surface levels of the EGFR.
To verify the above results obtained with the ELISA assay, we also assessed levels of cell surface-associated EGFR by immunofluorescence microscopy. (see Materials and Methods). The upper panels in Figure 1C display representative immunofluorescence microscopy images of the cells and the bar graph provides quantification of the cell surface immunofluorescence obtained by the MetaMorph analysis. We found that treatment of the cells with 100 ng/ml of EGF for 30 min caused about an 80% reduction and treatment with 20 μg/ml of EGCG for 30 min caused about a 60% reduction in cell surface-associated EGFR. These results confirm those obtained with the ELISA method (Figure 1A). Taken together, these findings suggested that EGCG might induce a change in cellular localization of EGFR, cause rapid degradation of the cell surface-associated EGFR or disrupt the binding of the anti-EGFR antibody to EGFR, although the latter possibility is unlikely, in view of the results obtained in Figure 1B and results described below.
EGCG causes internalization of the EGFR
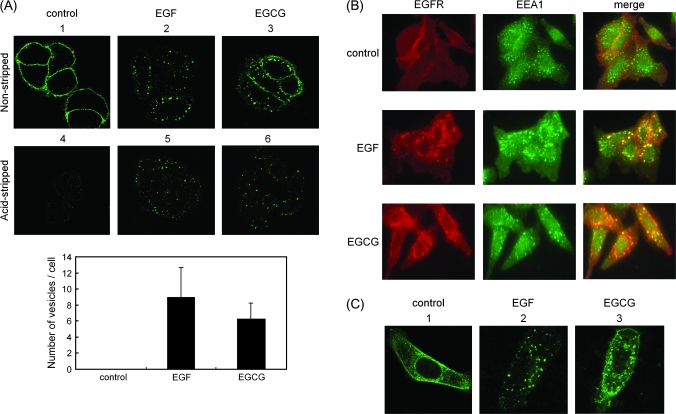
It is well known that EGF induces internalization of the EGFR via endocytosis and that this is associated with subsequent ubiquitin-mediated degradation of the EGFR (13). Therefore, we examined whether EGCG also induces changes in the cellular localization of the EGFR using confocal fluorescence microscopy and described in Materials and Methods. Figure 2A, panel 1, indicates that when the cells were not treated with EGF or EGCG, this procedure resulted in fluorescence staining of only the cell surface (Figure 2A, panel 1). However, treatment of the cells with EGF (100 ng/ml) for 30 min resulted in extensive internalization of the tagged EGFR into vesicles located beneath the plasma membrane (Figure 2A, panel 2). Treatment of the cells with EGCG (20 μg/ml) for 30 min also resulted in redistribution of the antibody-tagged EGFR to vesicles beneath the plasma membrane (Figure 2A, panel 3). To confirm that these vesicles were actually inside the cells, just prior to fixation the cells were briefly exposed to acid-stripping buffer to dissociate the bound antibody from the cell surface-associated EGFR. In the untreated control cells, this removed virtually all the cell surface fluorescence (Figure 2A, panel 4). However, the cells treated with EGF (Figure 2A, panel 5) and the cells treated with EGCG (Figure 2A, panel 6) retained the fluorescent vesicles, indicating that these vesicles were indeed inside the cells. Quantification analysis revealed that EGCG as well as EGF caused a marked increase in the number of vesicles in cells with respect to the control cells (Figure 2A lower bar graph). We next performed a colocalization study of the internalized EGFRs with the early endosome marker EEA1 to see whether there are differences in the endocytic pathways (Figure 2B) and found that the internalized EGFR induced by either EGF or EGCG clearly colocalized with EEA1, suggesting that the mechanisms of endocytosis are similar, at least at the stage of early endosomes, with respect to both agents.
Fig. 2.
Both EGF and EGCG cause internalization of the EGFR. (A) SW480 cells were first labeled for 15 min at 37°C with an anti-EGFR antibody that recognizes the extracellular domain of the EGFR. They were then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C, followed by fixation with paraformaldehyde. The fixed cells were then exposed to an Alexa 488-conjugated anti-mouse secondary antibody (green signal in upper panels 1–6), in the presence of 100 μg/ml of saponin to permeabilize the cells, to label the antibody–EGFR complex, and then examined by confocal microscopy (panels 1–3). In a second study (panels 4–6), the cells were treated as described above but they were exposed to acid-stripping buffer for 150 s before the fixation step (see Materials and Methods) to remove cell surface-associated EGFR antibody. Lower bar graph shows quantification data of the number of vesicles of the internalized EGFR shown in upper panels 4–6 (see details in Materials and Methods). (B) The cells were first labeled for 15 min at 37°C with an anti-EGFR antibody. They were then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C and then fixed with paraformaldehyde. After permeabilization of the cells with 0.1% Triton X-100, the cells were exposed to anti-EEA1 antibody (1:100 dilution) for 1 h and then treated with Alexa 546-conjugated anti-mouse secondary antibody for EGFR (red signal) and Alexa 488-conjugated anti-mouse secondary antibody for EEA1 (green signal). They were then examined by confocal microscopy. (C) SW480 cells were transfected for 24 h with a plasmid encoding EGFR–GFP, prior to stimulation with the indicated compound. Thirty minutes after the addition of EGF (100 ng/ml) or EGCG (20 μg/ml) at 37°C, followed by fixation with paraformaldehyde and then examined by confocal microscopy. Representative results from at least three independent experiments are shown.
To extend these studies, we transfected an EGFR–GFP fusion protein into SW480 cells and then GFP fluorescence was monitored by confocal fluorescence microscopy to track the cellular localization of EGFR. When the transfected cells were grown in serum-free medium in the absence of EGF, the EGFR–GFP protein was detected on the cell surface and there was also some diffuse staining within the cell (Figure 2C, panel 1). Treatment with EGF (100 ng/ml) for 30 min resulted in the appearance of prominent intracellular fluorescent vesicles (Figure 2C, panel 2), which is consistent with previous studies utilizing EGFR–GFP (31). Treatment with EGCG (20 μg/ml) for 30 min also caused the formation of prominent intracellular fluorescent vesicles (Figure 2C, panel 3), thus confirming the finding in Figure 2A that treatment of SW480 cells with EGCG causes internalization of EGFR into vesicles. The results obtained with EGFR–GFP also indicate that the findings shown in Figure 2A are not simply an artifact introduced by tagging the EGFR with an antibody.
In contrast to the effect of EGF, EGCG does not cause ubiquitination, tyrosine phosphorylation or degradation of the EGFR
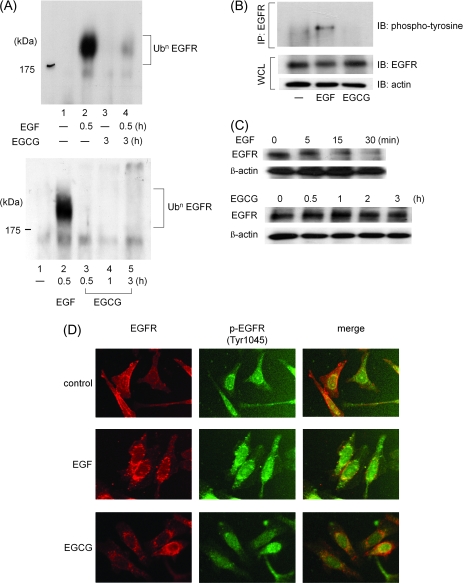
There is extensive evidence that following their activation by the respective ligand RTKs, including EGFR, undergo ligand-dependent ubiquitination (13,32,33). This ubiquitination provides a signal for endocytosis of RTKs and triggers their ubiquitin-mediated lysosomal degradation, thus causing downregulation of activated RTKs (32). Since we found that EGCG can cause endocytosis of the EGFR (Figure 2), we next examined whether or not EGCG induces ubiquitination of the EGFR (Figure 3A). These assays were done in the presence of the proteasome inhibitor MG132 to block the degradation of the ubiquitinated EGFR. We found that although 100 ng/ml of EGF induced, within 30 min, strong ubiquitination of the EGFR (Figure 3A, upper and lower panel lane 2, respectively), EGCG alone did not cause ubiquitination of the EGFR even when the cells were treated with EGCG for 0.5, 1 or 3 h (Figure 3A, lower panel lanes 3–5). Moreover, pretreatment with EGCG for 3 h inhibited EGF-induced ubiquitination of the EGFR (Figure 3A, upper panel lane 4), presumably because EGCG inhibits activation of the EGFR induced by EGF (26). We next performed immunoprecipitation–western blotting assays using an anti-phosphotyrosine antibody. As expected, we found that after treatment for 30 min, EGF (100 ng/ml) caused tyrosine phosphorylation of the EGFR, but EGCG (20 μg/ml) did not cause tyrosine phosphorylation of the EGFR (Figure 3B).
Fig. 3.
In contrast to the effects of EGF, EGCG does not cause ubiquitination and subsequent degradation of the EGFR. (A) Ubiquitination of the EGFR. The cells were pretreated with the proteasome inhibitor MG132 (10 μM) for 6 h and then exposed to EGF (100 ng/ml) for last 30 min or EGCG (20 μg/ml) for 0.5, 1 or 3 h at 37°C. Then, cell lysates (500 μg each) were prepared and incubated for 3 h at 4°C with an anti-EGFR antibody precoupled to anti-mouse IgG–agarose beads. The bound protein was then analyzed by western blotting with an anti-Ub antibody. (B) Phosphorylation at tyrosine residues of the EGFR. The cells were exposed to EGF (100 ng/ml) for 30 min or EGCG (20 μg/ml) for 30 min at 37°C. Each cell lysates were then prepared and incubated for 3 h at 4°C with an anti-EGFR antibody precoupled to anti-mouse IgG–agarose beads. The bound proteins were then analyzed by western blotting with an anti-phosphotyrosine antibody. The lower two panels indicate the corresponding whole cell lysates. (C) Rate of de novo proteolysis of the EGFR. The cells were treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for the indicated time in the presence of cycloheximide (10 μg/ml) to block new protein synthesis. Protein extracts were prepared and examined by western blotting using an anti-EGFR antibody. An antibody to β-actin was used to control for protein loading. (D) Phosphorylation of the internalized EGFR on Tyr 1045 residues. The cells were first labeled for 15 min at 37°C with an anti-EGFR antibody. They were then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C and then fixed with paraformaldehyde. After permeabilization of the cells with 0.1% Triton X-100, the cells were exposed to anti-phosphorylated EGFR (Tyr 1045) antibody for 1 h and then exposed to Alexa 546-conjugated anti-mouse secondary antibody for EGFR (red signal) and Alexa 488-conjugated anti-mouse secondary antibody for phosphorylated EGFR (Tyr 1045) (green signal). They were then examined by confocal microscopy. Representative results from at least three independent experiments are shown.
We next performed western blot analysis using an anti-EGFR antibody to examine the effects of treating SW480 cells with EGF or EGCG on degradation of the EGFR. The cells were treated in the presence of cycloheximide to block de novo synthesis of EGFR (Figure 3C). Treatment with EGF (100 ng/ml) resulted in extensive degradation of total cellular EGFR within 30 min (Figure 3C, upper panel). However, treatment with EGCG (20 μg/ml) did not cause degradation of the EGFR even after 3 h (Figure 3C, lower panel). These results are consistent with the ubiquitination results shown in Figure 3A. Taken together, these findings indicate that, in contrast to the effects seen with EGF, the internalization of EGFR by endocytosis that is induced by EGCG is not associated with ubiquitin-mediated degradation of the EGFR, at least within the first few hours.
Using an antibody specific for the phosphorylated (i.e. activated) form of EGFR and immunofluorescence microscopy, we found that, whereas the EGFR that was internalized after treatment of the cells with EGF for 30 min was phosphorylated on Tyr 1045, the EGFR that was internalized after treatment of the cells with EGCG was not phosphorylated on this residue (Figure 3D). These results are consistent with our western blot studies, described above (Figure 3B), indicating that EGCG does not induce phosphorylation of the EGFR at tyrosine residues.
The EGFR that is internalized following treatment of SW480 cells with EGCG can recycle back to the cell surface
Since the above studies indicated that the EGFR that is internalized in vesicles when SW480 cells were treated with EGCG was not ubiquitinated or degraded, it was of interest to follow the subsequent fate of these receptors. In the studies described in Figure 4A, the abundance of cell surface EGFRs was determined by the extent of binding of Alexa–EGF to intact cells, using flow cytometry (see Materials and Methods). The value obtained in untreated cells was expressed as 100% (Figure 4A, lane 2). After treating the cells with EGF (100 ng/ml) for 30 min, there was a 95% reduction in Alexa–EGF binding (Figure 4A, lane 3) and this marked reduction persisted for up to 2 h (Figure 4A, lanes 4 and 5). Presumably, this reflects the ubiquitin-mediated degradation of the EGFR induced by EGF (see Figure 3). When the cells were treated with EGCG (20 μg/ml) for 30 min, there was about a 70% reduction in Alexa–EGF binding (Figure 4A, lane 6), but after a 1 h incubation in serum-free growth medium lacking added EGCG this reduction was only 50% (Figure 4A, lane 7) and after 2 h it was only 30% (Figure 4A, lane 8). This apparent reappearance of EGFRs at the cell surface after treating the cells with EGCG was inhibited by treating the cells with monensin (Figure 4A, lanes 9–11), a known inhibitor of endosome recycling (21). Taken together, these results suggest that with the passage of time (1–2 h) the EGFRs that are initially internalized in vesicles in EGCG-treated cells can recycle back to the cell surface. In the same study, we found that when cells were incubated with EGCG (20 μg/ml) for 30 min at 4°C (Figure 4A, lane 13) rather than 37°C (lane 6) there was no reduction in Alexa–EGF binding. This indicates that EGCG does not act simply by physically preventing the binding of Alexa–EGF to the EGFR. Presumably, the requirement for incubation at 37°C reflects the active process of receptor internalization.
To confirm our evidence for recycling of the EGFR in EGCG-treated cells, we performed immunofluorescence microscopy studies (Figure 4B and C). SW480 cells were first labeled at 37°C with the anti-EGFR antibody that recognizes the extracellular domain of the EGFR and then treated with EGF (100 ng/ml) or EGCG (20 μg/ml) for 30 min at 37°C to cause internalization of the antibody-tagged EGFR. Residual antibody remaining on the cell surface was removed by acid stripping. Some of these cells were not further incubated and others were incubated for 1 h at 37°C in serum-free growth medium lacking added EGF or EGCG. The cells were then fixed and exposed to the Alexa 488-conjugated anti-mouse secondary antibody to highlight any previously tagged EGFR that appeared at the cell surface. Figure 4B displays representative immunofluorescence microscopy images of the cells and Figure 4C quantification of the cell surface immunofluorescence by the MetaMorph analysis. It is apparent that in the cells treated with EGF and then incubated for 1 h there was no significant increase in cell surface-associated EGFR (Figure 4C, lanes 3 and 4). However, in the cells pretreated with EGCG after 1 h there was a 2–3 fold increase in cell surface EGFR (Figure 4C, lanes 1 and 2). These results confirm those described in Figure 4A and provide strong evidence that the EGFR that is internalized when cells are treated with EGCG can recycle back to the cell surface, presumably via the vesicles displayed in Figure 2A. However, the EGFR that is internalized in the cells treated with EGF cannot recycle back to the cell surface, presumably because it undergoes degradation via an ubiquitin-mediated pathway (Figure 3). We should emphasize that in the studies described in Figure 4A and B after the cells were exposed to EGCG for 30 min, the medium was changed and the cells were then incubated for an additional 1 or 2 h in the absence of EGCG. If the cells were further incubated in the presence of EGCG, we did not observe recycling of the EGFR presumably due to the continued action of EGCG or its derivatives (S. Adachi and I.B. Weinstein, unpublished data).
Discussion
In 1997, Liang et al. (9) reported that EGCG inhibits activation of the EGFR in A431 cells suggesting that this natural product might exert its anticancer effects, at least in part, by blocking the function of this RTK in cancer cells. Since then, several studies have explored the mechanism responsible for this effect and the resulting effects on downstream signaling pathways (1,4,5,26,34). In addition, and as indicated in the Introduction, subsequent studies have shown that EGCG can also inhibit the activation of several other RTKs in various types of cancer cells (1,2,4,6–9). With respect to mechanism, it was found that in a subcellular system EGCG can inhibit the kinase activity of the EGFR in a cell extract (9). However, more detailed studies have not been done with a pure recombinant EGFR protein. Furthermore, in the present study, we found that EGCG did not inhibit binding of Alexa-labeled EGF to the EGFR in intact cells when assays were done at 4°C rather than at 37°C (Figure 4A). This suggests that EGCG is not simply competing with EGF for physical binding to the EGFR, and that a metabolic process is required for EGCG to inhibit EGF–EGFR binding.
The present study provides the first evidence that the ability of EGCG to inhibit binding of EGF to the EGFR, to inhibit activation of the EGFR by EGF and to thereby inhibit EGFR-related downstream signaling pathways (5) is due at least in part to the ability of EGCG to induce internalization of EGFR molecules via endocytosis. Presumably, this sequesters EGFRs within cells so that they are unavailable at the cell surface for activation by EGF or other EGFR ligands. In these studies, we used two different methods, an ELISA assay and confocal microscopy to demonstrate that EGCG caused a decrease in cell surface-associated EGFRs in SW480 cells (Figure 1A and C). Similar effects of EGCG were seen with HT29 colon cancer cells and A431 epidermoid cancer cells (S. Adachi and I.B. Weinstein, unpublished data) indicating that these results are not confined to a specific cell line. This effect, occurred within 30 min after exposure of the cells to EGCG, was dose dependent and was seen with as little as 1 μg/ml (∼2.2 μM) of EGCG (Figure 1A). On the other hand, EC, which lacks biologic activity, was inactive in these assays (data not shown). Furthermore, assays done in the presence of SOD or catalase provide evidence that this effect of EGCG is not secondary to the generation of reactive forms of oxygen (Figure 1B).
It is well established that when cells are treated with EGF, this results in rapid internalization of EGFRs into early endosomes (13). Using antibody-tagged EGFRs and immunofluorescence confocal microscopy, we obtained similar results when SW480 cells were treated with EGF for 30 min (Figure 2A). When the cells were treated with EGCG for 30 min, the above-described loss of cell surface-associated EGFRs (Figure 1) was associated with the appearance of numerous EGFR-containing vesicles within the cells (Figure 2A). These internalized EGFR induced by either EGF or EGCG colocalized with the early endosome marker EEA1, suggesting that the mechanisms of endocytosis induced by both agents are similar, at least at the stage of early endosomes (Figure 2B). Using SW480 cells transfected with an EGFR–GFP fusion protein, we confirmed that treatment of the cells with either EGF or EGCG caused, within 30 min, the appearance of numerous intracellular EGFR-containing vesicles (Figure 2C). Additional studies indicated that although both EGF and EGCG caused rapid internalization of EGFRs into endosomal vesicles, there were major differences between these two agents with respect to their effects on the EGFR. Thus, treatment of cells with EGF led to phosphorylation of the EGFR on Tyr 1045 (Figure 3D), as expected, but treatment with EGCG did not cause detectable phosphorylation of the EGFR at any tyrosine residues (Figure 3B and D). In addition, treatment with EGF caused ubiquitination of the EGFR, as expected (11–13), but treatment with EGCG did not have this effect (Figure 3A and B). Furthermore, treatment of the cells with EGF, but not EGCG, markedly enhanced the proteolytic degradation of cellular EGFRs (Figure 3C). These findings are consistent with evidence that EGF stimulates autophosphorylation of the EGFR, including Tyr 1045, and that this provides a docking site for the ubiquitin ligase c-Cbl, which then mediates the subsequent ubiquitin-mediated degradation of EGFRs (13). Since treatment with EGCG does not activate the EGFR and does not cause phosphorylation at tyrosine residues, including Tyr 1045 (Figure 3B and D), presumably there is no docking site for c-Cbl. Therefore, the ubiquitin-mediated pathway of degradation of the EGFR is not activated in EGCG-treated cells.
In view of the fact that when SW480 cells were treated with EGCG EGFRs were internalized into endosomal vesicles but the EGFRs were not degraded, we examined the subsequent fate of these molecules (Figure 4). As a control, we first examined the effects of treating the cells with EGF. As in Figure 1, treatment of the cells with 100 ng/ml of EGF led to almost a complete loss of cell surface-associated EGFRs and this decrease persisted even when the cells were subsequently incubated for 1 or 2 h in the absence of EGF (Figure 4, lanes 3–5). On the other hand, the decreased cell surface level of EGFRs caused by treatment with EGCG was partially restored when the treated cells were further incubated for 1 or 2 h in the absence of EGCG (Figure 4A, lanes 6–8). This reappearance of EGFRs at the cell surface was inhibited by monensin, which inhibits endosome recycling (Figure 4A, lanes 9–11). Confocal immunofluorescence microscopy studies employing EGFRs pretagged with an antibody (Figure 4B and C) confirmed the results obtained in Figure 4A. Furthermore, since in the latter study the original cell surface EGFRs were pretagged with an antibody, the reappearance of EGFRs at the cell surface is not due to the de novo synthesis of EGFR molecules. Taken together, these findings indicate that the EGFR molecules that are internalized in cells treated with EGCG can be recycled back to the cell surface. This finding is reminiscent of a previous study indicating that treatment of cells containing a kinase-negative EGFR with EGF causes internalization of the mutant EGFR but this protein is not degraded and can recycle back to the cell surface (35). We found that if the medium containing EGCG was not changed, we did not observe recycling of the EGFR (data not shown), presumably due to the continued action of EGCG or its derivatives. Therefore, recycling of the EGFR could present a limitation in the clinical use of EGCG as an anticancer agent, if tumor tissue levels of EGCG are not continuously maintained at a sufficient level. During the course of these studies, Mizuno et al. (36) published evidence that theaflavin-3-3′-digallate, a compound present in black tea, also induces internalization of the EGFR in A431 cells and JB6C141 cells. In contrast to the results, we obtained with EGCG, they found that theaflavin-3-3′-digallate also caused transient tyrosine kinase activation of the EGFR, as well as ubiquitination and downregulation of the EGFR (36). With prolonged exposure to EGCG, we have also seen downregulation, i.e. loss, of the EGFR protein in SW480 cells (S. Adachi and I.B. Weinstein, unpublished data).
Our findings with EGCG are consistent with the increasing evidence that a variety of agents, in addition to specific ligands like EGF and transforming growth factor-α, can induce internalization of EGFRs into endosomal vesicles. These agents include an antibody to the EGFR (19), oxidative stress (20), tumor necrosis factor-α (21), ultraviolet irradiation (21), gemcitabine (37) and cisplatin (28). The EGFR internalization process induced by EGCG described in the present study appears to differ in several respects from that induced by these agents, although this aspect requires further study. Since the effects of EGCG were not inhibited by SOD or catalase (Figure 1B) and the internalization of the EGFR induced by oxidative stress is associated with phosphorylation and activation of EGFR, which is not the case with EGCG, the effects of EGCG on the EGFR described in this study are not simply due to oxidative stress. Further studies are required to determine the protein components of the EGFR-containing endosomal particles induced by EGCG.
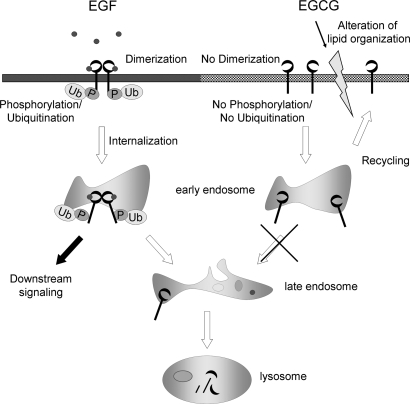
Hypothetical models that compare the internalization of the EGFR induced by EGF and by EGCG are shown in Figure 5. A major unsolved problem is the mechanism by which EGCG induces internalization of EGFRs into endosomal vesicles. This may be related to our previous finding that EGCG causes a rapid alteration of lipid organization in the plasma membrane of colon cancer cells (26), since this might trigger aberrant internalization of EGFRs. This association is speculative and further studies are required to determine the precise mechanism. Nevertheless, the present studies describe a unique cellular effect of EGCG. Additional studies are required to determine whether aberrant internalization of the EGFR plays an important role in the in vivo anticancer effects of EGCG and related compounds.
Fig. 5.
A schematic diagram of the mechanism by which the EGF causes activation and internalization of the EGFR, and a hypothetical mechanism by which EGCG causes internalization of the EGFR. After EGF binds to EGFR molecules on the cell surface, the receptor undergoes dimerization and autophosphorylation at tyrosine residues, and this triggers EGFR-related downstream signaling. The EGFR is also ubiquitinated and internalized into early endosomes that are EEA1 positive, which become late endosomes, and eventually the receptors are degraded in lysosomes. By contrast, when cells are treated with EGCG, the receptor is not dimerized (26), autophosphorylated or ubiquitinated. However, EGFR molecules are also internalized into EEA1-positive early endosomes, perhaps because of EGCG-induced alterations in lipid organization (26), and they are not degraded at early time point. With time, these EGFR molecules can be recycled back to the cell surface.
Funding
Entertainment Industry Foundation's-National Colorectal Cancer Research Alliance; T.J. Martell Foundation; National Foundation for Cancer Research and Polyphenon E International to I.B.W.; National Institutes of Health (DK27083) to F.R.M.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle’ medium
- EEA1
early endosome antigen-1
- EC
epicatechin
- EGCG
epigallocatechin gallate
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein
- RTK
receptor tyrosine kinase
- SOD
superoxide dismutase
References
- 1.Masuda M, et al. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 2.Pianetti S, et al. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62:652–655. [PubMed] [Google Scholar]
- 3.Paschka AG, et al. Induction of apoptosis in prostate cancer cell lines by the green tea component, (-)-epigallocatechin-3-gallate. Cancer Lett. 1998;130:1–7. doi: 10.1016/s0304-3835(98)00084-6. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M, et al. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 5.Khan N, et al. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, et al. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, et al. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 8.Sachinidis A, et al. Green tea compounds inhibit tyrosine phosphorylation of PDGF beta-receptor and transformation of A172 human glioblastoma. FEBS Lett. 2000;471:51–55. doi: 10.1016/s0014-5793(00)01360-0. [DOI] [PubMed] [Google Scholar]
- 9.Liang YC, et al. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, et al. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Waterman H, et al. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 13.Massie C, et al. The developing role of receptors and adaptors. Nat. Rev. Cancer. 2006;6:403–409. doi: 10.1038/nrc1882. [DOI] [PubMed] [Google Scholar]
- 14.Avrov K, et al. The role of c-Src in platelet-derived growth factor alpha receptor internalization. Exp. Cell Res. 2003;291:426–434. doi: 10.1016/j.yexcr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Kallal L, et al. Visualization of agonist-induced sequestration and down-regulation of a green fluorescent protein-tagged beta2-adrenergic receptor. J. Biol. Chem. 1998;273:322–328. doi: 10.1074/jbc.273.1.322. [DOI] [PubMed] [Google Scholar]
- 16.Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 17.Di Fiore PP, et al. Endocytosis and signaling: an inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- 18.McPherson PS, et al. Signaling on the endocytic pathway. Traffic. 2001;2:375–384. doi: 10.1034/j.1600-0854.2001.002006375.x. [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo ML, et al. Effect of the anti-receptor ligand-blocking 225 monoclonal antibody on EGF receptor endocytosis and sorting. Exp. Cell Res. 2006;312:2778–2790. doi: 10.1016/j.yexcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Khan EM, et al. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J. Biol. Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 21.Zwang Y, et al. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pike LJ, et al. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J. Biol. Chem. 2005;280:26796–26804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang L, et al. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 24.Puri C, et al. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol. Biol. Cell. 2005;16:2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pipalia NH, et al. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 26.Adachi S, et al. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 27.Naslavsky N, et al. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winograd-Katz SE, et al. Cisplatin induces PKB/Akt activation and p38(MAPK) phosphorylation of the EGF receptor. Oncogene. 2006;25:7381–7390. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- 29.Yang GY, et al. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 30.Yang GY, et al. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 31.Hayes N, et al. Green fluorescent protein as a tool to study epidermal growth factor receptor function. Cancer Lett. 2004;206:129–135. doi: 10.1016/j.canlet.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Haglund K, et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 33.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CS, et al. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2006;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- 35.Felder S, et al. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno H, et al. Theaflavin-3, 3′-digallate induces epidermal growth factor receptor downregulation. Mol. Carcinog. 2006;45:204–212. doi: 10.1002/mc.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng FY, et al. Role of epidermal growth factor receptor degradation in gemcitabine-mediated cytotoxicity. Oncogene. 2007;26:3431–3439. doi: 10.1038/sj.onc.1210129. [DOI] [PubMed] [Google Scholar]