Abstract
Tissue transglutaminase (TG2), an enzyme involved in protein cross-linking and overexpressed in ovarian tumors, has antiapoptotic effects in cancer cells and may play a role in response to chemotherapy. In this study, we investigated the role of TG2 in the sensitivity of ovarian cancer cells to cisplatin. By using stable knockdown and overexpression strategies, we demonstrate that the level of expression of TG2 regulates apoptosis induced by cisplatin in SKOV3 and OV-90 ovarian cancer cells. Interestingly, not only TG2 knockdown but also a TG2 enzymatic inhibitor (KCC009) sensitized SKOV3 cells to cisplatin. To understand the mechanism by which TG2 exerts its antiapoptotic role, we examined the effects of protein kinase B (Akt) and nuclear factor-kappa B (NF-κB), two survival pathways commonly involved in development of drug resistance. Overexpression of the constitutively active p65 subunit of NF-κB, but not constitutively active Akt, rescued cells with diminished TG2 expression from cisplatin-induced apoptosis. This implicates activation of NF-κB as the main cisplatin resistance mechanism downstream of TG2. Indeed, NF-κB activity is decreased and the level of the inhibitory subunit IκBα is increased in ovarian cancer cells engineered to express diminished levels of TG2 or treated with the enzymatic inhibitor, KCC009. Our data show that TG2 prevents apoptosis induced by cisplatin by activating the NF-κB survival pathway in ovarian cancer cells.
Introduction
Cisplatin, a DNA cross-linking agent, is the first line and mainstay of therapy for epithelial ovarian cancer (EOC) (1). Initially, most tumors and ovarian cancer cell lines are cisplatin sensitive, but invariably, after repeated exposure, drug resistance develops, limiting clinical outcome. Much effort has been directed to understand the mechanisms involved in cisplatin resistance (2). It is becoming accepted that one of the important causes of cisplatin resistance relates to aberrant functioning of the apoptotic machinery in cancer cells (3–5), with both protein kinase B (Akt)- and nuclear factor-kappa B (NF-κB)-regulated survival pathways being implicated in acquired EOC cisplatin resistance (4–6). In this study, we investigated whether tissue transglutaminase (TG2), an enzyme implicated in regulation of apoptosis and overexpressed in ovarian cancer cells (7), plays a role in this process.
TG2 cross-links proteins by acyl transfer between glutamine and lysine residues and participates in Ca++-dependent post-translational protein modification by incorporating polyamines into peptide chains (8). The enzyme has been linked to apoptosis, acting either as a promoter or as an antagonist, through mechanisms that are specific to different cellular contexts. In physiological conditions, the intracellular enzymatic activity of TG2 is negatively regulated by low concentrations of Ca2+ and high level of guanosine triphosphate. However, in the late phases of apoptosis, when massive intracellular Ca2+ influx occurs, the enzymatic function of TG2 is activated leading to cross-linking of cytosolic proteins and finalization of the cell death process (9). Recently identified, a pro-apoptotic TG2 isoform (TGase-S), lacking the 3′ C-terminal end, has been implicated in cell death (10). Interestingly, this isoform, inducible by tumor necrosis factor α and detectable in brain tissue from patients with Alzheimer disease, promotes apoptosis through formation of large-size oligomers, which are toxic to the cell. In contrast, TG2 has an antiapoptotic role in malignant cells. TG2 is overexpressed in epithelial cancers, such as pancreatic (11), breast (12) and non-small cell lung cancer (13) and its antiapoptotic role involves different mechanisms. For instance, in breast and pancreatic cancer cells, TG2 activates NF-κB by cross-linking the inhibitory subunit inhibitor of kappa B α (IκBα). This leads to its polymerization and displacement out of the complex with NF-κB (14,15). This process depends on TG2′s enzymatic activity and leads to constitutive activation of NF-κB. In leukemia HL60 cells, TG2-mediated transamidation protects the retinoblastoma gene product from caspase-induced degradation and promotes cell survival (16). In colon cancer cells HCT116, TG2 suppresses apoptosis by protecting the cleavage and activation of caspase-3, through protein cross-linking (17). Furthermore, TG2 is involved in anchoring epithelial cells to the extracellular matrix; this process leads to activation of ‘outside-in’ signaling that ultimately promotes cell survival (18). These observations have direct applications for understanding the process of chemotherapy resistance and have incited an interest in developing TG2 inhibitors as anticancer therapy (19).
We recently reported that TG2 is upregulated in transformed ovarian epithelial cells and tumors (7,20). Given TG2′s presumed antiapoptotic function in other tumors and the significance of cisplatin-induced apoptosis to clinical outcome of patients with EOC, we analyzed whether TG2 protects ovarian cancer cells from cisplatin-induced apoptosis and mechanisms of resensitizing EOC cells to platinum through TG2 inhibition.
Materials and methods
Materials
Cisplatin and methylthiazolyldiphenyl-tetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO). TG2 enzyme inhibitor, KCC009, was provided by Alvine Pharmaceuticals, San Carlos, CA.
Cell lines
Human SKOV3 and OV-90 ovarian carcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA), cultured in growth media containing 1:1 MCDB 105 media (Sigma) and M199 media (Cellgro, Herndon, VA) and supplemented with 10% heat-inactivated fetal bovine serum (Cellgro) and 1% antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). All cells were grown at 37°C in a humidified 5% CO2 atmosphere. Cells were treated with cisplatin at concentrations from 1 to 20 μM for 24 h or as indicated. Treatment with KCC009 at concentrations from 10 μM to 1 mM was carried out for 24–72 h.
Transfection
To overexpress TG2, OV-90 cells in the logarithmic phase of growth were transfected with wild-type TG2 cloned into the pcDNA3.1 vector using Fugene (Roche Applied Science, Indianapolis, IN). To knock down TG2, an antisense construct cloned into pcDNA3.1 vector was transfected in SKOV3 cells. As a control, cells were transfected with the pcDNA3.1 vector, carrying G418 resistance gene. Transfection efficiency in these conditions is typically 5–10% in OV-90 cells and 30–40% in SKOV3 cells, as determined by estimation of green fluorescent protein expression. Stable transfected clones were established by selection with G418 (Sigma) at concentrations of 600 μg/ml for SKOV3 cells and 150 μg/ml for OV-90 cells. Plasmids were generous gift from Prof. Janusz Tucholski, University of Alabama. Overexpression and knockdown of TG2 in selected clones were demonstrated by western blot analysis.
To reconstitute NF-κB and Akt activity in SKOV3 cells stably transfected with anti-sense tissue transglutaminase (AS-TG2) or vector, the NF-κB constitutively active p65 subunit (21) and a constitutively active form of Akt that lacks the pleckstrin homology domain (amino acids 4–129) (22) were transferred by retroviral infection. The empty retroviral vector (pQXIP) carrying only the puromycin resistance gene was transduced as control. After transduction in the presence of polybrene, pooled clones were selected with puromycin (Sigma).
Cell proliferation
Cell proliferation was assayed by MTT and BrdU assays. In brief, after treatment, cells were incubated with MTT (200 μl of 1 mg/ml in RPMI) for 4 h. The formazan crystals formed were solubilized in 200 μl acidified isopropanol (0.04 N HCl final) containing 1% Triton X-100 for 10 min, and the optical density was measured with an ELISA plate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA) at 570 nm. In addition, an enzyme-linked immunosorbent assay colorimetric method based on measurement of BrdU incorporation into DNA (Roche Diagnostics, Penzberg, Germany) assessed cell proliferation. In brief, after cisplatin treatment, cells were incubated with BrdU (10 μM) for 3 h, then fixed and denatured. Cells were subsequently treated with a peroxidase-labeled anti-BrdU antibody for 90 min and the colorimetric reaction was developed with a tetramethyl-benzidine-based substrate. The reaction product was measured at 370 nm. All assays were performed in quadruplicate and were repeated twice in independent conditions. Data are presented as means ± SEM.
Clonogenic assay
SKOV3 cells stably transfected with AS-TG2 or empty vector were seeded in six-well plates at a density of 100 cells per well. Triplicate wells were treated with 1, 2 and 5 μM cisplatin for 48 h after which the media was changed. Cells were allowed to grow for 10 days until visible colonies were formed. The colonies were fixed and stained with Hema 3 stain (Fisher) and counted. The number of colonies formed after treatment with cisplatin was expressed as a percentage of untreated controls (relative colony formation). Data are presented as means ± SDs.
Immunoblotting
Cells were lysed into RIPA buffer containing leupeptin (1 μg/ml), aprotinin (1 μg/ml), phenylmethylsulfonyl fluoride (400 μM) and Na3VO4 (1 mM). Cell lysates were sonicated and incubated on ice for 30 min. Cellular debris was removed by centrifugation for 15 min at 4°C. Protein concentration was measured by the Bradford method (Bio-Rad, Hercules, CA). Protein samples were dissolved in 5× loading buffer (62.5 mM Tris–HCl, pH 6.8, 2% sodium dodecyl sulfate, 100 mM dithiothreitol, 0.01% bromophenol blue and 10% glycerol) and heated to 100°C for 5 min. Equal amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membrane. The membranes were blocked with 1× tris-buffered saline tween-20 (TBS-T) containing 5% dry milk for 30 min and subsequently probed with desired primary antibodies diluted in blocking buffer overnight at 4°C with gentle rocking. Membranes were probed with anti-TG2 antibody (CUB 7402, Neomarkers, Fremont CA, 1:1000 dilution), anti-phospho FAK antibody (Cell Signaling, Denvers, MA, 1:1000 dilution), anti-pSer473Akt antibody (Cell Signaling, 1:1000 dilution), anti-cytochrome c antibody (BD Bioscience, San Jose, CA, 1:500 dilution), anti-Caspase-9 P35 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:500 dilution), anti-hILP/XIAP antibody (BD Bioscience, 1:500 dilution), anti-β-actin antibody (Sigma, 1:3000 dilution) and anti-GAPDH (Santa Cruz Biotechnology, 1:10 000 dilution) as loading control. After primary antibody incubation, membranes were washed thrice (5 min each) with TBS-T followed by incubation with corresponding horseradish peroxidase-labeled secondary antibodies. Antigen–antibody complexes were visualized using the enhanced chemiluminescence detection system, as recommended by the manufacturer (Amersham Biosciences, Piscataway, NJ).
TdT-mediated deoxyuridine triphosphate nick-end labeling assay
TdT (terminal deoxynucleotidyl transferase)-mediated deoxyuridine triphosphate nick-end labeling assay (Chemicon, Temecula, CA) was utilized to quantify cisplatin-induced apoptosis. In brief, after cisplatin treatment, cells were fixed in 1% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature and then in ethanol:acetic acid (2:1) for 5 min at −20°C. Fixed cells were blocked with equilibration buffer for 1 min at room temperature and treated with TdT enzyme and anti-digoxingenin conjugate. A mounting medium containing 4′,6-diamidino-2-phenylindole (Vectorshield, Vector Laboratories, Burlingame, CA) was used. Cells were viewed by fluorescence microscope and counted at high power magnification (×400). Five fields were counted in each of the duplicated wells. Data are presented as means ± SEM.
Fluorometric caspase-3 and -9 assays
Caspase-3 activity was measured in cell lysates by a fluorescence-based assay (Apo-ONE Homogeneous Caspase-3/7 Assay, Promega) following the manufacturer's protocol. The profluorescent substrate (Z-Asp-Glu-Val-Asp-R100) upon sequential cleavage and removal of the Asp-Glu-Val-Asp peptides by caspase-3 activity relives the rhodamine group which when excited at 499 nm becomes fluorescent. The fluorescent product was measured using a plate reader (SpectraMax M5 Microplate Reader). Recombinant active caspase-3 (Chemicon) was used as a positive control and denominator for analysis. Likewise, caspase-9 activity was measured in cell lysates using a fluorescence-based assay (BioVision, Mountain View, CA) according to the manufacturer's protocol. The assay is based on detection of cleavage of substrate LEHD–7-amino-4-trifluoromethyl coumarin (AFC). LEHD–AFC emits blue light; upon cleavage of the substrate by caspase-9, free AFC emits yellow–green fluorescence that is quantified using a SpectraMax M5 Microplate Reader. Comparison of the fluorescence of AFC from an apoptotic sample with an uninduced control allows determination of the fold increase in caspase-9 activity. The fluorescent units for caspase-3 and -9 were normalized to the amount of protein measured for each sample using the Bradford method.
NF-κB reporter assay
Dual-Luciferase Assay (Promega) was performed to quantify NF-κB activity in pcDNA3.1 and AS-TG2 SKOV3 cells, according to the manufacturer's instructions. In brief, cells were cotransfected with NF-κB promoter luciferase and Renilla plasmids at the ratio of 10:1 using DreamFect Gold transfection reagent (OZ Biosciences, Marseille, France). Luminescence was measured using TD-20/20 Luminometer (Turner Biosystems, Sunnyvale, CA) 48 h after transfection. The experiments were performed in duplicate and repeated in independent conditions. To control for transfection efficiency, values for NF-κB luminescence were normalized to Renilla activity.
Analysis of combined drug effects
Drug synergy was measured using the CalcuSyn software (Biosoft, Ferguson, MO). To perform these analyses, we used the data obtained from the growth inhibitory experiments, generated isobolograms and measured the combination index (CI) based on the median-effect principle of Chou et al. (23). The CI method is a mathematical representation that measures two-drug pharmacologic interaction. A CI of 1 indicates an additive effect between two agents, a CI <1 indicates synergism, whereas a CI >1 represents antagonism.
Statistical analysis
Values presented are the means ± standard errors from at least triplicate experiments. 50% inhibitory concentration (IC50) values for inhibition of cell proliferation by cisplatin and KCC009 were calculated as the concentration of drugs resulting in a 50% of reduction in viable cells compared with untreated controls by MTT or BrdU assays. The IC50 values and the combination effect of multiple drugs were determined using the Calcusyn software program for Windows (Version 1.2, 1996; Biosoft, Cambridge, UK). Comparisons between groups were performed using Student's t-test; P-values of <0.05 being considered significant.
Results
TG2 mediates response to cisplatin in EOC cells
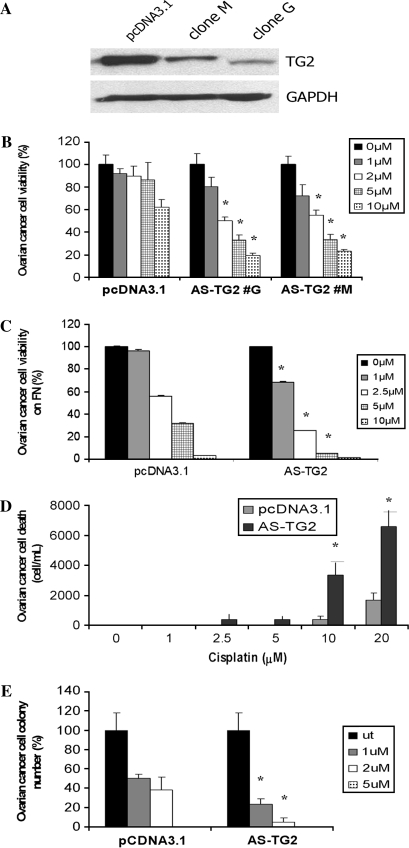
To understand the function of TG2 in ovarian cancer cells' response to cisplatin, we generated stable human cell lines, in which TG2 was overexpressed or knocked down. To knock down TG2, we used an antisense construct (AS-TG2) cloned into pcDNA3.1 (24) in SKOV3 ovarian cancer cells, which express abundant TG2. Two stable clones (G and M) were selected based on G418 resistance and screening by immunoblotting; decreased TG2 being noted in whole-cell lysate from both clones compared with vector-transfected cells (Figure 1A). Next, we determined whether TG2 plays a role in the response of ovarian cells to cisplatin. Cells stably transfected with AS-TG2 and control vector were treated with varying concentrations of cisplatin for 24 h. To avoid potential clonal bias, the effects of cisplatin were measured in two different clones expressing low TG2 levels. The knockdown of TG2 sensitized cells to cisplatin, with a 2-fold decrease in the inhibitory concentration (IC50), from 5 μM in control cells to 2.5 μM in AS-TG2 cells (Figure 1B; P-value = 0.0005). Results were confirmed by BrdU assay (data not shown). To mimic the interaction with the extracellular matrix, cells were plated on fibronectin and treated with cisplatin. Similar results were obtained, the IC50 being decreased from >5 μM in controls to 2.5 μM in AS-TG2 cells and data were verified by BrdU assay (P-value = 0.01; Figure 1C). This was accompanied by a corresponding increase in apoptotic or necrotic cells, as counted by Trypan blue exclusion, with >10-fold increase in the number of dead cells after exposure to cisplatin in AS-TG2 cells versus controls (P-value = 0.01; Figure 1D). We next assessed the effects of cisplatin on the clonogenic potential of SKOV3 cells transfected with vector or with AS-TG2. Colony formation by cells with low TG2 expression was suppressed more significantly by low concentrations of cisplatin (1 and 2 μM) compared with control cells (Figure 1E; P-value = 0.007). Along with the results of the proliferative assays, these observations support the role of TG2 as a survival protein in ovarian cancer cells.
Fig. 1.
TG2 knockdown in SKOV3 ovarian cancer cells enhances sensitivity to cisplatin. (A) TG2 was knocked down using stable transfection with an antisense (AS-TG2) construct cloned into pcDNA3.1. Stable transfectants were selected based on resistance to G418. Western blot demonstrates TG2 levels in SKOV3 cells stably transfected with pcDNA3.1 vector (control) and with AS-TG2 (clones #G and #M). (B) Effects of cisplatin on cell proliferation were assessed by MTT assay. SKOV3 cells transfected with pcDNA3.1 (control) and two AS-TG2 stable clones (#G and #M) were treated with different concentrations of cisplatin for 48 h. (C) Effects of cisplatin on cell proliferation in SKOV3 cells transfected with pcDNA3.1 (control) and AS-TG2, cells being plated on the matrix protein, fibronectin (FN). (D) Number of floating cells after 48 h treatment with cisplatin in AS-TG2 versus control cells. (E) Clonogenic assay assessed colony formation after 48 h exposure to cisplatin in SKOV3 cells transfected with AS-TG2 or pcDNA3.1. Data represent averages of triplicate experiments ± SEM.
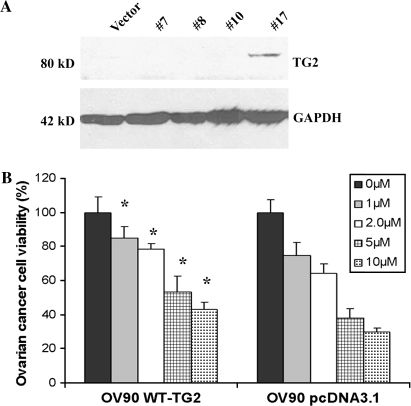
Next, we tested whether stable overexpression of TG2 decreases sensitivity to cisplatin. For this, an ovarian cancer cell line with low endogenous expression of TG2 (OV-90) was transfected with human full-length TG2. A stably transfected clone was identified by immunoblotting after selection with G418 (Figure 2A). Stable expression of TG2 increased resistance to cisplatin compared with cells transfected with empty vector (pcDNA3.1), with a 2-fold increase in IC50 from <5 μM in control cells to 10 μM in cells overexpressing TG2 (P-value < 0.001; Figure 2B). Coupled with the effects of TG2 knockdown, these experiments show that TG2 is important to ovarian cancer cells' response to cisplatin.
Fig. 2.
Overexpression of TG2 in the ovarian cancer cell line OV-90 induces resistance to cisplatin. (A) Wild-type (WT) TG2 was transfected in OV-90 ovarian cells. Stable transfectants were selected based on resistance to G418. Western blot shows levels of TG2 in several clones (clone #17 is positive) and in vector-transfected cells (controls). (B) Effect of cisplatin was measured by MTT assay. OV-90 cells transfected with pcDNA3.1 (control) and WT-TG2 were treated with different concentrations of cisplatin for 48 h. Data represent averages of triplicate experiments ± SEM.
TG2 protects against apoptosis induced by cisplatin in EOC cells
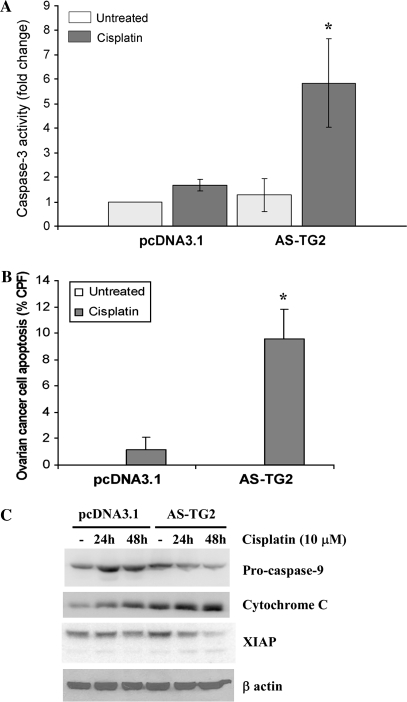
Knowing that cisplatin induces apoptosis in EOC cells, by activating the mitochondrial machinery, and having noticed a markedly increased number of detached cells in AS-TG2 cells exposed to cisplatin compared with controls, we measured apoptosis induced by cisplatin. Apoptosis was determined by measuring the activity of caspase-3 in ovarian tumor cells after exposure to cisplatin in AS-TG2 cells versus mock-transfected cells as controls. We observed increased activation of caspase-3 by treatment with cisplatin in cells with low levels of TG2 expression compared with untreated and even cisplatin-treated controls (Figure 3A; P-value = 0.01 and 0.02, respectively). Likewise, the percentage of TdT-positive cells measured by TdT-mediated deoxyuridine triphosphate nick-end labeling assay after exposure to cisplatin was 5-fold higher in cells stably transfected with AS-TG2 compared with control. Cisplatin increased the percentage of TdT-positive cells from 1% in controls to 9% in AS-TG2 cells (Figure 3B; P-value = 0.0005). These results demonstrate that apoptosis induced by cisplatin is enhanced in cells with decreased TG2 expression. Indeed, cleavage of pro-caspase-9, degradation of the X-linked inhibitor of apoptosis protein (XIAP) and cytoplasmic release of cytochrome c were more pronounced after cisplatin treatment in AS-TG2 cells compared with controls (Figure 3C), suggesting that TG2 exerts an ‘apoptotic brake’ in EOC cells exposed to cisplatin.
Fig. 3.
Level of TG2 expression modulates cisplatin-induced apoptosis. (A) Activation of caspase-3 was measured by a fluorometric assay. SKOV3 cells stably transfected with pcDNA3.1 and AS-TG2 were treated with 10 μM cisplatin for 24 h. Data represent averages of two independent experiments performed in duplicate ± SEM. (B) Percentage of apoptotic cells after treatment with cisplatin was assessed by TdT-mediated deoxyuridine triphosphate nick-end labeling assay. SKOV3 cells stably transfected with AS-TG2 or vector were treated with 10 μM cisplatin for 24 h. Percentage of TdT-positive cells was quantified by counting of 10 microscopic fields (×200) per specimen. Data represent average of duplicate experiments ± SEM. All counts were obtained in duplicate. The number of TdT-positive cells in wells not treated with cisplatin was close to zero. (C) Cisplatin-induced apoptosis was measured by western blotting using antibodies for pro-caspase-9, cytochrome c and XIAP. β-Actin was used as a control. SKOV3 cells stably transfected with pcDNA3.1 and AS-TG2 were treated with cisplatin (10 μM) for 24 and 48 h.
TG2 protects EOC cells from cisplatin-induced apoptosis through activation of NF-κB
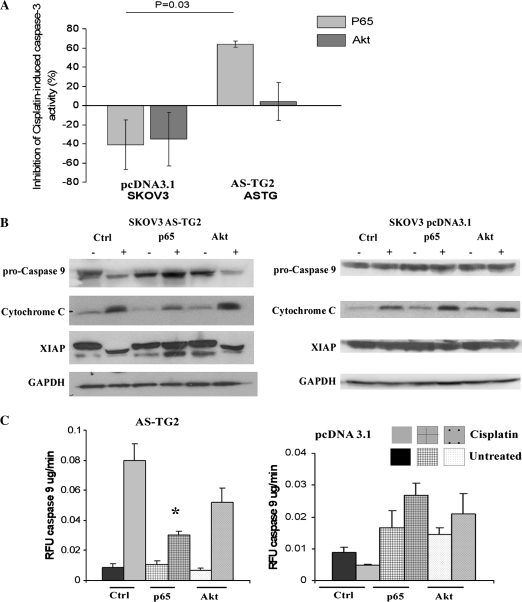
To understand the mechanism by which TG2 exerts its antiapoptotic role in this context, we examined the effects of Akt and NF-κB, two survival pathways commonly involved in development of drug resistance in EOC. For this, we overexpressed through retroviral transduction a constitutively active form of Akt (CA-Akt) and the p65 subunit of NF-κB in AS-TG2 and pcDNA3.1 (control) stably transfected cells and examined cisplatin-induced apoptosis. Overexpression of CA-Akt in pooled clones was verified by western blot with an antibody for pSer473Akt (data not shown). There was no statistically significant difference between cisplatin-induced caspase-3 activity in control cells transduced with empty vector and control cells tranduced with p65 (P = 0.1). Similarly, Akt overexpression failed to inhibit cisplatin-induced apoptosis in these control cells. In contrast, cisplatin-induced caspase-3 activation was rescued by overexpressing constitutively active p65, but not by CA-Akt in AS-TG2 cells (Figure 4A; P = 0.03), suggesting that the antiapoptotic effect of TG2 was exerted mainly through activation of NF-κB.
Fig. 4.
Reconstitution of NF-κB, but not of Akt, restores resistance to cisplatin in AS-TG2 cells. SKOV3 cells transfected with AS-TG2 or pcDNA3.1 were transduced with constitutively active p65, CA-Akt or vector (pQXIP) and treated with cisplatin. (A) Caspase-3 activity was measured by fluorometric assay at 24 h after cisplatin treatment. The results are expressed as inhibitory potency (mean % inhibition ± SEM; n = 2) of constitutively active p65 or CA-Akt on the caspase-3 activity induced by cisplatin treatment. Note the potent inhibitory effect of p65 overexpression on the cisplatin-induced caspase-3 activity in cells transfected with AS-TG2 (P-value = 0.01). All other treatments failed to significantly inhibit (or further activate) cisplatin-induced caspase-3 in either control vector- or AS-TG2-expressing cells. (B) Apoptosis was assessed by western blotting of cytochrome c, pro-caspase-9 and XIAP after 48 h cisplatin treatment. β-Actin was used as a control. (C) Caspase-9 activation was measured using a fluorometric assay in pcDNA3.1 or AS-TG2 cells transduced with constitutively active p65, CA-Akt and vector control (pQXIP) and treated with cisplatin for 24 h. Data are expressed as mean fold increase versus control caspase-9 activity (measured as fluorescent units per minute per milligram protein) ± SEM (n = 3; *P < 0.05 versus untreated cells). Note that only p65-overexpressing AS-TG2 cells failed to activate caspase-9 in response to cisplatin treatment.
Knowing that cisplatin-activated apoptosis proceeds primarily through activation of the mitochondrial pathway, we examined the effects of cisplatin on the mediators of this intrinsic apoptotic pathway. Indeed, we found that cisplatin-induced activation of caspase-9 in AS-TG2 cells was significantly inhibited by overexpression of p65 (P-value = 0.0006), but to a lesser extent by CA-Akt compared with vector-transfected cells (Figure 4C).
We next investigated by western blot the level of expression of other markers of apoptosis induced by cisplatin (pro-caspase-9, cytochrome c and XIAP) in AS-TG2 and control cells transduced with vector, p65 or CA-Akt. Overexpression of constitutively active p65 subunit prevented cleavage of pro-caspase-9 and XIAP in AS-TG2 cells treated with cisplatin (Figure 4B, left panel). Partial cleavage of XIAP after exposure to cisplatin was still detected in AS-TG2 cells transduced with p65, however, to lesser degree than in AS-TG2 cells transduced with empty vector. Cisplatin-induced cytochrome c release was also decreased by overexpression of p65 in AS-TG2 cells. These findings support the previous observations that p65 rescues AS-TG2 ovarian cancer cells from cisplatin-induced apoptosis. In contrast, CA-Akt did not affect cisplatin-induced cleavage of pro-caspase-9 or XIAP and release of cytochrome c in AS-TG2 cells, suggesting that Akt does not play a major role in TG2-mediated ovarian cancer cells' resistance to cisplatin. Interestingly, in control cells, expressing intact levels of TG2, cleavage of pro-caspase-9 or XIAP was not observed after 48 h exposure to cisplatin, consistent with a cisplatin-resistant phenotype. Overexpression of CA-Akt or p65 did not significantly alter basal or cisplatin-induced pro-caspase-9 or XIAP levels in control cells.
TG2 regulates NF-κB activity in EOC cells
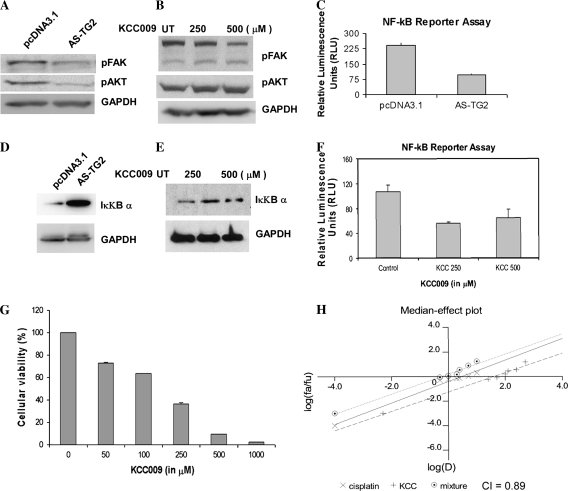
Having found that p65 rescues cisplatin-induced apoptosis in EOC cells to greater extent than CA-Akt, we next examined the function of the Akt and NF-κB pathways in EOC cells in the presence or absence of functional TG2. For this, we compared levels of phosphorylated Akt in SKOV3 cells stably transfected with AS-TG2 or vector in basal conditions. We found decreased activation of Akt in AS-TG2 cells compared with controls as measured by decreased phosphorylation at Ser473. This occurred in parallel to decreased phosphorylation of the focal adhesion kinase (FAK) in AS-TG2 cells compared with control cells (Figure 5A). Interestingly, inhibition of TG2 enzymatic activity by the TG2 inhibitor KCC009 did not affect activation of Akt, suggesting that the effects of TG2 on this survival pathway are independent of its enzymatic function (Figure 5B). However, FAK phosphorylation was modestly reduced after treatment with KCC009.
Fig. 5.
TG2 modulates Akt and NF-κB activity in ovarian cancer cells and TG2 inhibitors sensitize ovarian cancer cells to cisplatin. (A) Western blot for Ser473Akt, phosphorylated FAK and GAPDH in SKOV3 cells stably transfected with AS-TG2 and vector. (B) Western blot for Ser473Akt, phosphorylated FAK and GAPDH in SKOV3 cells treated with KCC009 for 24 h at a concentration of 250 and 500 μM. (C) NF-κB promoter activity measured by a reporter assay in SKOV3 cells stably transfected with AS-TG2 and vector. (D) Western blot for IκBα in SKOV3 cells stably transfected with AS-TG2 and vector. (E) Western blot for IκBα in SKOV3 cells treated with KCC009 for 24 h at a concentration of 250 and 500 μM. (F) NF-κB promoter activity measured by a reporter assay in SKOV3 cells treated with the TG2 inhibitor KCC009 at a concentration of 250 and 500 μM. (G) Effects of KCC009 on ovarian cancer cell proliferation were measured by MTT assay. Data represent average of triplicate experiments ± SEM. (H) KCC009 has synergistic effects when combined with cisplatin (CI = 0.89) in SKOV3 cells. Cell proliferation was measured by MTT assay after 48 h treatment with cisplatin, KCC009 and the combination of two drugs and the CI was measured based on the median-effect principle of Chou et al. (23). The median-effect plot was analyzed using Calcusyn.
In contrast, the activity of the NF-κB complex was affected by both expression level and enzymatic activity of TG2. Using a reporter assay, we found that the activity of the NF-κB complex was decreased 2-fold in cells stably transfected with AS-TG2 compared with controls (P-value = 0.02; Figure 5C). Likewise, inhibition of TG2 enzymatic function by KCC009 led to decreased NF-κB activity in SKOV3 cells (P-value = 0.04; Figure 5F). This inhibition of the NF-κB promoter activity was paralleled by a corresponding increase in expression levels of inhibitory IκBα level in cells transfected with AS-TG2 versus controls (Figure 5D). The expression level of IκBα was also increased by treatment with the TG2 enzyme inhibitor KCC009 (Figure 5E). This suggested that the expression and the enzymatic function of TG2 are essential in regulating the expression level of the inhibitory unit IκBα and subsequent level of activity of the NF-κB complex. The TG2 inhibitor KCC009 had antiproliferative effects in SKOV3 ovarian cancer cells, with an IC50 of <250 μM (Figure 5G). This effect may be partly explained by the inhibition of the NF-κB survival pathway, but other contributory mechanisms cannot be excluded.
These results suggested that inhibition of TG2 may serve as a novel modality to enhance sensitivity of ovarian cancer cells to cisplatin. We therefore tested whether the combination KCC009 and cisplatin exerts synergistic effects in SKOV3 cells. Measurement of growth inhibition indices after treatment with each agent and with the two-drug combination led to a calculated CI of 0.89 (Figure 5H), using the median-effect principle of Chou et al. (23). This CI value indicates a modest synergistic effect between cisplatin and the TG2 inhibitor.
Discussion
The present study shows that TG2 regulates apoptosis induced by cisplatin in ovarian cancer cells. The current findings follow our initial observations describing TG2 overexpression in a large proportion of ovarian tumors and its role in peritoneal dissemination (7). We demonstrate here that cisplatin-induced activation of pro-caspases-9 and -3 is increased in cells engineered to express reduced amounts of TG2 and that this activation can be prevented by reconstituting the activated p65 subunit of NF-κB. This is the first report implicating TG2 in cisplatin resistance and the first study to show that TG2 regulates NF-κB activity in EOC cells.
TG2 has been implicated in doxorubicin resistance in breast cancer cells (14,25,26) and its role in this process was linked to adhesion-mediated activation of intracellular signaling (25). Having demonstrated in prior work that TG2 plays an important role in ovarian cancer cell adhesion to fibronectin and collagen, through interaction with β1 integrins (7), we hypothesized that TG2 would affect integrin-mediated outside-in signaling converging on the phosphoinositide 3 kinase/Akt pathway and that this in turn would affect resistance to chemotherapy. Indeed, we found that TG2 knockdown led to decreased levels of Akt phosphorylation in SKOV3 cells, in parallel with decreased levels of FAK activation (Figure 5A). However, reconstitution of Akt activity through overexpression of CA-Akt in AS-TG2 cells altered only partially cisplatin-induced activation of caspases-9 and -3, suggesting that Akt was not the primary pathway through which TG2 protects cancer cells from chemotherapy-induced apoptosis. While activation of Akt by TG2 in SKOV3 cells may be related to facilitation of integrin engagement with subsequent FAK phosphorylation, our findings do not exclude other non-adhesion-mediated mechanisms leading to Akt activation. It is interesting that TG2 inhibition by KCC009 had discrepant effects on the levels of phosphorylated FAK compared with the levels of phosphorylated Akt in SKOV3 cells (Figure 5B), suggesting that perhaps Akt activation in these cells may be modulated through other pathways. Indeed, a recent report suggests that the phosphatase and tensin homolog (PTEN) is a direct TG2 substrate in pancreatic cancer cells (27); it is possible that such a mechanism is operative in ovarian cancer cells as well.
In contrast to the inability of CA-Akt to fully reduce cisplatin-mediated caspase-9 and -3 activation, overexpression of constitutively active p65 subunit of NF-κB alone significantly reduced caspase-9 and -3 activities induced by platinum in AS-TG2 cells (Figure 4). This finding implicates activation of the NF-κB survival pathway by TG2 as the main escape mechanism from cisplatin-induced apoptosis. NF-κB had been linked to cisplatin-induced apoptosis in ovarian cancer cells (6), but its link to TG2 is novel. Given that in certain cellular contexts Akt plays a role in activating NF-κB (28), the discrete roles played by the two pathways may not be fully separated.
We further examined the activity of the NF-κB complex in ovarian cancer cells and found that NF-κB activity is modulated by the level and function of TG2 (Figure 5C and F). This is consistent with prior studies in pancreatic cancer cells, where TG2 has been implicated in constitutive activation of the NF-κB complex, presumably through destabilization of IκBα. The inhibitory IκBα subunit appears to be a direct substrate for cross-linking mediated by TG2 in malignant pancreatic cells (15). Abnormally cross-linked IκBα would then be targeted for proteasomal degradation and displaced from the NF-κB complex, leading to its activation. We found increased levels of IκBα levels in SKOV3 cells where TG2 has been knocked down (Figure 5D), suggesting that a similar mechanism of TG2-mediated NF-κB activation is functional in EOC cells. Furthermore, we demonstrate for the first time that KCC009, a dihydroisoxazole inhibitor of TG2 (29,30), inhibits directly NF-κB and increases levels of IκBα in SKOV3 cells. KCC009 was investigated as a potential anticancer agent in glioblastoma, its effects being attributed to disruption of the extracellular matrix (30) and to inhibition of the Akt survival pathway and its targets (19). Interestingly, KCC009 did not significantly affect activation of Akt in ovarian cancer cells, contrasting the findings in glioblastoma (19). This may be due to the fact that Akt is constitutively active in SKOV3 cells (31,32) and in ovarian tumors (33) and thus may be less dependent on outside-in signaling compared with glioblastoma cells. Constitutive activation of Akt in ovarian cancer cells has been attributed to low levels of expression of the phosphatase PTEN (32). Our data suggest that the cytotoxic effects of KCC009 in SK0V3 ovarian cancer cells and the synergistic activity in combination with cisplatin may be due to its inhibitory effect on the NF-κB survival pathway (Figure 5G and H).
Our findings demonstrate that TG2′s enzymatic activity is important for modulating NF-κB function in ovarian cancer and highlight a new role for TG2 inhibitors, such as KCC009, as potent regulators of NF-κB activity. Knowing that TG is overexpressed in a high proportion of ovarian tumors and widely expressed in other cancers (34), the data presented here provide strong experimental rationale for investigating the role of TG2 inhibitors as sensitizers to chemotherapy.
Funding
VA Merit Award and Marsha Rivkin Research Fund to D.M.; National Institutes of Health (RO1 HL077328) to I.P; Walther Oncology Center scholarship to L.C.
Acknowledgments
We thank Alvine Pharmaceuticals for supplying the TG2 inhibitor, KCC009, and Dr David Donner from the University of California at San Francisco for careful review of the manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AFC
7-amino-4-trifluoromethyl coumarin
- Akt
protein kinase B
- AS-TG2
anti-sense tissue transglutaminase
- CI
combination index
- EOC
epithelial ovarian cancer
- FAK
focal adhesion kinase
- IC50
50% inhibitory concentration
- IκBα
inhibitor of kappa B α
- MTT
methylthiazolyldiphenyl-tetrazolium bromide
- NF-κB
nuclear factor-kappa B
- TdT
terminal deoxynucleotidyl transferase
- TG
transglutaminase
- XIAP
X-linked inhibitor of apoptosis protein
References
- 1.Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl J. Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, et al. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Henkels KM, et al. Induction of apoptosis in cisplatin-sensitive and -resistant human ovarian cancer cell lines. Cancer Res. 1997;57:4488–4492. [PubMed] [Google Scholar]
- 4.Yang X, et al. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 5.Asselin E, et al. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001;61:1862–1868. [PubMed] [Google Scholar]
- 6.Mabuchi S, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem. 2004;279:23477–23485. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 7.Satpathy M, et al. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 8.Pincus JH, et al. The specificity of transglutaminase. II. Structural requirements of the amine substrate. Arch. Biochem. Biophys. 1968;126:44–52. doi: 10.1016/0003-9861(68)90557-2. [DOI] [PubMed] [Google Scholar]
- 9.Lorand L, et al. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 10.Antonyak MA, et al. Two isoforms of tissue transglutaminase mediate opposing cellular fates. Proc. Natl Acad. Sci. USA. 2006;103:18609–18614. doi: 10.1073/pnas.0604844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobuzio-Donahue CA, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 12.Grigoriev MY, et al. Tissue transglutaminase expression in breast carcinomas. J. Exp. Clin. Cancer Res. 2001;20:265–268. [PubMed] [Google Scholar]
- 13.Martinet N, et al. In vivo transglutaminase type 1 expression in normal lung, preinvasive bronchial lesions, and lung cancer. Am. J. Respir. Cell Mol. Biol. 2003;28:428–435. doi: 10.1165/rcmb.2002-0114OC. [DOI] [PubMed] [Google Scholar]
- 14.Kim DS, et al. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006;66:10936–10943. doi: 10.1158/0008-5472.CAN-06-1521. [DOI] [PubMed] [Google Scholar]
- 15.Mann AP, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-{kappa}B in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–8795. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 16.Boehm JE, et al. Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J. Biol. Chem. 2002;277:20127–20130. doi: 10.1074/jbc.C200147200. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi H, et al. Tissue transglutaminase serves as an inhibitor of apoptosis by cross-linking caspase 3 in thapsigargin-treated cells. Mol. Cell. Biol. 2006;26:569–579. doi: 10.1128/MCB.26.2.569-579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akimov SS, et al. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan L, et al. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Mol. Cancer Ther. 2005;4:1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]
- 20.Matei D, et al. Gene expression in epithelial ovarian carcinoma. Oncogene. 2002;21:6289–6298. doi: 10.1038/sj.onc.1205785. [DOI] [PubMed] [Google Scholar]
- 21.Chua HL, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 22.Campbell RA, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J. Biol. Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 23.Chou TC, et al. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Tucholski J, et al. Tissue transglutaminase is essential for neurite outgrowth in human neuroblastoma SH-SY5Y cells. Neuroscience. 2001;102:481–491. doi: 10.1016/s0306-4522(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 25.Herman JF, et al. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene. 2006;25:3049–3058. doi: 10.1038/sj.onc.1209324. [DOI] [PubMed] [Google Scholar]
- 26.Antonyak MA, et al. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J. Biol. Chem. 2004;279:41461–41467. doi: 10.1074/jbc.M404976200. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, et al. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin. Cancer Res. 2008;14:1997–2005. doi: 10.1158/1078-0432.CCR-07-1533. [DOI] [PubMed] [Google Scholar]
- 28.Gustin JA, et al. Akt regulates basal and induced processing of NF-kappaB2 (p100) to p52. J. Biol. Chem. 2006;281:16473–16481. doi: 10.1074/jbc.M507373200. [DOI] [PubMed] [Google Scholar]
- 29.Choi K, et al. Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem. Biol. 2005;12:469–475. doi: 10.1016/j.chembiol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]
- 31.Altomare DA, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 32.Arboleda MJ, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 33.Cheng JQ, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl Acad. Sci. USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma A, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]