Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) and insulin resistance are common in overweight adolescents.
Objective
The purpose of this study was to determine the relation between NAFLD and insulin sensitivity in liver and skeletal muscle by studying overweight adolescents with a normal or high intrahepatic triglyceride (IHTG) content, who were matched for age, sex, body mass index (BMI; in kg/m2), and Tanner stage.
Design
Stable-isotope-labeled tracer infusion and the hyperinsulinemic-euglycemic clamp procedure were used to assess skeletal muscle and hepatic insulin sensitivity, and magnetic resonance spectroscopy was used to assess the IHTG content in 10 overweight (BMI = 35.9 ± 1.3) adolescents with NAFLD (IHTG = 28.4 ± 3.4%) and 10 overweight (BMI = 36.6 ± 31.5) adolescents with a normal IHTG content (3.3 ± 0.5%).
Results
The baseline plasma glucose concentration and the rate of appearance of glucose in plasma were the same in subjects with a normal (87.1 ± 1.2 mg/dL, 16.2 ± 1.1 μmol · kg fat-free mass−1 · min−1) or high (89.2 ± 2.5 mg/dL, 16.3 ± 1.2 μmol · kg fat-free mass−1 · min−1) IHTG content. However, compared with subjects who had a normal IHTG content, subjects with NAFLD had a lower hepatic insulin sensitivity index, based on baseline glucose kinetics and insulin concentrations (4.0 ± 0.5 compared with 2.4 ± 0.4; P < 0.05) and an impaired increase in glucose uptake during insulin infusion (169 ± 28.1% compared with 67 ± 9.6% above baseline; P < 0.01). In addition, the plasma triglyceride concentration was greater and the plasma HDL-cholesterol concentration was lower in subjects with NAFLD than in those with a normal IHTG content.
Conclusion
An elevated IHTG content in overweight adolescents is associated with dyslipidemia and with insulin-resistant glucose metabolism in both liver and skeletal muscle.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is characterized by an elevated intrahepatic triglyceride (IHTG) content, with varying degrees of inflammation and fibrosis. The prevalence of NAFLD is directly correlated with body mass index (BMI) (1). Therefore, it is likely that the marked increase in the prevalence of obesity is responsible for the increase in the prevalence of NAFLD. In the United States, almost 20% of adolescents (12–9 y old) are overweight (defined as BMI ≥ 95th percentile on the sex-specific BMI-for-age growth chart) (2, 3), and approximately one-third of overweight children and adolescents have NAFLD (4–7).
In adults, NAFLD is associated with insulin resistance in both liver (impaired suppression of insulin-mediated glucose production) and muscle (impaired stimulation of insulin-mediated glucose uptake) (8–10). Data from several studies demonstrate that NAFLD is also associated with insulin resistance in children and adolescents (11–13). However, these studies evaluated insulin sensitivity by evaluating basal plasma glucose and insulin concentrations (11, 13) or oral glucose tolerance (11, 12), which are unable to determine insulin action in specific tissues.
The purpose of the present study was to evaluate liver and skeletal muscle insulin sensitivity in overweight adolescents with an elevated (≥10%) and normal (≤5%) IHTG content. The hyperinsulinemic-euglycemic clamp technique and stable-isotope-labeled tracer infusion were used to determine basal glucose kinetics and insulin-mediated glucose metabolism in liver and muscle in vivo. We hypothesized that, compared with overweight adolescents who have a normal IHTG content, overweight adolescents with NAFLD have impaired insulin action in both the liver and skeletal muscle.
SUBJECTS AND METHODS
Study subjects
Subjects were recruited from outpatient clinics of the St Louis Children’s Hospital, local pediatric offices, and our current database of eligible volunteers. It was necessary to screen 28 subjects to find 10 subjects with a normal IHTG content and 10 subjects with an elevated IHTG content, who were matched for sex, age, Tanner stage, and BMI (Table 1). Twenty overweight (BMI ≥95th percentile for age and sex) adolescents participated in the study; 10 subjects had a normal IHTG content (≤5.6%) (14) and 10 had NAFLD (≥10% IHTG content). We chose an IHTG content ≥10% to define subjects who had NAFLD to ensure a clear separation between the NAFLD and control groups. There were 3 whites and 7 African Americans in the control group and 9 whites and 1 African American in the NAFLD group. No subjects with other racial or ethnic background participated in the study.
TABLE 1.
Characteristics and body composition of the study participants1
| Normal IHTG content (n = 8 M, 2 F) | NAFLD (n = 8 M, 2 F) | |
|---|---|---|
| Age (y) | 15.2 ± 0.5 | 16.5 ± 0.4 |
| Tanner stage | 4.0 ± 0.2 | 4.5 ± 0.2 |
| BMI (kg/m2) | 36.6 ± 1.5 | 35.9 ± 1.3 |
| BMI (percentile) | 98.8 ± 0.2 | 98.4 ± 0.3 |
| Body weight (kg) | 105 ± 6 | 111 ± 5 |
| Fat-free mass (kg) | 59.9 ± 4.6 | 66.4 ± 4.4 |
| Fat mass (kg) | 42.4 ± 3.1 | 42.0 ± 2.1 |
| Fat mass (%) | 43.3 ± 2.0 | 39.1 ± 1.7 |
| Total abdominal fat (cm3) | 4985 ± 291 | 4911 ± 423 |
| Subcutaneous abdominal fat (cm3) | 4358 ± 274 | 3739 ± 2892 |
| Intraabdominal fat (cm3) | 627 ± 50 | 1114 ± 1392,3 |
| IHTG (%) | 3.2 ± 0.5 | 28.4 ± 3.64 |
All values are x̄ ± SEM. Significance of differences between groups was evaluated by using the Student’s t test for independent samples. IHTG, intrahepatic triglyceride; NAFLD, nonalcoholic fatty liver disease.
Data represent values obtained from 9 of the 10 subjects who had NAFLD, because technical problems precluded obtaining images of abdominal fat in 1 subject.
Significantly different from overweight subjects with normal IHTG content, P < 0.01.
Significantly different from overweight subjects with normal IHTG content, P < 0.001.
All subjects underwent a medical evaluation, which included a history and physical examination and blood tests. Subjects who had impaired fasting glucose concentration, diabetes, severe hypertriglyceridemia (>400 mg/dL), or history of other liver diseases (Wilson disease, α1-antitrypsin deficiency, hepatitis B orC, and autoimmune hepatitis) were excluded. None of the subjects consumed alcohol, smoked tobacco products, or took medications known to cause steatosis or alter glucose or lipid metabolism.
The study was approved by the Human Research Protection Office and the General Clinical Research Center (GCRC) Advisory Committee of Washington University School of Medicine (St Louis, MO). All subjects agreed to participate in the study after a detailed explanation of the study was provided to them and their parents. Written informed consent was obtained from each subject’s parent(s), and written informed assent was obtained from each subject before being enrolled in the study.
Body-composition assessments
Total body fat mass and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Delphi W-densitometer equipped with version 12.4 software; Hologic, Waltham, MA) (15). Total abdominal, subcutaneous abdominal, and intraabdominal fat (IAF) volumes were determined by using magnetic resonance imaging with a 1.5 T scanner (Siemens, Iselin, NJ). Eight 10-mm thick axial images were obtained beginning at the L4–L5 interspace and analyzed for subcutaneous and IAF content by using Analyze 6.0 software (Mayo Foundation, Biomedical Imaging Resource, Rochester, MN); the volume of fat was calculated for each slice and the values were added. Readings were verified by a second investigator blinded to the initial readings; the values obtained by each of the 2 investigators were averaged for data analyses. IHTG content was determined by using proton magnetic resonance spectroscopy with a 1.5 T scanner (Magnetom Sonata; Siemens, Erlangen, Germany) (16, 17).
Hyperinsulinemic-euglycemic clamp
A one-stage hyperinsulinemic-euglycemic clamp procedure was performed 1–2 wk after assessment of body composition. Subjects were instructed to adhere to their regular diet and to refrain from exercise for 3 d before the study, to avoid caffeine for 1 d before the study, and to fast (except for water) for 12 h before their admission to the GCRC at 0600 on the morning of the study. At 0700, a catheter was inserted into an antecubital vein to infuse a stable-isotope-labeled glucose tracer, insulin, and dextrose. Another catheter was inserted into a contralateral hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized blood samples. At 0800, after a baseline blood sample was obtained to determine the background plasma glucose tracer-to-tracee ratio (TTR), a primed continuous infusion of [6,6-2H2]glucose (priming dose: 22.5 μmol/kg; in-fusion rate: 0.25 μmol · kg−1 · min−1), dissolved in 0.9% NaCl solution, was initiated and maintained for 360 min. At 180 min, a hyperinsulinemic-euglycemic clamp procedure was started and maintained for 180 min. Insulin was infused at a rate of 40 mU · m−2 · min−1 (initiated with a 2-step priming dose of 160 mU · m−2 · min−1 for 5 min followed by 80 mU · m−2 · min−1 for 5 min). Dextrose (20%) was infused at a variable rate to maintain plasma glucose concentration at 100 mg/dL. The dextrose solution was enriched with [6,6-2H2]glucose (≈2.5%) to minimize changes in plasma glucose TTR during the clamp procedure (18). The infusion rate of [6,6-2H2]glucose was decreased by 50% (to 0.125 μmol · kg−1 · min−1) during the clamp procedure (from 180 to 360 min after the start of the study) to account for the expected decline in hepatic glucose production. Blood samples were taken every 10 min during the last 30 min of the basal period and the clamp procedure to determine plasma glucose and insulin concentrations and glucose TTR. Blood samples were taken every 10 min between 190 and 360 min to monitor glucose concentrations. After the clamp procedure was completed, glucose tracer and insulin infusions were stopped and subjects were given a standard meal. The dextrose infusion was stopped after the subjects ate lunch, and the subjects were then discharged from the GCRC after confirming that blood glucose concentrations were stable for ≥1 h after the dextrose infusion was stopped. Female subjects were studied during the follicular phase of their menstrual cycle.
Sample analyses
Plasma glucose concentrations were measured by using an automated glucose analyzer (YSI 2300 STAT Plus; Yellow Spring Instrument Co, Yellow Springs, OH). Plasma insulin concentrations were measured by radioimmunoassay (Linco Research, St Louis, MO). Plasma free fatty acid (FFA) concentrations were quantified by using gas chromatography (HP 5890 Series II GC; Hewlett-Packard, Palo Alto, CA) after heptade-canoic acid was added to plasma as an internal standard (19). Plasma glucose TTR was determined by using electron impact ionization gas chromatography–mass spectrometry (Agilent Technologies/HP 6890 Series GC System 5973 Mass Selective Detector; Hewlett-Packard) as previously described (19). After heptafluorobutyryl derivative of glucose was formed, plasma glucose TTR was determined by selectively monitoring ions at mass-to-charge ratios of 519 and 521.
Calculations
Metabolic and isotopic steady states were achieved during the last 30 min of the basal period (ie, between 150 and 180 min) and the hyperinsulinemic-euglycemic clamp procedure (ie, between 330 and 360 min). Therefore, the total (endogenous and exogenous) glucose rate of appearance (Ra) in plasma during basal conditions and the hyperinsulinemic-euglycemic clamp procedure was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR between 150 and 180 min (basal) and 330 and 360 min (clamp), respectively. Basal endogenous glucose Ra was calculated by using Steele’s equations (20). It was assumed that glucose rate of disappearance (Rd) from plasma was equal to the total glucose Ra.
Hepatic sensitivity was assessed by using the Hepatic Insulin Sensitivity Index (HISI), which is the inverse of the product of the basal hepatic glucose production rate (in μmol · kg FFM−1 · min−1) and the fasting plasma insulin concentration (in mU/L) (21, 22). Skeletal muscle insulin sensitivity was determined by evaluating the ability of insulin to stimulate the glucose Rd, assessed as the relative increase above baseline in whole-body glucose Rd during insulin infusion.
Statistical analysis
Statistical analyses were performed by using SPSS (version 13.0; SPSS Inc, Chicago, IL). Student’s t test for independent samples was used to evaluate differences in characteristics of the 2 groups of study subjects and cardiometabolic variables in subjects with normal IHTG content and those with NAFLD. An analysis of variance with repeated measures was used to evaluate the statistical significance of differences in glucose Rd during basal conditions and insulin infusion between groups. An analysis of covariance was performed to help separate the contribution of IHTG content from its major covariates, IAF volume and plasma triglyceride concentration, to the metabolic outcome measures. The relation between IHTG content and IAF volume was assessed by using the Pearson correlation coefficient. A P value <0.05 was considered statistically significant. All data are presented as means ± SEMs.
RESULTS
Characteristics and body composition of the study participants
By design, subjects who had normal and those who had an elevated IHTG content were matched for sex, age, Tanner stage, and BMI (Table 1). Total body weight, FFM, fat mass, and total abdominal fat volume were not different between groups. However, IAF volume in subjects with NAFLD was almost double the value in those with a normal IHTG content. The IHTG content in subjects with NAFLD was almost 10 times that in subjects with a normal IHTG content (Table 1). No significant relations were detected between IHTG content and BMI (r = −0.08, NS) or percentage body fat (r −0.33, NS). In contrast, the IHTG content correlated directly with IAF volume (Figure 1; r = 0.81, P = 0.01).
FIGURE 1.
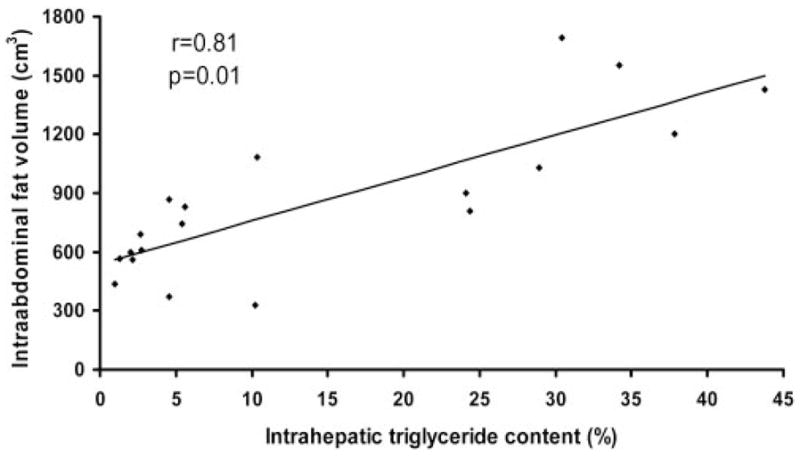
Relation between intrahepatic triacylglycerol content and intraabdominal fat volume in overweight adolescents (n = 19).
Metabolic variables
The basal plasma glucose concentration was not different between subjects with NAFLD and those with a normal IHTG content (Table 2). However, the mean plasma insulin concentration in subjects with NAFLD was almost twice that in subjects who had a normal IHTG content. Mean plasma triglyceride, total cholesterol, and LDL-cholesterol concentrations were greater and the HDL-cholesterol concentration was lower in subjects with NAFLD than in those with a normal IHTG content. Plasma transaminase (serum aspartate and alanine aminotransferase) concentrations were greater in subjects with NAFLD than in subjects with a normal IHTG content. However, 2 subjects with NAFLD (20%) had normal plasma transaminase concentrations.
TABLE 2.
Basic metabolic characteristics of the study participants1
| Normal IHTG content (n = 10) | NAFLD (n = 10) | |
|---|---|---|
| Glucose (mg/dL) | 87.1 ± 1.2 | 89.2 ± 2.5 |
| Insulin (mU/L) | 18.4 ± 3.0 | 33.3 ± 5.52 |
| Free fatty acids (μmol/L) | 432 ± 38 | 475 ± 29 |
| Triglyceride (mg/dL) | 75.5 ± 6.6 | 173.1 ± 19.53 |
| Total cholesterol (mg/dL) | 135.9 ± 6.7 | 169.6 ± 7.04 |
| LDL cholesterol (mg/dL) | 74.9 ± 6.7 | 99.9 ± 7.12 |
| HDL cholesterol (mg/dL) | 45.7 ± 3.1 | 35.1 ± 2.72 |
| Aspartate aminotransferase (IU/L) | 23.9 ± 2.3 | 58.3 ± 12.14 |
| Alanine aminotransferase (IU/L) | 24.6 ± 3.6 | 105.4 ± 23.74 |
| Systolic blood pressure (mm Hg) | 119 ± 3 | 123 ± 5 |
| Diastolic blood pressure (mm Hg) | 67 ± 3 | 79 ± 9 |
All values are x̄ ± 3 SEM. Significance of differences between groups was evaluated by using the Student’s t test for independent samples. IHTG, intrahepatic triglyceride; NAFLD, nonalcoholic fatty liver disease.
Significantly different from overweight subjects with normal IHTG content:
P < 0.05,
P < 0.001,
P < 0.01.
Glucose kinetics and insulin sensitivity
Basal endogenous glucose Ra (ie, endogenous glucose production) was not different in subjects with NAFLD than in subjects with normal IHTG content (16.2 ± 1.1 and 16.3 ± 1.2 μmol ·kg FFM−1 · min−1, respectively; P = 0.99). However, the mean HISI value was much lower in subjects with NAFLD than in those with a normal IHTG content (Figure 2). Although basal glucose Rd was not different between groups, insulin infusion caused a greater increase in glucose Rd in subjects with a normal IHTG content than in subjects with NAFLD (44.9 ± 5.5 compared with 28.8 ± 2.9 μmol · kg FFM−1 · min−1: 168.6 ± 28.1% compared with 67.4 ± 9.6% above the basal value (P < 0.01) (Figure 3). Plasma glucose and insulin concentrations during the hyperinsulinemic-euglycemic clamp procedure were not different between subjects with a normal IHTG content and those with NAFLD (glucose concentration: 96.6 ± 1.7 and 97.2 ± 0.7 mg/dL, respectively; insulin concentration: 103 ± 6 and 109 ± 9 mU/L, respectively).
FIGURE 2.
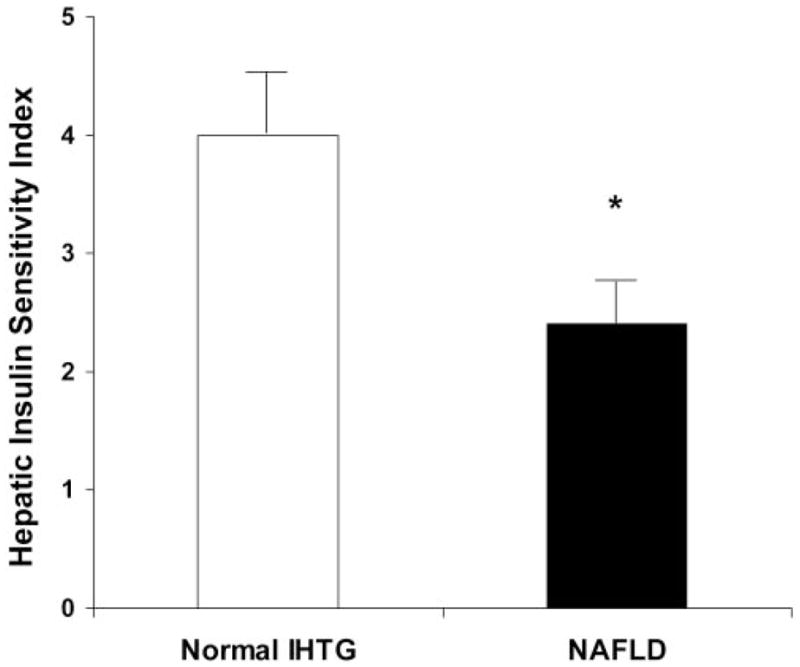
Mean (±SEM) hepatic insulin sensitivity index in overweight adolescents with a normal intrahepatic triacylglycerol (IHTG) content and those with nonalcoholic fatty liver disease (NAFLD). n = 10 per group. *Significantly different from corresponding value in subjects with normal IHTG, P < 0.05 (Students t test for independent samples).
FIGURE 3.
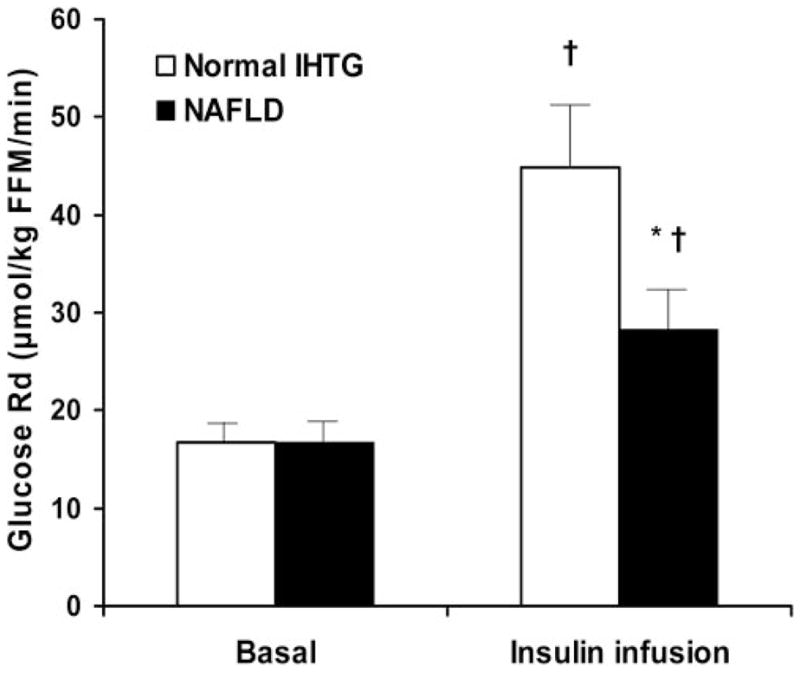
Mean (±SEM) glucose rate of disappearance (Rd) during basal conditions and the hyperinsulinemic-euglycemic clamp in overweight adolescents with a normal intrahepatic triacylglycerol (IHTG) content and those with nonalcoholic fatty liver disease (NAFLD). n = 10 per group. ANOVA with repeated measures was used to evaluate the statistical significance of differences in glucose Rd during basal conditions and insulin infusion between groups. Analysis of the group × time (stage) interaction showed the following: *Significantly different from subjects with a normal IHTG content, P < 0.001; †Significantly different from value during basal conditions, P < 0.01. FFM, fat-free mass.
Multivariate regression analyses of both insulin-mediated glucose disposal and the HISI with IHTG content, IAF volume, and triglyceride concentration as independent variables found that there was no longer any significant difference between the normal and NAFLD groups when more than one independent variable was included in the analysis. However, the power to determine an independent effect of IHTG content in our study was very low, because of the small sample size and colinearity between these variables, unless there was an extraordinary effect of IHTG content.
DISCUSSION
NAFLD occurs commonly in overweight children and adolescents. Although NAFLD is associated with insulin-resistant glucose metabolism, it is not clear which tissues are responsible for insulin resistance in adolescents. In the present study, the hyperinsulinemic-euglycemic clamp procedure and stable-isotope-labeled tracer infusion were used to evaluate liver and skeletal muscle insulin sensitivity in overweight adolescents with an elevated (≥10%) and normal (≤5%) IHTG content, who were carefully matched for sex, age, Tanner stage, and BMI. Our data show that subjects with NAFLD have a greater resistance to insulin-mediated glucose metabolism in both liver (decreased HISI) and skeletal muscle (impaired insulin-mediated glucose Rd) than do subjects with a normal IHTG content, despite normal fasting blood glucose and oral glucose tolerance in both groups. These results indicate that NAFLD is an important marker of impaired insulin action in multiple organs, which might not be detected by a standard medical examination.
Our findings suggest that fat accumulation in the liver identifies a category of overweight adolescents who are at high risk of developing diabetes, hypertension, and coronary heart disease (CHD) in the future. Although measures of adiposity (ie, BMI, body fat mass, and percentage body fat) were similar in our 2 groups of subjects, those with NAFLD had evidence of abnormalities in multiple metabolic risk factors for CHD. Compared with the normal IHTG group, the NAFLD group had higher plasma triglyceride, total cholesterol, and LDL-cholesterol concentrations; a lower plasma HDL-cholesterol concentration; and a trend toward higher systolic and diastolic blood pressures. These results are consistent with findings in adults, which found that NAFLD is frequently associated with metabolic risk factors for CHD and the metabolic syndrome (23, 24). Our data show that NAFLD in adolescents should be considered part of a constellation of co-occurring metabolic abnormalities that are associated with increased CHD risk in adults. Therefore, an elevated IHTG content identifies overweight adolescents who are likely to have multiple metabolic abnormalities associated with obesity. In contrast, the subjects in our study who had a normal IHTG content had a normal metabolic profile, and even the effect of insulin on muscle glucose uptake was similar to that reported in healthy, lean adolescents (25). These data suggest that the future medical outcomes in overweight adolescents might differ on the basis of IHTG content. Adults with NAFLD have a high incidence of CHD mortality, diabetes, dyslipidemia (elevated triglyceride and low HDL-cholesterol concentrations), and hypertension (23).
An elevated IHTG content is associated with abnormalities in other triglyceride depots. The IHTG content in our study subjects was directly correlated with their IAF volume. This relation is similar to findings observed in both adult (1, 26) and pediatric (27, 28) populations and helps explain the similarities in metabolic abnormalities observed in persons with abdominal obesity and NAFLD (9, 23, 24, 29). In addition, data from a recent study found that IHTG was directly correlated with the intramyocellular triglyceride content (30). It is not known whether an excessive IHTG content, intramyocellular triglyceride content, and IAF volume are a result of the metabolic abnormalities associated with abdominal obesity, or are directly involved in the pathogenesis of these abnormalities.
The mechanisms responsible for the relation between elevated IHTG content and skeletal muscle insulin resistance in our obese adolescents is not clear. Moreover, this observation is robust and we recently found a linear inverse correlation between IHTG content and insulin-mediated glucose uptake in adults across a wide range of IHTG content (31). It has been hypothesized that excessive fatty acid release from adipose tissue is responsible for many of the metabolic abnormalities associated with obesity (32). Increased release of fatty acids from subcutaneous adipose tissue into the systemic circulation can cause the following: 1) insulin resistance in liver and muscle, resulting in impaired insulin-mediated suppression of hepatic glucose production and insulin-mediated muscle glucose uptake (32); 2) dyslipidemia resulting from elevated hepatic VLDL-triglyceride secretion and elevated HDL-cholesterol clearance (33, 34); and 3) an elevated triglyceride content in liver and IAF because of increased fatty acid uptake and esterification to triglyceride (35). Therefore, elevations in liver and IAF could simply be a marker of deranged fatty acid metabolism. Another hypothesis is that a decreased capacity for storing triglyceride in subcutaneous adipose tissue results in “ectopic” triglyceride distribution in other organs, such as the liver, muscle, and IAF (36). Elevated intrahepatic and intraabdominal triglycerides can cause hepatic insulin resistance because of increased intrahepatic availability of fatty acids and inflammatory adipokines; IHTG releases fatty acids within the liver and IAF releases fatty acids and adipokines into the portal circulation, which are then delivered directly to the liver (31, 37, 38). An elevated intramyocellular triglyceride content is directly associated with insulin-resistant glucose metabolism in skeletal muscle (30).
In summary, an excessive IHTG content in overweight adolescents is associated with insulin-resistant glucose metabolism in liver and skeletal muscle and an abnormal plasma lipid profile. These results suggest that measurement of the IHTG content could be a useful clinical tool to help identify a subset of overweight adolescents who are at increased risk of developing type 2 diabetes and CHD and, therefore, should be targeted for regular medical monitoring and aggressive weight-loss therapy.
Acknowledgments
We thank the nursing staff of the GCRC for their help in performing the studies; Freida Custodia, Jennifer Shew, Adewole Okunade, and Gary Skolnick for their technical assistance; and the study subjects for their participation. None of the authors had a financial conflict of interest to disclose.
Footnotes
Supported by National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), DK 077653 (Ruth L Kirschstein National Research Service Award), RR-00954 (Biomedical Mass Spectrometry Resource), and RR-00036 (General Clinical Research Center).
References
- 1.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Arslan N, Buyukgebiz B, Ozturk Y, Cakmakci H. Fatty liver in obese children: prevalence and correlation with anthropometric measurements and hyperlipidemia. Turk J Pediatr. 2005;47:23–7. [PubMed] [Google Scholar]
- 5.Louthan MV, Theriot JA, Zimmerman E, Stutts JT, McClain CJ. Decreased prevalence of nonalcoholic fatty liver disease in black obese children. J Pediatr Gastroenterol Nutr. 2005;41:426–9. doi: 10.1097/01.mpg.0000177314.65824.4d. [DOI] [PubMed] [Google Scholar]
- 6.Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409–15. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 8.Bugianesi E, Pagotto U, Manini R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of non-alcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 10.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 11.Ciba I, Widhalm K. The association between non-alcoholic fatty liver disease and insulin resistance in 20 obese children and adolescents. Acta Paediatr. 2007;96:109–12. doi: 10.1111/j.1651-2227.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- 12.Perseghin G, Bonfanti R, Magni S, et al. Insulin resistance and whole body energy homeostasis in obese adolescents with fatty liver disease. Am J Physiol Endocrinol Metab. 2006;291:E697–703. doi: 10.1152/ajpendo.00017.2006. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 15.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 17.Petersen KF, West AB, Reuben A, Rothman DL, Shulman GI. Noninvasive assessment of hepatic triglyceride content in humans with 13C nuclear magnetic resonance spectroscopy. Hepatology. 1996;24:114–7. doi: 10.1002/hep.510240119. [DOI] [PubMed] [Google Scholar]
- 18.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–24. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–8. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 20.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–30. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 21.Gastaldelli A, Miyazaki Y, Pettiti M, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–21. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 22.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 23.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 25.Lange A, Moran CM, Palka P, Fenn LN, Sutherland GR, McDicken WN. The variation of integrated backscatter in human hearts in differing ultrasonic transthoracic views. J Am Soc Echocardiogr. 1995;8:830–8. doi: 10.1016/s0894-7317(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 26.Westerbacka J, Corner A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 27.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–94. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 28.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–71. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 29.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–51. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JH, Stein DT, Barzilai N, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–9. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 31.Korenblat KFE, Mohammed BS, Klein S. Liver, skeletal muscle and adipose tissue insulin resistance is directly related to intrahepatic triglyceride content in obese men and women. Gastroenterology. 2008;134:1369–75. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 33.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–40. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin North Am. 1998;27:503–19. doi: 10.1016/s0889-8529(05)70023-2. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]


