Abstract
Curcumin (diferuloylmethane) is the major active ingredient of turmeric (curcuma longa) used in South Asian cuisine for centuries. Curcumin has been shown to inhibit the growth of transformed cells and to have a number of potential molecular targets. However, the essential molecular targets of curcumin under physiological conditions have not been completely defined. Herein, we report that the tumor cellular proteasome is most likely an important target of curcumin. Nucleophilic susceptibility and in silico docking studies show that both carbonyl carbons of the curcumin molecule are highly susceptible to a nucleophilic attack by the hydroxyl group of the N-terminal threonine of the proteasomal chymotrypsin-like subunit. Consistently, curcumin potently inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50=1.85 µM) and cellular 26S proteasome. Furthermore, inhibition of proteasome activity by curcumin in human colon cancer HCT-116 and SW480 cell lines leads to accumulation of ubiquitinated proteins and several proteasome target proteins, and subsequent induction of apoptosis. Furthermore, treatment of HCT-116 colon tumor–bearing ICR SCID mice with curcumin resulted in decreased tumor growth, associated with proteasome inhibition, proliferation suppression and apoptosis induction in tumor tissues. Our study demonstrates that proteasome inhibition could be one of the mechanisms for the chemopreventive and/or therapaeutic roles of curcumin in human colon cancer. Based on its ability to inhibit the proteasome and induce apoptosis in both HCT-116 and metastatic SW480 colon cancer cell lines, our study suggests that curcumin could potentially be used for treatment of both early stage and late stage/refractory colon cancer.
Keywords: Curcumin, polyphenols, proteasome inhibitors, colon cancer, apoptosis
Introduction
Curcumin (diferuloylmethane) is the active ingredient of turmeric, derived from the rhizome of Curcuma longa. Besides its traditional use as a food coloring and flavoring agent, curcumin also has a well-documented history in medicine in India and Southeast Asia. Curcumin is used to treat various diseases, including respiratory conditions, inflammation, liver disorders, diabetic wounds, cough and certain tumors (1).
Although curcumin has been shown to inhibit the growth of transformed cells and all three stages of colon carcinogenesis (initiation, promotion and progression) in carcinogen-induced rodent models, the underlying mechanisms are not fully understood. Many molecular targets have been suggested including various transcription factors, inflammatory enzymes, cytokines, adhesion molecules and cell survival proteins (2).
The ubiquitin-proteasome pathway is essential for many fundamental cellular processes, including the cell cycle, apoptosis, angiogenesis and differentiation (3, 4). The proteasome contributes to the pathological state of several human diseases including cancer and AIDS, in which some regulatory proteins are either stabilized due to decreased degradation or lost due to accelerated degradation (5). The 20S proteasome, the proteolytic core of 26S proteasome complex, contains multiple peptidase activities including the chymotrypsin-like (CT-like), trypsin-like (T-like) and peptidylglutamyl peptide hydrolyzing-like (PGPH-like) (6). It has been shown that inhibition of tumor cellular chymotrypsin-like activity is a strong stimulus that induces apoptosis (7, 8). The possibility of therapeutically targeting the ubiquitin-proteasome pathway was initially met with great skepticism, since this pathway plays an important role in normal cellular homeostasis as well. However, after the demonstration that actively proliferating cancer cells are more sensitive to apoptosis-inducing stimuli, including proteasome inhibition, proteasome inhibitors became the subject of further investigation (9–11). With the finding that proteasome inhibitors were well tolerated and had activity in experimental models of human malignancies in vivo (12), the proteasome inhibitor bortezomib (Velcade/ PS-341) was introduced into a Phase I clinical trial (13). Although the data from the bortezomib trials showed significant clinical benefit, some toxicity was observed (14). The most common side effects include nausea, fatigue, and diarrhea, while more adverse events are thrombocytopenia, peripheral neuropathy, neutropenia, lymphopenia, and hyponatremia. Among them, peripheral neuropathy is the one that often causes an early cessation of the treatment (4). Therefore, there is a need to search for other proteasome inhibitors with less or no toxic side-effects, e.g., from naturally occurring or nutritional compounds, mostly because they are generally more tolerable in human body (15).
The benefit and chemopreventative role of dietary curcumin was observed in a variety of rodent models of carcinogenesis. As a logical consequence, curcumin is currently in clinical trials for treatment of various cancers, including multiple myeloma, pancreatic cancer, and colon cancer (16). The fact that colon cancer is one of the leading cancer-related deaths in Western countries and that current treatment options have only limited efficacy against advanced colon cancer contributes to the increasing interest for the use of curcumin in colon cancer prevention (17). Moreover, curcumin has been shown to inhibit the expression of cyclooxygenase-2 (COX-2) that plays a significant role in colon carcinogenesis (18). It is also important to emphasize that to date no toxicity associated with curcumin in either experimental animals or humans, even at very high doses, has been observed (2).
Curcumin-induced suppression of carcinogenesis is thought to be due to inhibition of NF-κB, which is controlled by the proteasome-mediated proteolytic degradation pathway (19), and subsequent inhibition of pro-inflammatory pathways (20). Additionally, curcumin was shown to down-regulate cyclin D1, cyclin E and MDM2, up-regulate p21, p27, and p53, and to induce apoptosis (2).
A recent report revealed that curcumin enhanced the anti-tumor activity of celecoxib, indicating their synergistic effect on the growth of colorectal cancer cells (21). Trials currently underway include an evaluation of colon cancer patients treated with gemcitabine in combination with curcumin and celecoxib (NCT00295035; Tel-Aviv Sourasky Medical Center). Recently, we have reported that in colon cancer cells, curcumin acts synergistically with FOLFOX (5-fluorouracil, oxaliplatin and leucovorin), a chemotherapeutic regimen that, together with FOLFIRI (5-fluorouracil, irinotecan and leucovorin), remains the backbone of colon cancer treatment (22). Taken together, the results suggest that chemotherapeutics in combination with curcumin may provide a superior therapeutic strategy in the clinical setting for treatment of refractory tumors.
In the present study, we have studied the potential molecular target of curcumin in human colon cancer cells. We first show that a computer docking model predicts curcumin to inhibit proteasomal chymothypsin-like activity. We then demonstrate that curcumin indeed directly inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50 = 1.85 µM) and 26S proteasome in human colon cancer HCT-116 and SW480 cell lines. Following proteasome inhibition, apoptotic cell death was induced. Furthermore, treatment of colon cancer–bearing ICR SCID mice with curcumin (500 mg/kg/d, intragastric, for 21 days) resulted in tumor growth suppression, associated with in vivo proteasome inhibition, growth arrest and apoptosis induction. Our results suggest that curcumin has proteasome-inhibitory activity in vitro and in vivo and could effectively be used for the prevention and treatment of human colon cancer.
Materials and Methods
Materials
Curcumin, ethanol, and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMEM/F12, McCoy’s 5A, penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA). Purified rabbit 20S proteasome and fluorogenic peptide substrates Suc-LLVY-AMC, Z-LLE-AMC, Z-ARR-AMC (for the proteasomal CT-like, PGPH-like, T-like activities, respectively) and Ac-DEVD-AMC (for the caspase-3/7 activity) were from Calbiochem (San Diego, CA, USA). Mouse monoclonal antibody against human poly(ADP-ribose) polymerase (PARP) was purchased from BIOMOL International LP (Plymouth Meeting, PA). Mouse monoclonal antibodies against Bax (B-9), p27 (F-8), ubiquitin (P4D1), rabbit polyclonal antibody against IκB-α (C-15), goat polyclonal antibody against actin (C-11), and secondary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Nucleophilic susceptibility analysis and computational modeling
Molecules were constructed using the CAChe Workstation (Fujitsu, inc.) as described previously (23). The AutoDock 3.0 suite of programs was used for the docking calculations and the output from AutoDock was rendered with PyMOL as described previously (23).
Cell cultures and whole cell extract preparation and Western blot analysis
The human colon carcinoma HCT-116 (a generous gift from Dr. Charles D. Lopez, Oregon Health & Science University) and metastatic SW480 cell lines (a generous gift from Prof. Satya Narayan, UF Shands Cancer Center, Gainesville, FL, USA) were grown in DMEM/F12 and McCoy’s 5A medium, respectively, both supplemented with 10% FBS, 100 units/mL of penicillin, and 100 µg/mL of streptomycin at 37°C in a humidified incubator with an atmosphere of 5% CO2. A whole cell extract preparation and Western blot analysis were done as previously described (24). Densitometry was quantified using AlphaEase FC software (Alpha Innotech Corporation, San Leandro, CA).
Inhibition of purified 20S proteasome activity
Purified rabbit 20S proteasome (17.5 ng) was incubated with 10 µM of the various substrates in 100 µl of assay buffer (25 mM Tris–HCl, pH 7.5) in the presence of curcumin at various concentrations or equivalent volume of solvent ethanol as a control. After 2 h incubation at 37°C, inhibition of each proteasomal activity was measured by the release of hydrolyzed AMC groups, as previously described (24).
Inhibition of the proteasomal chymotrypsin-like activity in intact cells
HCT-116 or SW480 cells were cultured in 96-well plates (1 × 104 cells/well) and treated for various time points with different concentrations of curcumin or MG132. After the additional 2 hour incubation with the fluorogenic peptide substrate specific for the proteasomal CT-like activity, production of hydrolyzed AMC groups was measured, as described above.
Caspase-3/-7 activity assay
Proteins extracted from treated cells were incubated in 100 µl of assay buffer (25 mM Tris-HCl, pH 7.5) with 20 µmol/L fluorogenic substrate specific for caspase-3/-7 activity. After 3 hour incubation at 37°C, release of hydrolyzed AMC groups was measured as described above.
Cellular morphology analysis and Terminal deoxynecleotydyl transferase-mediated dUPT-biotin nick end-labeling (TUNEL) assay
A Zeiss Axiovert 25 microscope was used for all microscopic imaging with phase contrast for cellular morphology as described previously (25). TUNEL assay was performed using the BD Biosciences Pharmingen, APO-DIRECT™ kit (San Diego, CA). Cells were treated with various curcumin concentrations for 24 hours, harvested, fixed and stained according to the protocol.
Mice and tumor cell implantation
Six-week old female homozygous ICR SCID mice were purchased from Taconic Farms (Germantown, NY). The mice were adapted to animal housing and HCT-116 xenografts were developed as described earlier (26). Prior to treatment, curcumin was dissolved in DMSO (final volume 0.05%) and further diluted in sesame oil. Tumor growth deduced as volume of the tumor in each group was determined by twice-weekly caliper measurements. All mice were euthanized one day following the last dose of treatment. H&E staining confirmed the presence of tumor.
Determination of proliferating cell nuclear antigen (PCNA) immunoreactivity, apoptosis by TUNEL assay, immunostaining, and Hematoxylin and Eosin (H & E) assays using tumor tissue samples
Determination of PCNA immunoreactivity, as a measure of proliferative activity, was done as described previously (27). TUNEL assay was done to detect apoptotic cells using the In situ Cell Death Detection kit from Roche Applied Science according to the manufacturer's instructions. Immunostaining of p27 was done as described previously (28). Hematoxylin and Eosin (H & E) staining in tumor tissues were performed following manufactory protocols.
Statistical analysis
Data are represented as mean ± SD for the absolute values as indicated in the vertical axis legend of Figures. The statistical significance of differential findings between curcumin-treated and control mice was determined by Student’s ‘t’ test as implemented by Excel 2000 (Microsoft Corp., Redmond, WA). P values smaller than 0.05 were considered statistically significant.
Results
Curcumin adopts a proteasome-inhibitory pose in an in silico model
We have reported that the ester bond carbon of β-lactone, the green tea polyphenol (−)-epigallocatechin-3-gallate [(−)-EGCG], (−)-EGCG analogues, and tannic acid is responsible for the potent and specific proteasomal chymotrypsin-like inhibition (29–31). Moreover, our recent data suggest that the carbonyl carbon of tea polyphenols and flavonoids confers their proteasome-inhibitory potencies (32–33). Since curcumin contains two carbonyl carbons (Fig. 1A) we hypothesized, and found both of them to be the sites of nucleophilic susceptibility (Fig. 1B).
Figure 1.
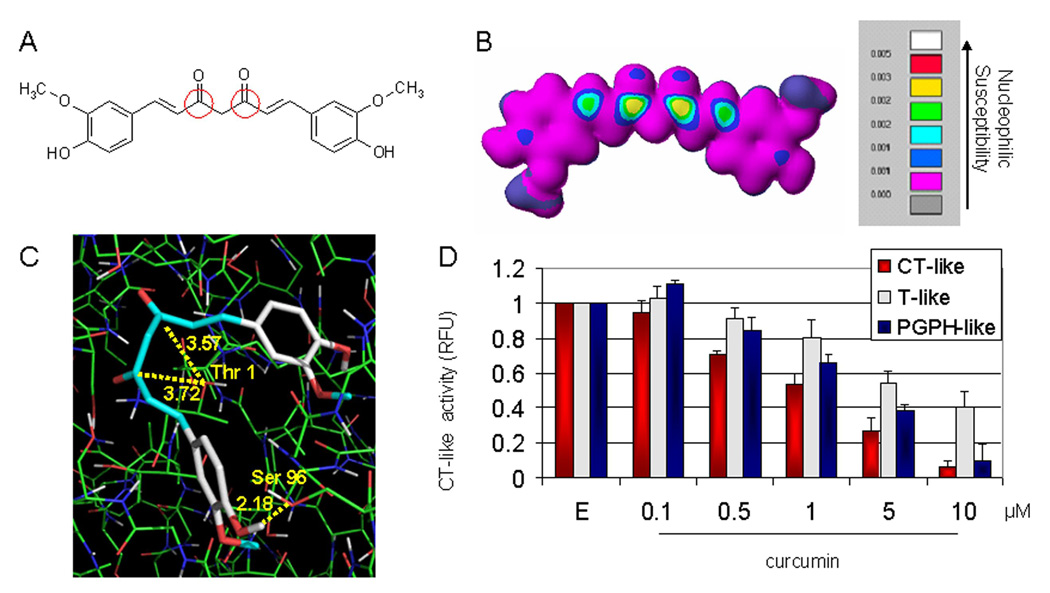
In silico and in vitro proteasome inhibition by curcumin. A, the chemical structure of curcumin. The regions with carbonyl carbons that have the SAR (structure activity relationship) are marked with red circles. B, molecular orbital energy analysis is demonstrated by drawing and electron density isosurface and coloring by nucleophilic susceptibility. The yellow center signifies the highest area of susceptibility. C, docking analysis of curcumin, which is represented by the stick structure and the colors are representative of atom type (carbon, gray; oxygen, red; hydrogen, white; methyl, light blue). The dotted line in yellow represents the distance, in angstroms, of each of the carbonyl carbons to Thr 1, indicative of potential nucleophilic attack, and to Ser 96, indicative of potential hydrogen bonding. D, in vitro analysis using a 20S proteasome indicates that curcumin inhibits chymotrypsin-like, trypsin-like and PGPH-like activities with IC50 values of 1.85±0.35, 6.23±0.22 and 3.68±0.19 µM, respectively. Ethanol was used as a control (E). Columns, mean of representative independent triplicate experiments; bars, SD.
We then proposed that curcumin is susceptible to nucleophilic attack by N-terminal threonine (Thr 1) of the β5 chymotrypsin-like subunit of the proteasome and is able to be oriented in an inhibitory pose within this subunit. To test this, curcumin was docked to the proteasomal β5 subunit, and cluster analysis was performed. The predictability of binding was determined based on the frequency of curcumin to adopt an inhibitory pose (a pose that places the curcumin carbonyl carbon within 4.0 Å of Thr 1). Curcumin docked with an average of 80 out of 100 poses (80%) that placed at least one of the carbonyl carbons in a suitable position to undergo nucleophilic attack and the most representative pose is shown in Fig. 1C. Furthermore, due to exposed hydroxyl groups, curcumin could potentially form a hydrogen bonds with the surrounding amino acids, such as Serine 96 with a distance of 2.18 Å in the β5-subunit (Fig. 1C), strengthening its binding potential. Therefore, the computer docking results predict that curcumin binds to Thr 1 of the proteasomal β5-subunit, which might cause inhibition of the proteasomal chymotrypsin-like activity.
Inhibition of purified 20S proteasome chymotrypsin-like activity by curcumin
To provide direct evidence for proteasome inhibition by curcumin, we carried out a cell-free proteasome activity assay by incubating a purified rabbit 20S proteasome with different concentrations of curcumin. As predicted by the in silico model, we found that the β5-mediated chymotrypsin-like activity of the purified 20S proteasome was significantly inhibited by curcumin with an IC50 value of 1.85 µM (Fig. 1D). To investigate whether curcumin specifically inhibits the proteasomal chymotrypsin-like activity, its effects on the trypsin-like and PGPH-like activities of the purified 20S proteasome were also examined. When used at 1 µM, curcumin inhibited the chymotrypsin-like, trypsin-like, and PGPH-like activities of the purified 20S proteasome by 46%, 20%, and 34%, respectively (Fig. 1D), whereas at 5 µM, it inhibited these three proteasomal activities by 73%, 46%, and 59%, respectively (Fig. 1D). The IC50 values of curcumin to the chymotrypsin-like, trypsin-like, and PGPH-like activities of the purified 20S proteasome were determined to be 1.85, 6.23 and 3.68 µM, respectively. Taken together, our results show that curcumin possesses the ability to inhibit all the three proteasomal activities but with the highest potency to the chymotrypsin-like activity.
Curcumin inhibits proteasomal activity and induces apoptosis in human colon cancer HCT-116 and SW480 cells in a dose-dependent manner
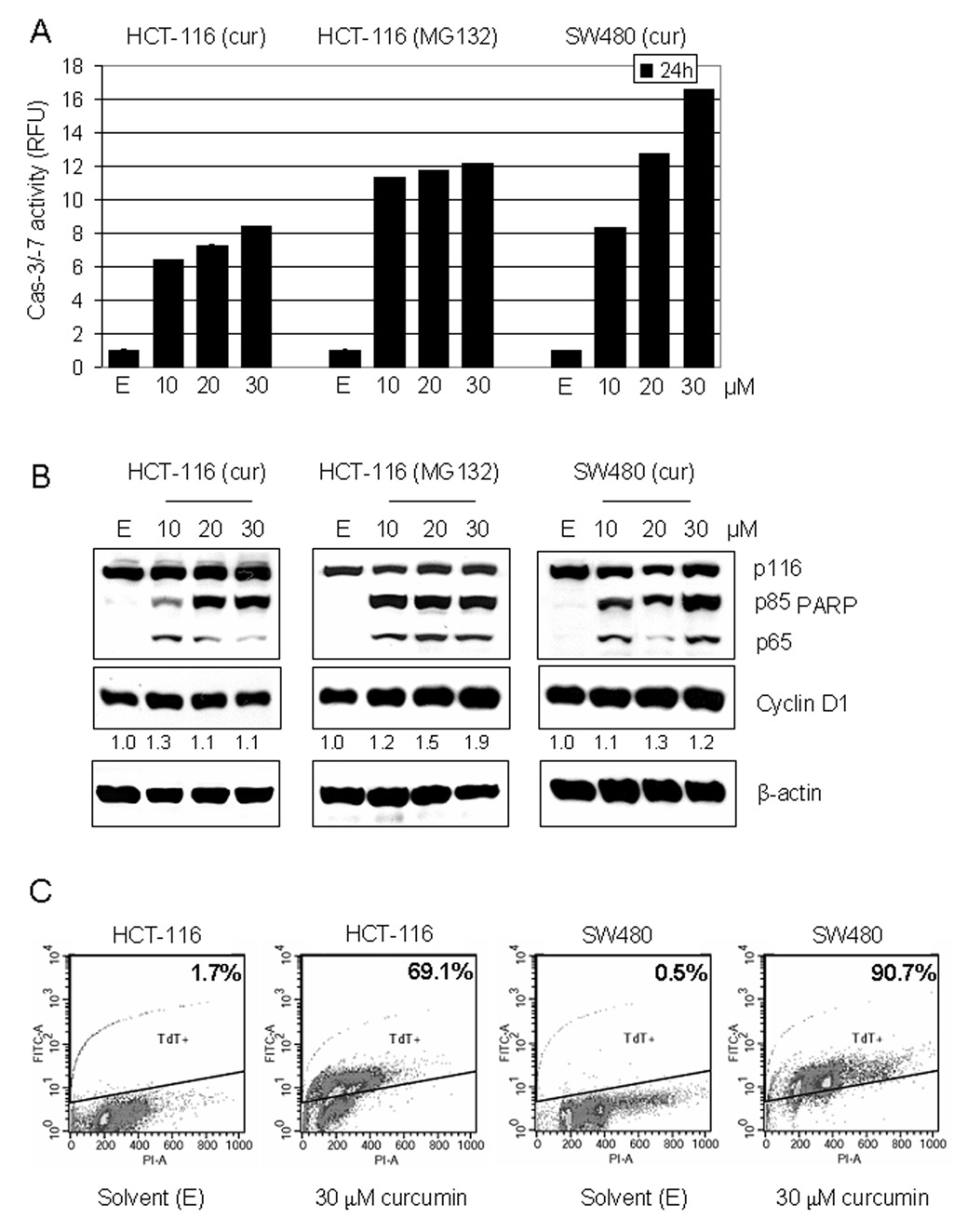
After demonstrating that curcumin inhibits the purified 20S proteasome (Fig. 1), the next set of experiments was performed to determine if curcumin would also target and inhibit the tumor cellular 26S proteasome. Human colon cancer HCT-116 and SW480 cells were plated in 96-well plates and treated with various concentrations of curcumin for 24 hours, followed by 2 hours of additional incubation with a specific peptide substrate for the proteasomal chymotrypsin-like activity. We found a dose-dependent inhibition induced by curcumin in both colon cancer cell lines (Fig. 2A). When used at a 10 µM concentration, curcumin induced 32% and 42% inhibition in HCT-116 and SW480 cells, respectively, while at 30 µM concentration 65% and 81% inhibition was induced in HCT-116 and SW480 cells, respectively (Fig. 2A).
Figure 2.

Inhibition of proteasomal chymotrypsin-like activity in human HCT-116 and SW480 colon cancer cells by curcumin. A, inhibition of proteasomal chymotrypsin-like activity by curcumin in intact HCT-116 and SW480 cells. HCT-116 and SW480 cells were treated with different curcumin concentrations (10, 20, and 30 µM) for 24 h, followed by measurement of proteasomal CT-like activity. Proteasome inhibitor MG132 was used as comparison with curcumin and the solvent, ethanol (E), was used as a control. B, Western blot analysis. HCT-116 and SW480 cells were treated with different curcumin concentrations (10, 20, and 30 µM) for 24 h, and used for whole cell extract preparation. Cell extract was analyzed by Western blot for accumulation of ubiquitinated proteins, IκB-α, and Bax (right and left panels). Proteasome inhibitor MG132 was used as comparison to curcumin (middle panel) and the solvent, ethanol (E), was used as a control. Actin was used as a loading control. Change in the levels of ubiquitinated IκB-α and p36/Bax proteins in the cells treated with curcumin, and the levels of ubiquitinated IκB-α and p21/Bax proteins in the cells treated with MG132 was analyzed by densitometry and quantified using AlphaEase FC software.
We also compared curcumin to a well-known proteasome inhibitor MG132. When used at 10 or 30 µM concentrations in HCT-116 cells, MG132 induced 53% and 70% inhibition, respectively (Fig. 2A), showing similar potency to curcumin.
To further confirm the proteasomal inhibition caused by curcumin, HCT-116 and SW480 cells were treated for 24 hours, harvested, and used for cell extraction, followed by Western blot analysis. We found a dose-dependent accumulation of ubiquitinated proteins in both cell lines (Fig. 2B, left and right panels). We have previously reported an ubiquitinated form of IκB-α protein with molecular weight of ~56 kD (34). Dose-dependent accumulation of a similar p56 band was detected in both cell lines, after 24 hours of treatment (Fig. 2B, left and right panels). It is well known that ubiquitinated IκB-α protein is recognized and degraded by the proteasome leading to release of NF-κB and its translocation to the nucleus, where it activates genes involved in cell proliferation and survival. However, when the proteasomal activity is inhibited, ubiquitinated IkB-α stays bound to NF-κB, preventing its transcriptional activity. Therefore, accumulation of ubiquitinated form of IkB-α upon curcumin treatment confirms proteasomal inhibition. As a comparison, treatment of HCT-116 cells with MG132 had similar effect on accumulation of ubiquitinated proteins and ubiquitinated IkB-α (Fig. 2B, middle panel).
To investigate whether the proteasomal inhibition by curcumin is associated with apoptosis induction, caspase-/-7 activation and PARP cleavage were measured, and TUNEL assay was performed. After 24 hours of treatment with curcumin, a dose-dependent increase in caspase-3/-7 activity was detected in both HCT-116 and SW480 cells (Fig. 3A). At 10 µM concentration curcumin induced a 6.4-fold and 8.2-fold increase in HCT-116 and SW480 cells, respectively, while at 30 µM it induced 8.4-fold and 16.6-fold increase in HCT-116 and SW480 cells, respectively (Fig. 3A). It has been shown that caspase-3/-7 activation is associated with production of the p85 cleaved PARP fragment (35), which we detected by Western blot analysis, together with a p65 PARP fragment (Fig. 3B, left and right panels), which was previously shown to be a product of calpain cleavage (36). The calpain involvement is further supported by the accumulation of p36/Bax, a homodimer of p18/Bax (a calpain cleaved product of p21/Bax) (37–38), found in the cells treated with all three concentrations of curcumin in both cell lines (and to some extent in the cells treated with MG132) (Fig. 2B). Our results demonstrate that curcumin is able to inhibit the proteasomal chymotrypsin-like activity and activate caspase-3/-7 and calpain, thereby inducing apoptosis in human colon cancer HCT116 and SW480 cells. Treatment with MG132 had similar apoptosis-inducing effect in HCT-116 cells (Fig. 3A and 3B, middle panels). Dose-dependent apoptosis induction by curcumin was confirmed with TUNEL assay (Fig. 3C and data not shown). At the highest concentration used (30 µM), curcumin induced production of 69.1% apoptotic HCT-116 and 90.7% apoptotic SW480 (Fig. 3C). Curcumin also induced apoptosis-related changes in cell morphology (shrunken cells and blebbing) in a concentration-dependent manner (data not shown). Therefore, SW480 cells are slightly more sensitive to curcumin treatment than HCT-116 cells.
Figure 3.
Induction of apoptosis in human HCT-116 and SW480 colon cancer cells by curcumin. A, A cell-free caspase-3/-7 activity assay. HCT-116 and SW480 cells were treated with different curcumin concentrations (10, 20, and 30 µM) for 24 h, harvested and prepared whole cell extract was used for fluorescent detection of caspase-3/-7 activity (see Materials and Methods). Proteasome inhibitor MG132 was used as comparison with curcumin and the solvent, ethanol (E), was used as a control. Columns, mean of representative independent triplicate experiments; bars, SD. B, Western blot analysis. Cell extract from HCT-116 and SW480 cells treated with curcumin was analyzed by Western blot for PARP cleavage and Cyclin D1 detection (left and right panels). Proteasome inhibitor MG132 was used as comparison with curcumin (middle panel) and the solvent, ethanol (E), was used as a control. Actin was used as a loading control. Change in Cyclin D1 protein level was analyzed by densitometry and quantified using AlphaEase FC software. C, For TUNEL assay, HCT-116 and SW480 cells were treated for 24 h with 30 µM curcumin or ethanol (solvent) as a control. The TUNEL assay was performed by using an APO-DIRECT kit and the number of apoptotic (TdT positive) cells is indicated.
Since curcumin has been previously shown to induce cell cycle arrest (16), we wanted to investigate if under our experimental conditions, in addition to apoptosis induction, curcumin also affected cell cycle progression. Western blot analysis was used to examine the levels of Cyclin D1, which is essential for G1/S transition. 24 hour treatment with either concentration of curcumin did not significantly affect Cyclin D1 protein expression (Fig. 3B, left and right panels), indicating that under these conditions curcumin-mediated proteasome inhibition mainly triggers apoptosis. Under the same experimental condition, 30 µM MG132 induced about 2-fold increase in Cyclin D1 protein level (Fig. 3B, middle panel). MG132 was also found to have stronger anti-proliferative effect against HCT-116 cells compared to curcumin, while the ability of curcumin to inhibit proliferation of HCT-116 and SW480 cells was very similar, as measured by MTT assay (data not shown).
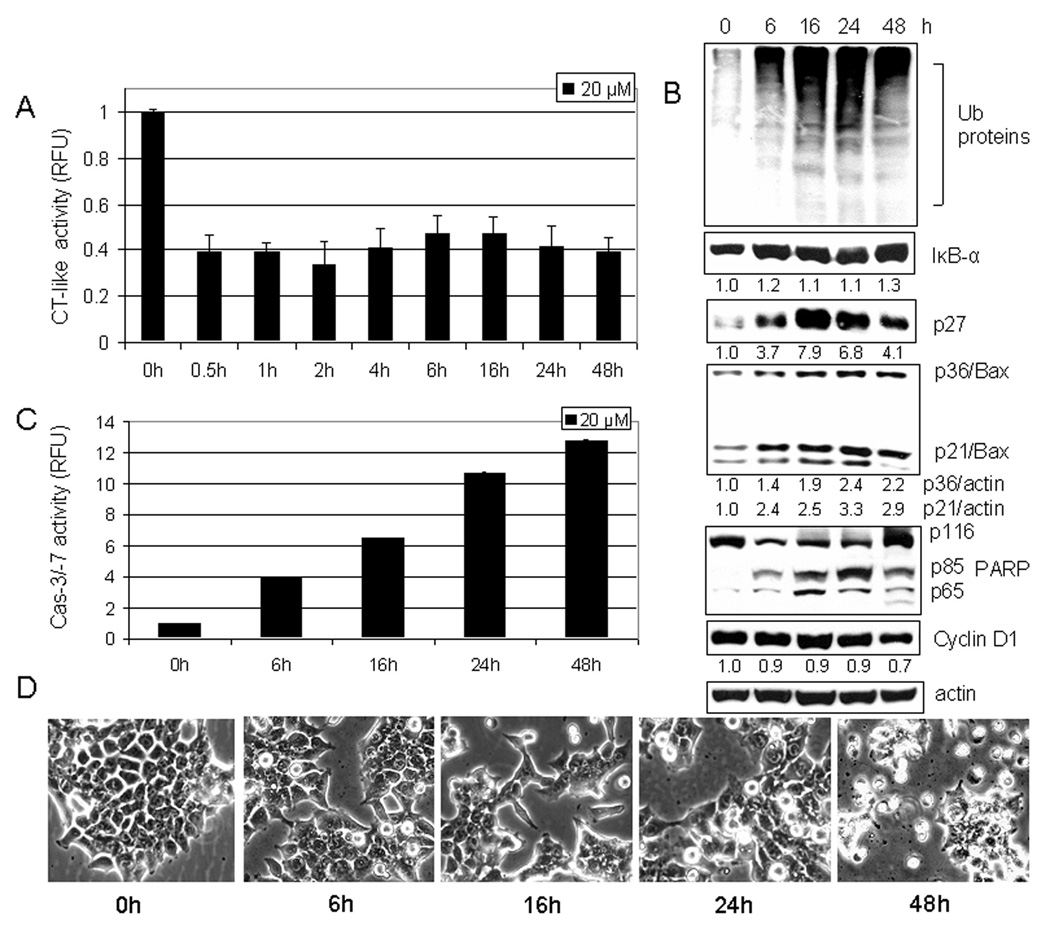
Curcumin-induced proteasome inhibition and apoptosis induction are time-dependent
To further study the effect of curcumin in human colon cancer cells, HCT-116 cells plated in 96-well plate were treated with 20 µM curcumin for various time points (0.5–12 h). We found that 60% of the proteasomal chymotrypsin-like activity was inhibited as early as 0.5 hour after the addition of curcumin and remained inhibited at about the same extent for the rest of the experimental period (Fig. 4A). Proteasomal inhibition was confirmed with Western blot analysis that showed an increased level of ubiquitinated proteins after 6 hours of treatment, together with the accumulation of the proteasomal target proteins, IκB-α, p27, and p21/Bax (Fig. 4B).
Figure 4.
Kinetic effect of curcumin on HCT-116 cells. A, HCT-116 cells were treated with 20 µM curcumin for 0.5 to 48 h, followed by the proteasomal chymotrypsin-like activity assay using Z-GGL-AMC. B, HCT-116 cells treated with 20 µM curcumin for 6 to 48 hours were used for whole cell extract preparation. Columns, mean of representative independent triplicate experiments; bars, SD. Cell extract analyzed by Western blot confirmed proteasomal inhibition by accumulation of ubiquitinated proteins, and proteasome target proteins IκB-α, p27, and p21/Bax. Apoptosis induced by curcumin treatment was confirmed by PARP cleavage (B), caspase-3/7 activation (C), and apoptotic morphological changes (D). Actin was used as a loading control. Columns, mean of independent triplicate experiments; bars, SD. Change in the level of IκB-α, p27, p21/Bax, p36/Bax and Cyclin D1 proteins was analyzed by densitometry and quantified using AlphaEase FC software.
In the same kinetic experiment, apoptosis-associated morphological changes were observed after first 6 hours of treatment and these changes were greatly increased after 48 hours (Fig. 4D). To confirm apoptosis induction, we measured the activity of caspase-3/-7 and found a 4, 6.5, 11, and 13-fold increase at 6, 16, 24, and 48 hours, respectively (Fig. 4C). Apoptosis was also confirmed with Western blot analysis by the presence of PARP cleavage (Fig. 4B). The appearance of p65 cleaved PARP fragment and accumulation of p36/Bax were also detected during the treatment (Fig. 4B), again supporting activation of calpain. Additionally, slight down-regulation (~30%) of Cyclin D1 by curcumin was found at the later time point (48 h) (Fig. 4C).
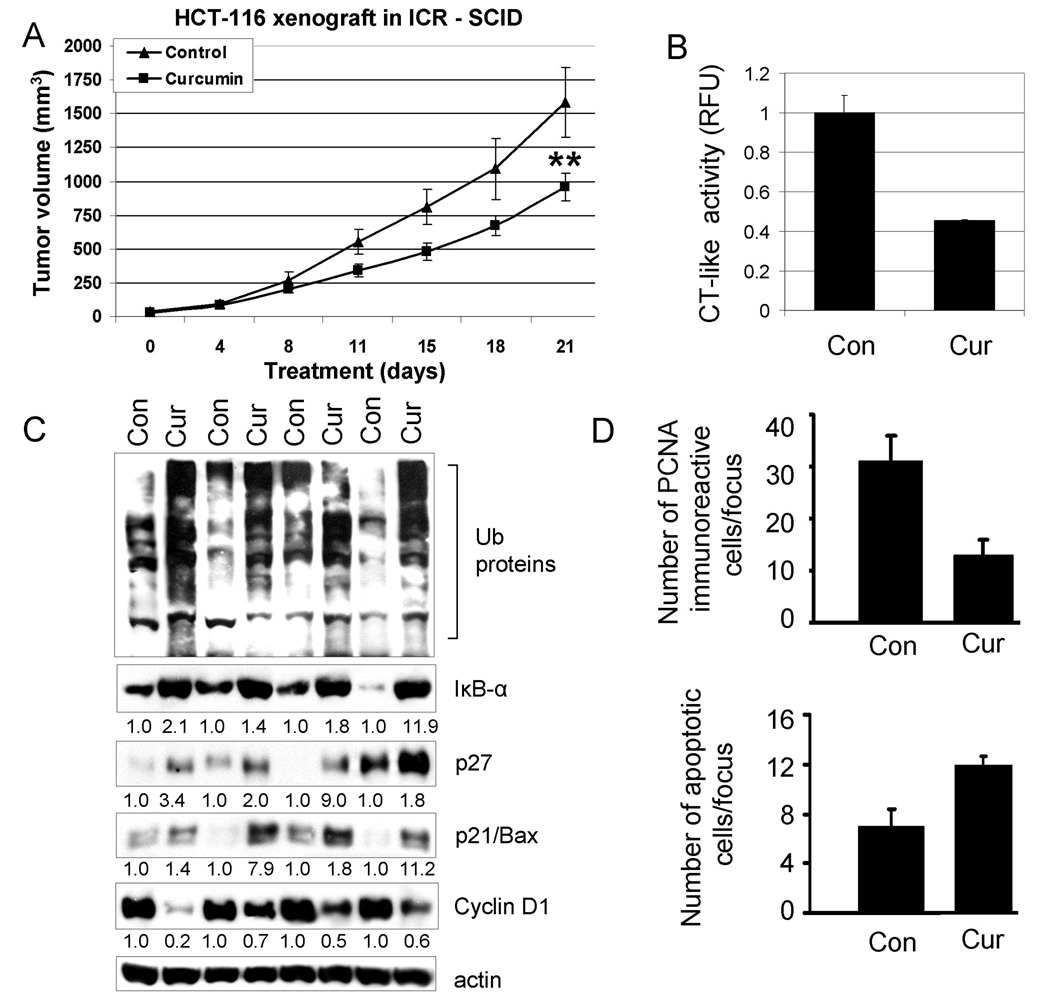
Curcumin suppresses the growth of human colon cancer HCT-116 xenografts, associated with proteasome inhibition, proliferation suppression and apoptosis induction
After we demonstrated that curcumin inhibited proteasomal activity and induced apoptosis in cultured colon cancer cells (Fig. 3–Fig. 4), we investigated whether the anti-tumor activity of curcumin could, at least partly, be attributed to inhibition of the proteasomal activity and induction of apoptosis in vivo. To do so, we implanted HCT-116 cells (3 × 106) subcutaneously into ICR SCID mice (both flanks). When the tumors began to enlarge, mice were randomized into the 2 groups for treatment of vehicle control and curcumin (500 mg/ kg body weight given intragastric every day for 3 weeks). Inhibition of tumor growth by curcumin was observed after a 21 day–treatment, confirming the anti-tumor activity of curcumin (Fig. 5A). Control tumors grew to an average size of 1587 mm3, and curcumin–treated tumors grew to 959 mm3, corresponding to 40% inhibition (P <0.01; Fig. 5A).
Figure 5.
Curcumin treatment leads to inhibition of the proteasomal chymotrypsin-like activity, proliferation suppression and apoptosis induction in vivo. Female homozygous ICR SCID mice bearing HCT-116 tumors were treated with either control solvent or curcumin at 500 mg/kg/d to day 21. A, inhibition of HCT-116 tumor growth by curcumin. Points, mean tumor volume in each experimental group containing 6 mice; bars, SD; **, P <0.01. B–D, effects of curcumin at the end point of the experiment. Tumors were collected after 21 days of treatment, and the prepared tissues were analyzed by the proteasomal chymotrypsin-like activity assay (B), Western blotting (C), and immunohistochemistry (D). Inhibition of proteasome activity (B), accumulation of ubiquitinated proteins, p27, IκB-α, and p21/Bax proteins and down-regulation of Cyclin D1 (C) were found in all four tumors treated with curcumin (Cur), compared to control mice (Con) treated with the control solvent alone. The slides prepared from the tumors treated with the control solvent or curcumin were used for PCNA immunostaining and TUNEL (D). Bars represent mean numbers of PCNA immunoreactive cells per focus, SD (D, upper panel), and mean numbers of apoptotic cells per focus, SD (D, lower panel). Change in protein level was analyzed by densitometry and quantified using AlphaEase FC software.
To determine if the observed anti-tumor effect of curcumin was associated with its proteasome-inhibitory activity, we extracted proteins from the tumor remnants and used them for multiple assays. We found that the proteasomal chymotrypsin-like activity was inhibited by 55% in the tumors from mice treated with curcumin, compared to vehicle-treated mice (Fig. 5B). This was associated with the accumulation of ubiquitinated proteins and the proteasome target proteins p27, IκB-α and p21/Bax (Fig. 5C). Increased expression of p27 was confirmed with immunostaining only in the tumors from mice treated with curcumin (Fig. 6A). The results show that curcumin is able to inhibit tumor proteasome activity in vivo.
Figure 6.
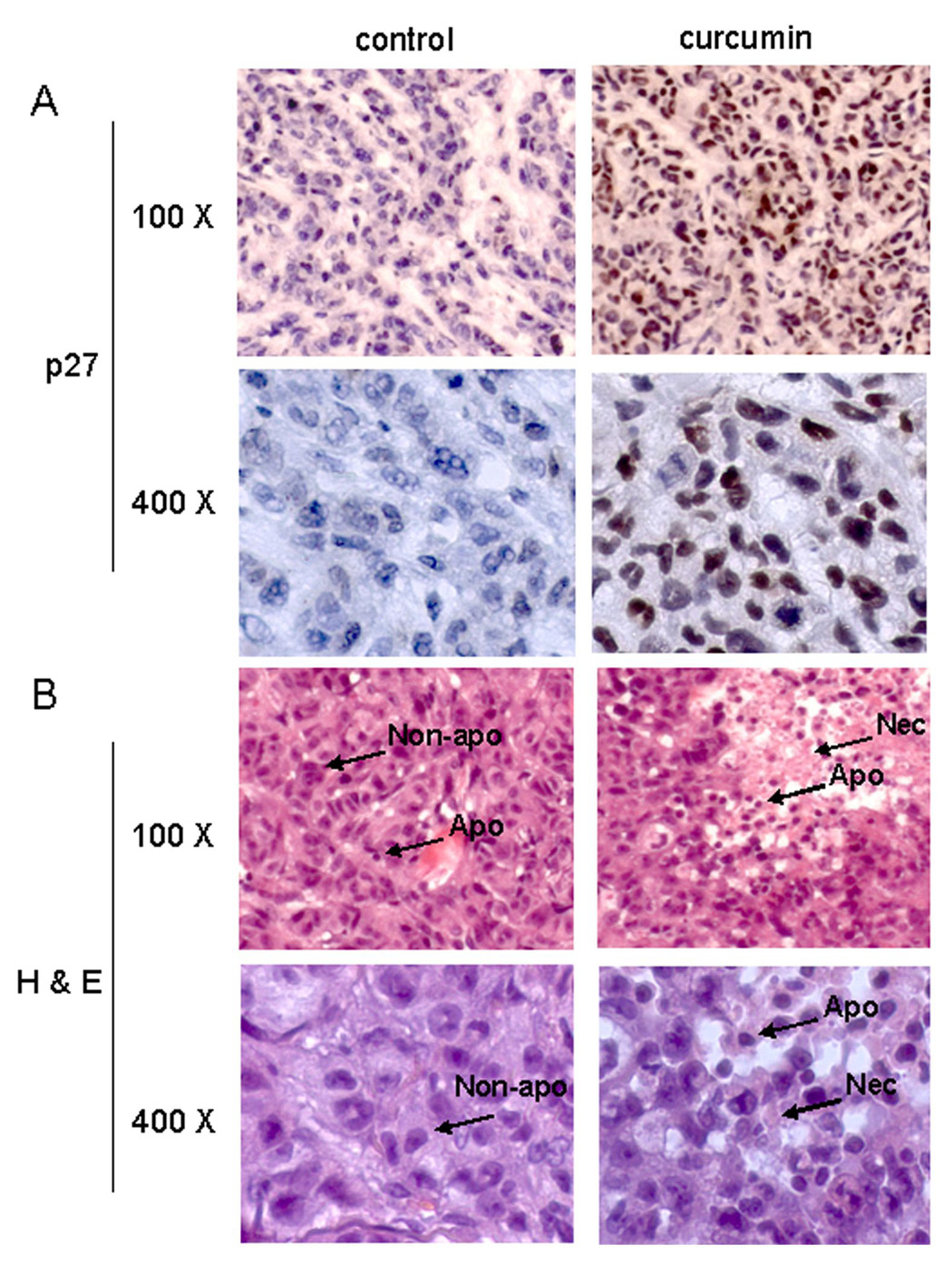
Immunohistochemistry p27 and Hematoxylin and Eosin (H & E) staining assays using mouse tumor samples. Tumors were collected after 21-day treatment (see Fig. 5 legend), and the prepared tissue slides were used for immunostaining with p27 antibody (A), and H & E staining assays (B). Stronger or/and more p27 positive cells (A), more apoptotic-condensed nuclei (Apo with arrow), and more necrotic tumor cells (Nec with arrow) were found in tumor tissue from mice treated with curcumin. Only few apoptotic cells and much more non-apoptotic cells (Non-apo with arrow) were found in tumor tissue from mice treated with solvent. Magnifications are 100X and 400X as indicated.
The formalin-fixed tumor tissue was analyzed to determine whether curcumin-induced proteasomal inhibition found in colon cancer caused a consequent inhibition of proliferation and stimulation of apoptosis. There was a 3-fold reduction in PCNA immunoreactivity (a marker of cell proliferation) in the tumor remnants of curcumin-treated ICR SCID mice compared with the vehicle-treated controls (Fig. 5D, upper panel). Consistent with growth inhibition in vivo by curcumin, Western blot analysis showed down-regulation of Cyclin D1, another marker of cell proliferation, in the tumors from mice treated with curcumin, compared to control (Fig. 5C), which was not observed in cultured cells treated with curcumin (Fig. 3 & Fig. 4).
Apoptosis was then determined using the TUNEL assay, which showed a 2-fold increase in the number of apoptotic cells in the tumors from mice treated with curcumin compared to the corresponding vehicle-treated control (Fig. 5D, lower panel). High levels of condensed apoptotic nuclei, another apoptotic feature, together with necrotic tumor cells were observed in tumors from curcumin-treated animals following Hematoxylin and Eosin staining (Fig. 6B). As a comparison, in tumor tissue from mice treated with solvent, only few apoptotic cells and much more non-apoptotic cells were found. Taken together, these data show that curcumin has the ability to regress colon cancer growth in ICR SCID mice bearing HCT-116 xenografts by inhibiting the proteasomal chymotrypsin-like activity, resulting in proliferation inhibition and apoptosis induction.
Discussion
Interest in potential cancer chemopreventive and therapeutic properties of dietary ingredients, which are generally more tolerable in the human body (15), has increased in recent years, especially for the cancers that usually do not respond well to currently available therapies (17). Therefore, it is not surprising that chemoprevention of colon cancer, the leading cancer-related death in Western countries, is becoming more attractive. One of the dietary ingredients that possess anti-inflammatory and/or antioxidant properties is curcumin. Epidemiological data suggest that curcumin may be responsible for the lower colon cancer rate in India and Southeast Asia (39). Based on that and its long history of consumption without any adverse health effects, curcumin is considered to be a safe chemopreventative agent (15).
Although it was suggested previously that curcumin-induced apoptosis is mediated through the impairment of the ubiquitin-proteasome pathway (40), the exact mechanism was not fully elucidated. While the anti-oxidative property of curcumin is well-documented, it has also been shown that at higher concentrations (50 and 100 µM) curcumin could induce apoptosis though production of reactive oxygen intermediates (41). Therefore, it was hypothesized that curcumin could induce proteasomal malfunction through the oxidative stress generation (40). Another possible mechanism for the proteasomal inhibition induced by curcumin was proposed based on its chemical structure. Since it belongs to a class of compounds with α,β-unsaturated ketones and two sterically accessible β carbons, curcumin was suggested to inhibit the ubiquitin isopeptidase activity located at the 19S regulatory subunit of the 26S proteasome (42).
On the other hand, the chemical structure of curcumin led us to the hypothesis of curcumin’s direct binding to the 20S proteasome. By using an in silico model, we found that both carbonyl carbons are indeed susceptible to nucleophilic attack by Thr 1 within the β5 subunit of the proteasome (Figs. 1B & C), suggesting that curcumin binds to Thr 1 with high predictability. The proteasomal inhibition by curcumin was confirmed by an in vitro experiment that showed a significant inhibition of the chymotrypsin-like activity of a purified 20S proteasome with an IC50 value of 1.85 µM (Fig. 1D). Taken together, these data indicate that inhibition of proteasomal chymotrypsin-like activity induced by curcumin is mediated through its direct binding.
In our study, we used physiological concentrations of curcumin (10–30 µM), thereby excluding the possibility that curcumin-induced reactive oxygen species production is involved in proteasomal inhibition. We have further confirmed that this inhibition is physiologically functional by demonstrating accumulation of ubiquitinated proteins and proteasome target proteins IκB-α, p27, and p21/Bax. More importantly, our data demonstrate that intragastric treatment of HCT-116 tumor–bearing IRA SCID mice with curcumin resulted in the inhibition of proteasomal chymotrypsin-like activity in the tumors (Fig. 5B, 5C & Fig. 6A), associated with a 40% inhibition of tumor growth (Fig. 5A), suggesting that the effective plasma levels of curcumin have been reached. Consistent with previous reports (7–8), we found that inhibition of the proteasomal chymotrypsin-like activity, induced by curcumin in colon cancer cells and xenografts, was associated with the induction of apoptosis (Fig. 3–Fig. 6). We have also found that inhibition of tumor growth was caused not only by apoptosis induction but also by induction of cell cycle arrest, most probably G1 arrest, as shown by decreased levels of two proliferation markers PCNA (Fig. 5D) and Cyclin D1 (Fig. 5C), and accumulation of p27 (Fig. 5C & Fig. 6A). However, this G1 arrest was not observed in cultured cells treated with curcumin (Fig. 3 & Fig. 4).
When comparing the in vitro and in vivo potencies of curcumin, we found that although 1.85 µM curcumin was sufficient to inhibit 50% of the chymotrypsin-like activity of purified 20S proteasome (Fig. 1D), a much higher concentration of curcumin (10 µM) produced only 32% – 42% inhibition of the chymotrypsin-like activity in colon cancer cells (Fig. 2A). This finding is similar to previous reports with other natural compounds with proteasome-inhibitory activities (30), suggesting that natural compounds have more than one cellular target and/or might not be very stable in cells. Furthermore, our in silico data show that two hydroxyl groups could potentially form hydrogen bonds with surrounding amino acids in the β5-subunit, strengthening curcumin’s potential to bind the proteasome. Modification of these two hydrogen groups, such as methylation, could also explain the lower potency of curcumin to bind and inhibit the proteasome within the cells, as opposed to the purified 20S proteasome.
We have also shown that curcumin inhibited about ~60% of the proteasomal activity within the first 30 minutes of the treatment, which did not change much until the end of 48 hour treatment (Fig. 4A). However, due to the cumulative effect of this continuous inhibition, the level of proteasomal target proteins did increase after 6 hours of treatment. Once the level of accumulated pro-apoptotic proteins reached the critical level for apoptosis induction and caspase-3/-7 activation, the cells became committed to apoptosis, resulting in time-dependent caspase-3/-7 activation. We also found that although the proteasome activity was not completely inhibited in cultured tumor cells (30–50% inhibition), apoptosis was still induced by curcumin, supporting the idea of the proteasome being an important cellular target for curcumin. Moreover, curcumin was shown to be a highly pleiotropic molecule that can interact with numerous molecular targets, thereby affecting various biochemical cascades and pathways, including caspase cascade and apoptosis (2).
We have published that some natural compounds with proteasome-inhibitory activity such as (−)-EGCG are unstable under physiologic conditions and could be rapidly degraded or metabolized through interactions with the hydroxyl groups on the phenol rings (43). Knowing that curcumin contains two hydroxyl groups, it is possible that the same mechanism could be applied on curcumin. Indeed, a number of studies over the past three decades have shown that poor absorption, rapid metabolism, and rapid systemic elimination of curcumin are responsible for its poor bioavailability (44). In an attempt to improve the bioavailability of curcumin, different approaches have been undertaken, such as combining curcumin treatment with piperine, a known inhibitor of hepatic and intestinal glucuronidation. This combinational treatment was found to significantly enhance serum concentration, absorption level and bioavailability of curcumin in both rats and humans with no adverse effects (45).
Since curcumin undergoes a rapid and efficient metabolism and biotransformation during absorption in the intestinal tract (17), it would be expected that different parts of the intestine, including the colon would accumulate a higher concentration of curcumin in the metabolically active form, compared to other organs. Numerous trials investigating the chemopreventative and therapeutic effects of curcumin alone or in combination with other natural or synthetic compounds have been and continue to be carried out (2). Curcumin has been shown to potentiate the anti-tumor effects of gemcitabine in pancreatic cancer cells growing in vitro or in vivo (46) and synergize with FOLFOX in colon cancer cells in vitro (22). It has also been demonstrated that curcumin potentiates the effect of gemcitabine, paclitaxel, TNF and TRAIL against bladder cancer cells (47). These results are consistent with that proteasome inhibition could chemosensitize human cancer cells (48). On the other hand, curcumin has also been found to significantly inhibit the chemotherapy-induced effect of camptothecin, mechlorethamine and doxorubicin in breast cancer cells in vitro, and cyclophosphamide-induced tumor regression in vivo (1). Therefore, to design a better and more efficient combination treatment that includes curcumin, it is necessary not only to identify its molecular targets but also to demonstrate that curcumin could reach them in vivo.
It has been shown that over-expression of COX-2 in intestinal epithelium is associated with increased colon cancer incidence and progression, and that curcumin has potential to specifically inhibit COX-2 expression at the RNA and protein level (49). Although the underlying mechanism of COX-2 inhibition by curcumin is not well understood, it seems to be different from the class of COX-2 inhibitors that act through direct binding to the COX-2 enzyme (50). Different mechanisms of action are supported by the fact that no toxicity associated with curcumin treatment, even when used at very high doses, has been observed (2). On the other hand, the observed toxicity associated with COX-2 inhibitor treatment seems to be result of their specific chemical properties and are unrelated to COX-2 binding and inhibition (51). Since both colon cancer cell lines used in our study (HCT-116 and SW480) do not express COX-2, our finding is consistent with previously published data, which indicate that growth inhibition of colon cancer cells by curcumin is independent of COX-2 expression (49) and supported by recently published finding that DHA (docosahexaenoic acid) stimulates degradation of β-catenin and induces apoptosis in the same cell lines independently of COX-2 inhibition (52). Our data showing that curcumin is able to inhibit the proteasome and induce apoptosis in both, HCT-116 and metastatic colon cancer SW480 cells, suggests its possible use for treatment of both early stage and late stage/refractory colon cancer.
In summary, our data reveal the proteasome as an important cellular target of curcumin in vitro and in vivo. We have demonstrated that inhibition of the proteasomal activity (especially, the chymotrypsin-like activity) by curcumin is a strong apoptotic stimulus for both colon cancer HCT-116 and SW480 cell lines in vitro and that SW480 cells are slightly more sensitive to curcumin treatment than HCT-116 cells. We have also shown that intragastric administration of curcumin to ICR SCID mice bearing xenografts of colon cancer HCT-116 cells resulted in significant inhibition of tumor growth, as a consequence of proteasomal inhibition, inhibition of cell proliferation and stimulation of apoptosis. The fact that curcumin is able to target the human colon tumor proteasome and inhibit human colon tumor growth in vivo provides a strong impetus for using curcumin as a chemopreventative and/or chemotherapeutic agent for human colon cancer.
Acknowledgments
We thank to Dr. Charles D. Lopez, of Oregon Health & Science University for providing colon cancer HCT-116 cell lines, Prof. Satya Narayan, from UF Shands Cancer Center for providing metastatic colon cancer SW480 cell lines and Dr. Di Chen and the Karmanos Cancer Institute Pathology Core Facility for assisting in TUNEL and Immunohistochemistry assays.
Grant support: Karmanos Cancer Institute of Wayne State University (to Q. P. Dou), National Cancer Institute Grants (CA120009, CA112625 to Q. P. Dou.), and the NCI/NIH Cancer Center Support Grant (to Karmanos Cancer Institute).
References
- 1.Somasundaram S, Edmund NA, Moore DT, et al. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 2.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 4.Landis-Piwowar KR, Milacic V, Chen D, et al. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist Updat. 2006;9:263–273. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. Embo J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seemuller E, Lupas A, Stock D, et al. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 7.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 8.Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 9.Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Update. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 10.Adams J. Potential for proteasome inhibition in the treatment of cancer. Drug Discov Today. 2003;8:307–315. doi: 10.1016/s1359-6446(03)02647-3. [DOI] [PubMed] [Google Scholar]
- 11.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 12.Orlowski RZ, Eswara JR, Lafond-Walker A, et al. Tumor growth inhibition induced in a murine model of human Burkitt’s lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–4348. [PubMed] [Google Scholar]
- 13.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 14.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 17.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–470. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 18.Voutsadakis IA. Pathogenesis of colorectal carcinoma and therapeutic implications: the roles of the ubiquitin-proteasome system and Cox-2. J Cell Mol Med. 2007;11:252–285. doi: 10.1111/j.1582-4934.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Lahav-Baratz S, Ciechanover A. Two distinct ubiquitin-dependent mechanisms are involved in NF-kappaB p105 proteolysis. Biochem Biophys Res Commun. 2006;345:7–13. doi: 10.1016/j.bbrc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 21.Lev-Ari S, Strier L, Kazanov D, et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 22.Patel BB, Sengupta R, Qazi S, et al. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Mediating Growth Inhibition of Colon Cancer Cells by Modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 23.Smith DM, Daniel KG, Wang Z, et al. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins. 2004;54:58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- 24.Milacic V, Chen D, Ronconi L, et al. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006;66:10478–10486. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, Cui QC, Yang H, et al. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Banerjee S, Wang Z, et al. Antitumor activity of epidermal growth factor receptor–related protein is mediated by inactivation of ErbB receptors and nuclear factor-κB in pancreatic cancer. Cancer Res. 2006;66:1025–1032. doi: 10.1158/0008-5472.CAN-05-2968. [DOI] [PubMed] [Google Scholar]
- 27.Xiao ZQ, Moragoda L, Jaszewski R, et al. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev. 2001;122:1849–1864. doi: 10.1016/s0047-6374(01)00323-2. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Nam S, Lee CS, et al. CEP1612, a dipeptidyl proteasome inhibitor, induces p21WAF1 and p27KIP1 expression and apoptosis and inhibits the growth of the human lung adenocarcinoma A-549 in nude mice. Cancer Res. 2001;61:1280–1284. [PubMed] [Google Scholar]
- 29.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 30.Nam S, Smith DM, Dou QP. Tannic acid potently inhibits tumor cell proteasome activity, increases p27 and Bax expression, and induces G1 arrest and apoptosis. Cancer Epidemiol Biomarkers Prev. 2001;10:1083–1088. [PubMed] [Google Scholar]
- 31.Smith DM, Wang Z, Kazi A, et al. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–392. [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Daniel KG, Chen MS, et al. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Daniel KG, Landis-Piwowar KR, Chen D, et al. Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome beta5 subunit. Int J Mol Med. 2006;18:625–632. [PubMed] [Google Scholar]
- 34.Chen D, Peng F, Cui QC, et al. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 35.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 36.Pink JJ, Wuerzberger-Davis S, Tagliarino C, et al. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during β-Lapachone-mediated apoptosis. Exp Cell Res. 2000;255:144–155. doi: 10.1006/excr.1999.4790. [DOI] [PubMed] [Google Scholar]
- 37.Gao G, Dou QP. N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes Bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem. 2000;80:53–72. doi: 10.1002/1097-4644(20010101)80:1<53::aid-jcb60>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 41.Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 42.Mullally JE, Fitzpatrick FA. Pharmacophore model for novel inhibitors of ubiquitin isopeptidases that induce p53-independent cell death. Mol Pharmacol. 2002;62:351–358. doi: 10.1124/mol.62.2.351. [DOI] [PubMed] [Google Scholar]
- 43.Landis-Piwowar KR, Huo C, Chen D, et al. A Novel Prodrug of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate as a Potential Anticancer Agent. Cancer Res. 2007;67:4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 44.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 45.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 46.Kunnumakkara AB, Guha S, Krishnan S, et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 47.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 48.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- 49.Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 50.Soliva R, Almansa C, Kalko SG, Luque FJ, Orozco M. Theoretical studies on the inhibition mechanism of cyclooxygenase-2. Is there a unique recognition site? J Med Chem. 2003;46:1372–1382. doi: 10.1021/jm0209376. [DOI] [PubMed] [Google Scholar]
- 51.Mason RP, Walter MF, McNulty HP, et al. Rofecoxib increases susceptibility of human LDL and membrane lipids to oxidative damage: a mechanism of cardiotoxicity. J Cardiovasc Pharmacol. 2006;47 Suppl 1:S7–S14. doi: 10.1097/00005344-200605001-00003. [DOI] [PubMed] [Google Scholar]
- 52.Calviello G, Resci F, Serini S, et al. Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis. 2007;28:1202–1209. doi: 10.1093/carcin/bgl254. [DOI] [PubMed] [Google Scholar]