Abstract
Src family tyrosine kinases (SFK) play an important role in growth and metastasis of many types of human malignancies. However, their significance in Ewing’s sarcoma remains to be elucidated. The purpose of this study was to evaluate the role of Lyn, one member of the SFK, in Ewing’s sarcoma growth and metastasis and to determine whether a SFK inhibitor can induce Ewing’s tumor regression. Lyn was expressed and activated in TC71, A4573, and SK-ES human Ewing’s sarcoma cells. Lyn expression was seen in 13 of 15 patient tumor samples, 6 of which showed Lyn activation. Specific inhibition of Lyn using small interfering RNA significantly decreased primary tumor growth and lytic activity, and also reduced lung metastases in vivo. Down-regulation of Lyn resulted in decreased invasive capacity of tumor cells in vitro. AP23994, a small-molecule SFK inhibitor, decreased Lyn kinase activity and suppressed TC71 cell growth in vitro in a dose-dependent manner. Furthermore, treatment of mice bearing s.c. TC71 tumors with AP23994 or with polyethylenimine/Lyn-small interfering RNA gene therapy resulted in reduced Lyn kinase activity and significant tumor growth suppression. EWS/FLI-1, which is translocation fusion protein associated with Ewing’s sarcoma, regulated Lyn gene expression and kinase activity. These data suggest that targeting Lyn may be a new therapeutic approach in treatment of Ewing’s sarcoma.
Introduction
Ewing’s sarcoma is a poorly differentiated pediatric and young adult cancer of bone and soft tissue. The presence of the EWS/ETS fusion protein, arising from translocation of EWS gene on chromosome 22 with a member of the ETS family of developmentally regulated genes on chromosome 11, is characteristic of Ewing’s sarcomas (1). The EWS/ETS fusion protein has altered transcriptional activity. Modulation of downstream target genes through transcriptional activation is thought to contribute to development of Ewing’s sarcoma. EWS/FL-1 is the most common chromosome translocation associated with Ewing’s sarcomas (1). Rapid tumor growth and extensive bone destruction are typical features of this tumor (2, 3). Clinically, ∼25% of patients have metastases at diagnosis (4). Similar to several other sarcomas, Ewing’s sarcoma displays an aggressive behavior with a tendency toward recurrence following treatment with a combination of surgery, radiation, and chemotherapy. Despite these multimodal approaches, the survival rate remains poor: 50% at 5 years and <30% at 10 years (5, 6). Cure rates have remained stagnant for >15 years particularly for those patients who present with metastatic disease underscoring the need for new innovative treatment modalities.
Src family tyrosine kinases (SFK) mediate signal transduction from several receptor tyrosine kinases, such as epidermal growth factor receptor, platelet-derived growth factor receptor, HER-2/neu, and fibroblast growth factor receptor, and are involved in the regulation of tumor cell proliferation, adhesion, motility, and invasion. SFK contribute to tumor progression, metastasis, and angiogenesis. As a member of SFK, Lyn was found to be activated in blasts from patients with acute myelogenous leukemia (7). Overexpression of Lyn has been linked to resistance of the Bcr-Abl kinase inhibitor STI571 (8). Down-regulation of Lyn by small interfering RNA (siRNA) induced apoptosis in primary and drug-resistant Bcr-Abl-positive leukemia cells (9). However, studies with Lyn mutant mice showed a predisposition to myelogenic tumor development (10). These studies indicate the complexity of Lyn kinase. Despite numerous studies, the role of Lyn in the B-cell-mediated immune response, B-cell leukemia, lymphoma, and solid tumors is poorly understood (11-14).
High c-Src kinase activity has been reported in Ewing’s sarcoma cell lines compared with normal human fibroblasts and other tumor cell lines (15). Recently, Shor et al. reported that the small-molecule SFK inhibitor, dasatinib, induced apoptosis in Ewing’s sarcoma cells despite the low levels of c-Src activation detected in these cells (16). Dasatinib is a Src/Abl inhibitor that targets Src family kinases in addition to c-Src. Therefore, other Src kinases may play a role in Ewing’s sarcoma tumorigenesis. This hypothesis is supported by the fact that Lyn kinase activity was found to account for >90% of pan-Src kinase activity in glioblastoma and for ∼30% of pan-Src kinase activity in other tumors, indicating that activation Lyn kinase can promote the tumorigenesis (12).
Here, we determined the role of Lyn kinase in Ewing’s sarcoma. We showed that down-regulation of Lyn kinase activity by siRNA or by treatment with the SFK inhibitor AP23994 inhibited tumor growth and the metastatic behavior in vitro and in vivo. Furthermore, we found that EWS/FLI-1 up-regulated Lyn gene expression.
Materials and Methods
Expression Plasmids
EWS/FLI-1 cDNA was amplified from Ewing’s sarcoma TC71 cells using Expand long template PCR system (Roche Applied Science). The specific primers used were sense ATGGCGTCCACGGATTACAGTACCTATAGC and anti-sense CTAGTAGTAGCTGCCTAAGTGTGAAGGCAC. The amplified fragment was inserted into the TA PCR2.1 vector (Invitrogen/Life Technologies). The 1.6-kb HindIII/EcoRV fragment was then subcloned into pcDNA4/TO/myc-His A expression vector (Invitrogen/Life Technologies). siRNA expression vector pSilencer2.1-U6 hygro was purchased from Ambion. siRNA-expressing plasmids targeting human Lyn (Lynsi) or EWS/FLI-1 (EWSsi) were constructed as described previously (17). Briefly, complementary DNA oligonucleotides targeting human Lyn mRNA AAUGGUGGAAAGCAAAGUCCC (18) or EWS/FLI-1 mRNA ACGGGCAGCAGAACCCTTC (19) were annealed at 90°C for 3 min, cooled to 37°C, and incubated for 1 h. The annealed dsDNA oligonucleotides were ligated between BamHI and HindIII sites on the pSilencer2.1-U6 hygro vector. The control vector (si-) was constructed by inserting a sequence that expresses a siRNA with limited homology to sequences in the human and mouse genomes.
Cell Culture and Transfection
TC71 and A4573 human Ewing’s sarcoma cells were cultured in Eagle’s MEM (Invitrogen/Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 1 mmol/L sodium pyruvate, 2× MEM vitamins, 1× nonessential amino acids, and 2 mmol/L glutamine (Invitrogen/Life Technologies) at 37°C, 5% CO2 in a humidified incubator. SK-ES human Ewing’s sarcoma cells were obtained from the American Type Culture Collection and cultured in McCoy’s 5A medium with 10% fetal bovine serum. NIH3T3 murine fibroblast cells were maintained in DMEM supplemented with 10% fetal bovine serum. Cells were free of Mycoplasma, as screened by Mycoplasma Plus PCR Primer Set (Stratagene), and verified to be free of pathogenic murine viruses (National Cancer Institute-Frederick Cancer Research & Development Center). For all experiments, cells were ≤80% confluent. Transfection was done with Superfect (Qiagen) as directed by the manufacturer and selected in hygromycin B (Invitrogen/Life Technologies) containing medium at 400 μg/mL for TC71 cells or zeocin (Invitrogen/Life Technologies) at 800 μg/mL for NIH3T3 cells. Stable transfected cell clones were tested for Lyn or EWS/FLI-1 expression by Western blot.
Proliferation and Cytostasis Assay
To determine the effect of AP23994 on cell proliferation, TC71 cells were seeded into 96-well cell culture plates (5,000 per well) and allowed to adhere for 5 h before various concentrations of AP23994 or DMSO were added. Cells were cultured and treated in triplicate. The anti-proliferative activity was determined 48 h later by the MTT assay. The percentage of proliferation was normalized by comparison with the untreated control cells.
Western Blotting
Cells at 80% confluence were washed with cold PBS buffer, lysed in radioimmunoprecipitation assay buffer (1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS in PBS) containing aprotinin (2 μg/mL), leupeptin (2 μg/mL), pepstatin A (1 μg/mL), and phenylmethylsulfonyl fluoride (100 μg/mL; Sigma), and then placed on ice for 30 min. Cells were centrifuged at 13,000 rpm for 10 min to eliminate cell debris. Tumor samples from mouse model or patients were homogenized in radioimmunoprecipitation assay buffer with PRO250 homogenizer (PRO Scientific) and placed on ice for 30 min before centrifuge to eliminate cell debris. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories). The protein (50 μg) was boiled for 5 min before being loaded onto a 10% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane (Amersham Biosciences). The specific protein bands were detected with polyclonal anti-human Lyn (Ab-3; Santa Cruz), E-cadherin (Abcam), cadherin 11 (Zymed Laboratories), c-Src (Santa Cruz Biotechnology), FLI-1 (Santa Cruz Biotechnology), proliferating cell nuclear antigen (Cell Signaling Technology), or β-actin (Sigma) using the Enhanced Chemiluminescence Western blotting analysis system (Amersham Biosciences) according to the manufacturer’s instructions. Densitometric analysis was done and values were normalized with β-actin loading control.
In vitro Kinase Assay
To determine kinase activity of Lyn or other members of SFK, 200 μg cell lysate protein was immunoprecipitated with 2 μL polyclonal anti-human Lyn, c-Src, Lck, Hck, c-Fgr, or Fyn antibody followed by immobilization on protein A-Sepharose 4B beads (Pharmacia Fine Chemicals). After intensive washing, the immunocomplexes were incubated 20 min in kinase buffer [25 mmol/L HEPES (pH 7.4), 1 mmol/L DTT, 10 mmol/L MgCl2, 10 mmol/L MnCl2,1 μmol/L ATP] containing 10 μCi [γ-32P]ATP (3,000 Ci/mmol; 1 Ci = 37 GBq). Immunocomplexes were boiled for 5 min and separated on 10% SDS-PAGE gels. Autoradiography was done for 1 to 18 h on blue basic auto-rad film (ISC BioExpress).
Invasion Assay
Transwell (Corning Costar) were first coated with 100 μL Matrigel (BD Biosciences) diluted 1:10 in serum-free MEM. Mouse lung extract (25 mg lung tissue/mL PBS) was placed into lower chamber of Transwell as chemoattractant. Following Matrigel solidification, 2 × 104 TC71 cells in 200 μL serum-free MEM were added into Transwell and incubated for 46 h. Then, the cells on the top side of the Transwell filter were removed by cotton swab, cells on the bottom side of the filter were stained, and the cells were counted. The invading cells were counted in at least five randomized high-power microscopy fields. The mean and SD were calculated in each group.
Plasmid/Polyethylenimine Formulations
Polyethylenimine (PEI; 25 kDa, branched form; Aldrich) was prepared at a concentration of 0.1 mol/L in water. A PEI/plasmid mixture (1.29:1 PEI/DNA weight ratio) was prepared as described previously (20) by slowly adding the plasmid to the PEI solution while vortexing vigorously. The solution was then allowed to incubate at room temperature for 15 to 20 min before use.
AP23994 Formulations
The small-molecule SFK specific inhibitor AP23994 was obtained from Ariad Pharmaceuticals. For in vitro experiments, AP23994 was diluted in DMSO (Sigma) to the desired concentrations. For oral gavage, AP23994 was dissolved in 15% N,N-dimethylacetamide, 14% TPGS (vitamin E), 5% Tween 80, 26% polyethylene glycol 400, and 40% water.
In vivo Studies
Four- to 5-week-old specific pathogen-free athymic nude mice were purchased from Charles River Breeding Laboratories. The mice were maintained in an animal facility approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the U.S. Department of Agriculture, the Department of Health and Human Services, and the NIH. Animals were housed for 1 week before any experiments were begun. All animal protocols were approved by the Institutional Animal Care and Use Committee.
To assess bone tumor formation and lung metastasis, 2 × 105 TC71, TC/si-control, TC/Lynsi clone 8, or TC/Lynsi clone 10 cells were injected into the right tibia under anesthesia. Four weeks later, digitized radiographic images were taken using a MX-20 Specimen Radiograph System (Faxitron X-ray). A grading system for bone lysis with numeric values ranging from 0 to 4 was used to determine the extent of bone destruction (21). Grade 0 represented no bone lysis, grade 1 represented minimal but visible bone lysis within the medullary canal, grade 2 represented moderate bone lysis in the medullary canal with preservation of the cortex, grade 3 represented severe bone lysis with cortical disruption, and grade 4 represented massive destruction. The bone tumors were then removed by amputation under anesthesia and the mice allowed to recover. The mice were sacrificed 8 weeks after amputation and lungs were removed to quantify lung metastasis.
To determine the effect of AP23994 on Ewing’s sarcoma tumor growth in vivo, 2 × 106 TC71 cells were injected s.c. into nude mice. Three days later, when the tumors were palpable, the mice were divided into three groups. Group 1 received no therapy (controls). Group 2 received vehicle control orally twice weekly for 4 weeks. Group 3 received oral AP23994 50 mg/kg twice weekly for 4 weeks. Tumor size was measured and tumor tissue removed to determine proliferation by Ki-67 staining and apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining.
To assess the effect of EWS/FLI-1 siRNA (EWSsi) gene therapy, 2 × 106 TC71 cells were injected s.c. into nude mice. Three days later, when the tumors were palpable, the mice were divided into three groups. Group 1 mice received no treatment (controls). Group 2 received intratumor injections (20 μg/mouse) of PEI:si-control twice weekly for various time periods. Group 3 received intratumor injections with 20 μg PEI:EWSsi twice weekly as described for Group 2. The tumors were measured every 4 days with calipers and the diameters were recorded. Tumor volume was calculated by the formula: a2b / 2, where a and b are the two maximum diameters. Tumor tissue was homogenized for protein extraction to determine Lyn, EWS/FLI-1, and proliferating cell nuclear antigen expression by Western blot.
Immunohistochemical Analysis
Tumor sections were stained with H&E. Frozen sections were fixed with acetone, incubated in 3% H2O2 in methanol for 10 min to block endogenous peroxidase, and then incubated in 5% normal horse serum plus 1% normal goat serum in PBS for 20 min to block nonspecific protein. Expression of Ki-67 was detected using a mouse anti-human Ki-67 as the primary antibody (Dako); a rabbit anti-mouse horseradish peroxidase was the second antibody followed by incubation with chromogen diaminobenzidine. Apoptotic cells were quantified using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining. Formalin-fixed paraffin-embedded sections were dewaxed before being permeabilized with proteinase K for 15 min at room temperature. After blocking endogenous peroxidase in 3% H2O2, the fragmented DNA was labeled with biotin-16-dUTP with terminal transferase at 4°C overnight in terminal deoxynucleotidyl transferase buffer before reacted with streptavidin followed by incubation with chromogen diaminobenzidine.
Statistical Analysis
Two-tailed Student’s t test was used to statistically evaluate the tumor volumes, invasion, and proliferation of TC71 cells before and after Lyn down-regulation by siRNA or inhibition by AP23994. χ2 test was used to statistically evaluate the incidence of lung metastases in Fig. 2. P < 0.05 was considered statistically significant.
Figure 2.
Effect of inhibition of Lyn expression on Ewing’s sarcoma tumor growth, tumor-induced bone lysis, and lung metastases in vivo. A, cell clones were obtained from TC71 cells after transfection with a control vector (TC/si-control) or Lyn siRNA-expressing plasmid (TC/Lynsi-8 and TC/Lynsi-10). Lyn inhibition was confirmed by Western blot and kinase assay. The relative fold was determined by densitometry. Each band was adjusted with the β-actin loading control. B, TC71, TC/si-control, or TC/Lynsi clones 8 and 10 cells (2 × 105) were injected into the right tibia of nude mice. Radiographic images were taken 3 wk later. Representative bone tumor images from each group. C, TC71, TC/si-control, or TC/Lynsi clones 8 and 10 cells were inoculated into the tibias of nude mice. Four weeks later, digitized radiographic images were taken using a MX-20 Specimen Radiograph System. †, each osteolytic bone tumor lesion was graded from 0 to 4 as described in Materials and Methods (with 4 being the most lytic). The average severity of bone destruction in each group was the mean grading score of five different animals. The incidence of lung metastases was determined at 8 wk. ‡, number of mice with lung tumors/total number of mice. The percentage of mice with metastases is in parentheses. §, P < 0.05; **, P = 0.058, compared with TC/si-control mouse group using χ2 test. D, mice received a s.c. injection of TC71 cells. Three days later, mice were divided into three groups and given intratumoral injections of PEI:si-control, PEI:Lynsi, or no treatment. Tumor size was measured at various time points. *, P < 0.01, compared with the untreated or PEI:si-control group.
Results
Lyn Expression and Lyn Kinase Activity in Ewing’s Sarcoma Cell Lines and Patient Samples
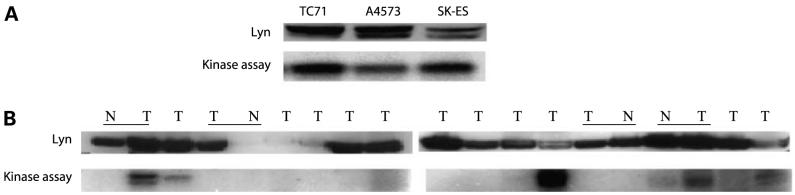
We first determined Lyn expression and kinase levels in three different Ewing’s sarcoma cell lines. Lyn protein expression were detected in TC71, A4573, and SK-ES cells (Fig. 1A). The high kinase activity in TC71, A4573, and SKES cells was further confirmed using a kinase assay. By contrast, kinase activity of c-Src, Fyn, Fgr, Hck, and Lck was not detectable in these cell lines (data not shown). These data suggest that Lyn is the predominant cellular Src kinase.
Figure 1.
Expression of Lyn protein and kinase activity in Ewing’s sarcoma cell lines and patient tumor specimens. A, total protein was extracted from TC71, A4573, or SK-ES cells and assessed by immunoblot for total Lyn. Lyn kinase activity was determined using immunoprecipitation from cell lysates. B, tissue lysates were obtained from patient specimens. Lyn protein level and kinase activity were then determined by Western blot and kinase assay, respectively. T, tumor tissue; N, relative normal surrounding tissue; underline, a pair of tissues.
To determine the level of Lyn kinase activity in patients’ samples, we obtained 15 Ewing’s sarcoma tumor specimens from a tumor bank. Four of the 15 had adjacent normal tissue as a control. Lyn protein was expressed in 13 of 15 tumor samples (Fig. 1B, top). However, we observed a difference in Lyn protein expression between tumor tissue and adjacent normal tissue in only 1 patient. Lyn kinase activity was detected in 6 of the 15 tumor samples (Fig. 1B, bottom). By contrast, negligible or no Lyn kinase activity was detected in the 4 normal tissues. The reason for positive Lyn kinase activity in only 6 samples may be due to the fact that these patient tumor specimens were banked rather than fresh tissue. Thus, kinase activity may not have been preserved as not all tumors were processed in the same way. In several specimens, a lower migrating form of Lyn reacted in the assay, suggesting that some degradation had occurred. Although the limited number of paired samples is not adequate to make a statistical comparison, these data suggest that many Ewing’s tumors are increased in Lyn kinase.
Down-regulation of Lyn Expression Inhibits Tumor Growth, Tumor-Induced Bone Lysis, and Metastasis to the Lungs
A siRNA-expressing plasmid specific for human Lyn (Lynsi) was constructed and transfected into TC71 cells (TC/Lynsi). Several clones were isolated following stable transfection. Western blot confirmed the inhibition of Lyn protein levels by 60% to 70% in clones 8 and 10 (Fig. 2A). Lyn kinase activity was also inhibited (Fig. 2A). To determine the role of Lyn in primary tumor growth and metastatic potential, TC71 parental, TC/si-control, and TC/Lynsi clones 8 and 10 cells were injected into the tibia of nude mice. Bone tumor lysis was assessed using a standardized methodology (21). There was no difference in the incidence of bone tumor development 4 weeks after tumor cell inoculation. However, the bone tumors induced by TC/Lynsi clones 8 and 10 cells were significantly less lytic than those induced by TC/si-control or TC71 parental cells (Fig. 2B). Osteolytic activity was graded as a score from 0 to 4 (21). The average severity of bone destruction was significantly decreased from 3.1 to 1.6 or 2.2 (Fig. 2B and C). Four weeks after tumor cells were inoculated, the tumor-bearing leg was amputated but the mice were kept alive. Eight weeks following amputation, all mice were sacrificed for evaluation of presence of lung metastases. The incidence of lung metastasis in mice injected with the TC/Lynsi cloned cells was significantly lower than in mice injected with TC/si-control cells (Fig. 2C; P < 0.05). We next investigated the effect of down-regulating Lyn expression on tumor growth in vivo using gene therapy with our Lynsi plasmid. We elected to use PEI as our gene delivery vector because of our previous experience with this nonviral vector (20). TC71 cells were injected s.c. into nude mice. Three days later, the palpable tumors were injected with PEI:Lynsi or PEI:si-control. As shown in Fig. 2D, PEI:Lynsi gene therapy significantly inhibited tumor growth in mice compared with the PEI:si-control. These data indicate that down-regulation of Lyn resulted in suppression of tumor growth, less bone destruction, and decreased metastatic potential.
Lyn Kinase Activity in Other Tumor Cell Lines
We have shown that inhibition of Lyn in TC71 cells resulted in less bone destruction after intrabone injection. To explore the correlation of Lyn with the lytic phenotype, we determined Lyn kinase activity in nine other tumor cell lines that form tumors when injected into the bone. The results are summarized in Table 1. High Lyn kinase activity was shown in all seven cell lines that form osteolytic primary bone or metastasis bone tumors in vivo. By contrast, no Lyn kinase activity was detected in the osteoblastic LM7 and SOAS-2 osteosarcoma cells. The Lyn kinase activity was also much lower in LNCaP cells, which form tumors with both osteolytic and osteoblastic characteristics. These data indicate that there is a correlation between Lyn kinase activity and the lytic phenotype of tumor cells.
Table 1. Lyn kinase activity in various tumor cells.
| Cell line | Cancer type | Tumor phenotype | Lyn kinase activity |
|---|---|---|---|
| TC71 | Ewing’s sarcoma | Lytic | +++ |
| PM3 | Ewing’s sarcoma | Lytic | ++++ |
| LM7 | Osteosarcoma | Blastic | - |
| SOAS-2 | Osteosarcoma | Blastic | - |
| KRIB | Osteosarcoma | Lytic | +++ |
| MDA-MB-231 | Breast cancer | Lytic | +++ |
| LNCaP | Prostate cancer | Lytic + blastic | + |
| DU145 | Prostate cancer | Lytic | ++ |
| RBMI-IT4 | Renal cell carcinoma | Lytic | ++ |
| A375 | Melanoma | Lytic | +++ |
NOTE: Cell lysates (200 μg) were assayed for Lyn kinase activity by in vitro kinase assay.
Effects of Down-regulating Lyn on Cell Invasion and Expression of E-Cadherin and Cadherin 11 Proteins
Increased c-Src kinase activity has been associated with metastatic and advanced-stage tumors (22). This suggests that SFK may play a role in the metastatic process. We showed that inhibiting Lyn affected the metastatic potential of TC71 cells. We therefore next analyzed the effect of inhibiting Lyn on the invasive properties of TC71 cells. The invasive ability of TC/Lynsi cells was significantly reduced compared with TC71 or TC/si-control cells (Fig. 3A). Integrins and cadherins are involved in tumor metastasis. For example, decreased E-cadherin and increased cadherin 11 have been shown to correlate with decreased invasion, cell-matrix adhesion, and increased cancer metastasis potential (23, 24). We therefore determined the expression of these genes by Western blot and flow cytometry. Analysis of TC/Lynsi cells showed higher expression of E-cadherin and lower expression of cadherin 11 compared with TC71 or TC/si-control (Fig. 3B). No difference was seen in α4β1 and α5β1 expression by flow cytometry (data not shown). Taken together, these data indicate that inhibition of Lyn leads to decreased invasion and metastatic potential of TC71 cells and is associated with the expected alteration in cadherin 11 and E-cadherin expression.
Figure 3.
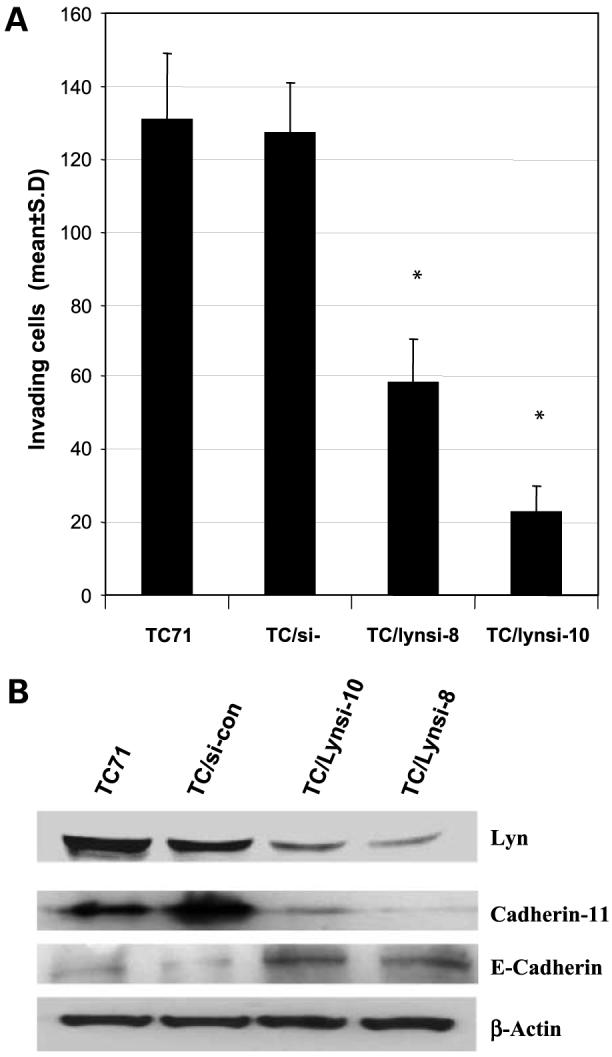
Effect of Lyn on TC71 cell invasion and expression of cadherins. A, parental TC71 cells or TC71 cell clones transfected with control vector or Lyn siRNA-expressing plasmid were assayed for their ability to invade Matrigel. *, P < 0.01, compared with TC/si-control group. B, parental TC71, TC/si-control-transfected, and TC/Lynsi-transfected cells were assayed by Western blot for E-cadherin or cadherin 11. β-Actin was used as the loading control.
Effect of AP23994 on Lyn Kinase Activity and Ewing’s SarcomaTumor Growth In vivo
The experiments described above showed that Lyn contributes to the growth and metastases of Ewing’s sarcoma cells and therefore may be a novel therapeutic target for the treatment of this disease. Because Lyn is a member of the Src kinase family, Src inhibitors will also target Lyn. We chose to investigate AP23994, a small-molecule Src inhibitor, for its in vivo efficacy against TC71 Ewing’s sarcoma tumors. This compound has been shown to selectively inhibit multiple Src family kinases as well as other kinases including Abl, Flt1, Flt3, KDR, and epidermal growth factor receptor (25). In vitro studies with TC71 cells showed a dose-dependent down-regulation of Lyn kinase activity in TC71 cells treated with AP23994 (Fig. 4A). Treatment of TC71 cells with AP23994 inhibited cell proliferation in a dose-dependent manner (Fig. 4B).
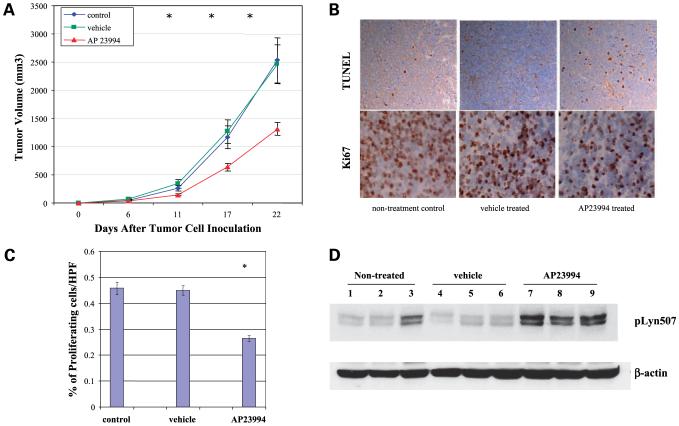
Figure 4.
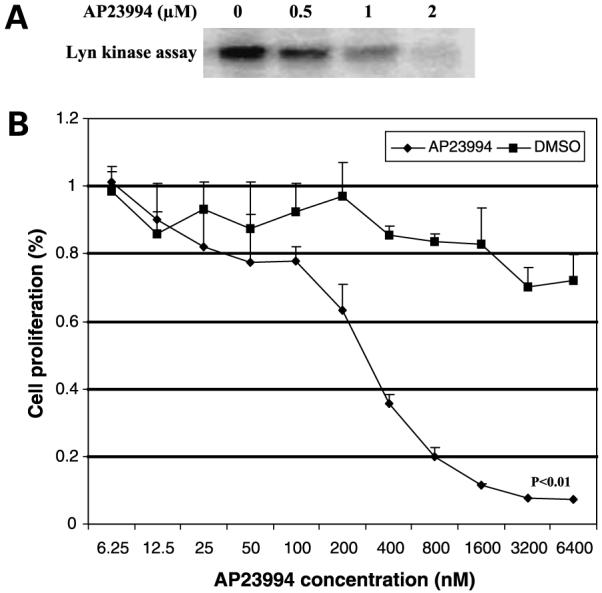
Effect of the SFK inhibitor AP23994 on Lyn kinase activity and cell proliferation. A, TC71 cells were treated with 0, 0.5, 1, or 2 nmol/L AP23994 for 1 h. Cell lysates were collected and assayed for Lyn kinase. B, TC71 cells were treated with various doses of AP23994 or DMSO for 48 h and then assayed by MTT.
To determine the effect of AP23994 in vivo, TC71 cells were injected s.c. into the flanks of nude mice. Three days after tumor cell inoculation, when the tumors were palpable, the mice were treated orally with either control vehicle or AP23994 at 50 mg/kg/d for 3 weeks. Treatment with AP23994 significantly inhibited Ewing’s sarcoma tumor growth compared with nontreated or control vehicle-treated group (Fig. 5A; P < 0.01). Tumors were excised and assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and Ki-67 staining (Fig. 5B and C). AP23994 treatment induced apoptosis and inhibited the number of proliferating tumor cells by 60% compared with the nontreated tumors or those treated with vehicle alone (P < 0.01). Total protein was extracted from these tumors and assayed for Lyn kinase activity by Western blot using anti-Lyn (Tyr507) antibody, which detects the inactivated form of Lyn. Inactivated Lyn was seen in tumors following treatment with AP23994 but not in the vehicle-treated or untreated controls (Fig. 5D). These data suggest that AP23994 inhibited the growth of TC71 tumors and that inactivating Lyn kinase activity may have contributed to this growth inhibition.
Figure 5.
AP23994 inhibited TC71 tumor growth in vivo. A, TC71 cells were inoculated into nude mice. Starting on day 3, mice were treated orally with AP23994, PBS, or vehicle alone daily for 3 wk. Tumor size was measured and the average tumor volume was calculated in each group. *, P < 0.01, compared with control or vehicle-treated tumors. B, apoptosis and proliferation of excised tumors were analyzed using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling or Ki-67 staining, respectively. C, Ki-67-positive cells were quantified under high-power field. Mean ± SD of five randomized fields from different samples in each group. *, P < 0.05. D, total protein was extracted from the tumors of each group. Lyn inactivation was determined by Western blot using anti-Lyn (Tyr507) antibody.
EWS/FLI-1 Regulates Lyn Expression and Kinase Activity
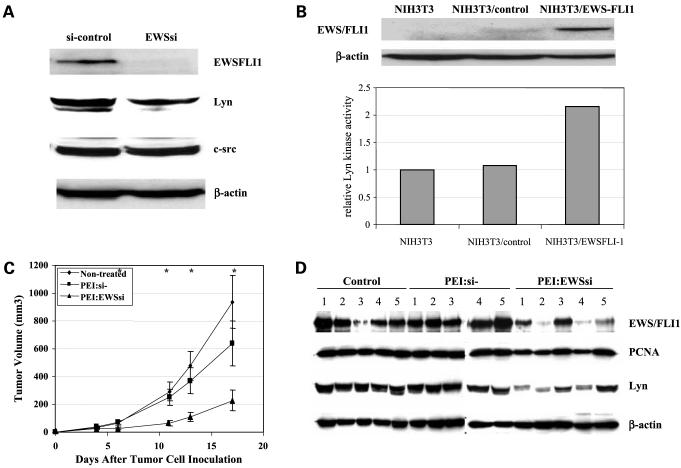
EWS/FLI-1 is thought to transform cells by acting as an aberrant transcriptional factor for specific target genes. We next determined if Lyn kinase activity was affected by EWS/FLI-1. A siRNA-expressing plasmid specific for EWS/FLI-1 (EWSsi) was constructed. EWS/FLI-1 protein levels were significantly decreased in TC71 cells transfected with EWSsi compared with those transfected with the sicontrol vector (Fig. 6A). Lyn expression was also decreased in EWSsi-transfected cells. Conversely, transfection of EWS/FLI-1 into NIH3T3 murine fibroblast cells resulted in up-regulation of Lyn kinase activity (Fig. 6B). These data indicate that Lyn activity may be regulated by EWS/FLI-1.
Figure 6.
EWS/FLI-1 regulates Lyn expression and kinase activity in vitro and in vivo. A, EWSsi or control plasmids were transiently transfected into TC71 cells. Cell lysates were collected 48 h later. Lyn and c-Src protein levels were determined by Western blot. β-Actin was used as the internal control. B, EWS/FLI-1 expression plasmid or control vector was stably transfected into NIH3T3 cells. EWS/FLI-1 expression was confirmed by Western blot. Lyn kinase activity was determined by kinase assay. C, TC71 cells were injected s.c. into mice. Three days later, mice were divided into three groups and given intratumoral injections of PEI:si-control, PEI:EWSsi, or no treatment. Tumor size was measured at various time points. *, P < 0.01, compared with the untreated or PEI:si-control group. D, total protein was extracted from tumors. EWS/FLI-1, Lyn, and proliferating cell nuclear antigen protein levels were determined by Western blot.
Effect of Down-regulation of EWS/FLI-1 on TC71 Tumor Growth and Lyn Expression In vivo
We next investigated whether EWS/FLI-1 siRNA (EWSsi) affected tumor growth. TC71 cells were injected s.c. into nude mice. Three days later, the palpable tumors were injected with PEI:EWSsi or PEI:si-control. EWSsi treatment significantly inhibited tumor growth compared with the PEI:si-control (Fig. 6C). Tumors were removed at the end of therapy and total protein was extracted. Western blot was done to determine the effect of PEI:EWSsi treatment on EWS/FLI-1 and Lyn expression. PEI:EWSsi effectively down-regulated both EWS/FLI-1 and Lyn expression in most tumors (Fig. 6D). Tumor cell proliferation was also inhibited as assessed by proliferating cell nuclear antigen protein levels.
Discussion
Lyn is a Src family protein tyrosine kinase expressed preferentially in hematopoietic cells and B cells but not T cells (22). Lyn expression has also been reported in solid tumors, including prostate cancer (11), pancreatic cancer (13), colon carcinoma (14), and Kaposi’s sarcoma (26). High Src kinase activity has been detected in Ewing’s sarcoma TC106 and 6647 cell lines when compared with other human tumor cell lines and fibroblasts (15). Shor et al. also reported high levels of phosphorylated Src in human osteosarcoma and Ewing’s sarcoma cell lines (16). These results prompted us to explore targeting Lyn as a therapeutic approach against Ewing’s sarcoma. In this study, we showed elevated Lyn kinase activity in Ewing’s sarcoma cell lines and numerous patient samples. We were unable to detect c-Src, Fyn, Fgr, Hck, or Lck kinase activity. Similar to our results, other investigators have shown that Lyn kinase activity accounts for the majority of the pan-Src kinase activity in tumor tissue (12). Inhibition of Lyn expression in TC71 cells with siRNA reduced bone tumor growth and metastatic potential in vivo. Furthermore, intratumoral gene therapy with PEI:Lynsi significantly inhibited the growth of established TC71 tumors. AP23994, a specific SFK inhibitor, was also effective in suppressing tumor growth. These data suggest that activated Lyn may contribute to the growth of Ewing’s sarcoma tumors.
Lyn activation plays a critical role in B-cell receptor signaling, apoptosis, and attenuation of the immune response (27, 28). Other studies, however, showed that activation of Lyn stimulates cell proliferation and inhibits apoptosis (29, 30). In addition, the sequence-based Lyn-specific peptide inhibitor, KRX-123, was found to inhibit cellular proliferation in three hormone-refractory prostate cancer cell lines (11). These data suggest that Lyn may either stimulate or block the growth signaling and apoptosis pathways depending on the cell line (31). Our studies showed for the first time that inhibition of Lyn kinase by siRNA gene therapy or a small-molecule inhibitor resulted in inhibition of Ewing’s sarcoma tumor growth in vivo (Figs. 2D and 5A). Inhibition Lyn also reduced the lytic phenotype of the tumor (Fig. 2B and C).
SFK has been shown to be important in tumor migration, invasion, and metastasis (22). The role of Lyn in these processes is poorly understood. Lyn activation is required in class A scavenger receptor-mediated macrophage cell adhesion, platelet-endothelial adhesion, and B-cell migration (32, 33). Dasatinib has been shown to inhibit Src and Lyn kinase activity, FAK and Crk substrate signaling, and the adhesion, migration, and invasion of prostate cancer cells (34). Recently, Lyn was shown to be activated through the CXCR4 chemokine receptor. Lyn activation transiently destabilized β2-integrin-mediated adhesion facilitating CD34+ cell movement outside the bone marrow in response to the chemokine SDF-1 (35). These data suggest that prolonged up-regulation of Lyn activity may promote cellular detachment and release into the peripheral circulation. Therefore, prolonged Lyn activation may similarly promote the detachment of tumor cells from the primary tumor allowing release into the circulation and metastasis. Indeed, we showed the correlation between high Lyn kinase activity, the ability of Ewing’s sarcoma cells to invade into Matrigel, and their ability to metastasize to the lung. Inhibition of Lyn led to a down-regulation of cadherin 11 and up-regulation of E-cadherin. Such changes have been shown to inhibit matrix metalloproteinase expression (36), decrease tumor cell invasion, and stabilize tumor cell adhesion to the local microenvironment, which should in turn prevent escape into the circulation and lessen metastatic potential (23, 24). Our findings of fewer lung metastases in the Lynsi-transfected cells support this hypothesis.
Src tyrosine kinases have been shown to play an important role in osteoclast function. Src-/- mice are osteopetrotic with defective osteoclastic resorption (37). We showed that down-regulation of Lyn resulted in less tumor-induced bone destruction (Fig. 2B). These results suggest that activation of Lyn may contribute to tumor-induced bone lysis. Increased lytic activity may provide a more conducive microenvironment for the tumor to grow, making it easier to invade the bone stroma, enter the circulation, and metastasize. Indeed, when Lyn was inhibited, tumors were less lytic and metastasized poorly to the lung (Fig. 2).
Understanding how kinases are activated may help develop new targeted therapies. EWS/FLI-1 is the most common translocation found in Ewing’s sarcoma/peripheral neuroectodermal tumors. The oncogene has been shown to transform cells by acting as a transcriptional factor to modulate a cohort of target genes. To date, there is no information linking EWS/FLI-1 with SFK expression. Previous studies have shown that a threshold level of EWS/FLI-1 is necessary to maintain tumorigenic properties (38, 39). EWS/FLI-1 can act as a transcriptional activator or suppressor depending on the target gene’s promoter (40, 41). Down-regulation of EWS/FLI-1 with siRNA inhibited Lyn protein production but not c-Src protein levels (Fig. 6A). Thus, the effect of EWS/FLI-1 on Lyn was specific and did not affect all SFK. Furthermore, intratumoral injection of PEI:EWSsi resulted in decreased Lyn protein levels and reduced tumor growth (Fig. 6D).
In summary, we have shown that Lyn is activated in Ewing’s sarcoma and affects the lytic phenotype of the tumor. Down-regulation of Lyn inhibited Ewing’s sarcoma tumor growth and metastasis to the lung. The results of Lyn activation in numerous Ewing’s sarcoma patient samples further support the consideration of Lyn as a potential therapeutic target for the treatment of patients with this disease.
Acknowledgments
We thank William C. Shakespeare and Tomi K. Sawyer (Ariad Pharmaceuticals) for providing AP23994 and Liz Han, Janet E. Price, Justin M. Summy, Quansheng Zhu, and Nadezhda V. Koshkina for helpful discussions and suggestions.
Grant support: Kayton Fund, Lindner Fund, and NIH Core grant CA16672.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Delattre O, Zucman J, Plougastel B, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 2.Mirra JM, Picci P, Gold RH, Goorin AM. Bone tumors: clinical, radiological and pathological correlation. Lea & Febiger; Philadelphia: 1989. pp. 1087–117. [Google Scholar]
- 3.Dorfman HD, Czerniak B. Bone tumors. Mosby; St. Louis: 1998. pp. 607–49. [Google Scholar]
- 4.Avigad S, Yaniv I. Novel approaches for the management of patients with Ewing sarcoma. Future Oncol. 2006;2:659–65. doi: 10.2217/14796694.2.5.659. [DOI] [PubMed] [Google Scholar]
- 5.Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Scotlandi K. Targeted therapies in Ewing’s sarcoma. Adv Exp Med Biol. 2006;587:13–22. doi: 10.1007/978-1-4020-5133-3_2. [DOI] [PubMed] [Google Scholar]
- 7.Roginskaya V, Zuo S, Caudell E, et al. Therapeutic targeting of Src-kinase Lyn in myeloid leukemic cell growth. Leukemia. 1999;13:855–61. doi: 10.1038/sj.leu.2401429. [DOI] [PubMed] [Google Scholar]
- 8.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–8. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 9.Ptasznik A, Nakata Y, Kalota A, Emerson SG, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat Med. 2004;10:1187–9. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- 10.Harder KW, Parsons LM, Armes J, et al. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–15. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg-Furmanov M, Stein I, Pikarsky E, et al. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–66. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 12.Stettner MR, Wang W, Nabors LB, et al. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65:5535–43. doi: 10.1158/0008-5472.CAN-04-3688. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Zagozdzon R, Avraham R, Avraham HK. CHK negatively regulates Lyn kinase and suppresses pancreatic cancer cell invasion. Int J Oncol. 2006;29:1453–8. [PubMed] [Google Scholar]
- 14.Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via Lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61:5275–83. [PubMed] [Google Scholar]
- 15.Rosen N, Bolen JB, Schwartz AM, et al. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986;261:13754–9. [PubMed] [Google Scholar]
- 16.Shor AC, Keschman EA, Lee FY, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67:2800–8. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 17.Guan H, Zhou Z, Wang H, et al. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing’s sarcoma growth in a xenograft mouse model. Clin Cancer Res. 2005;11:2662–9. doi: 10.1158/1078-0432.CCR-04-1206. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Stewart J, Olman MA, Klobe MR, Gladson CL. The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of glioblastoma cells is affected by the integrin engaged. J Biol Chem. 2003;278:39882–91. doi: 10.1074/jbc.M304685200. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Higashino F, Aoyagi M, et al. EWS/ETS fusions activate telomerase in Ewing’s tumors. Cancer Res. 2003;63:8338–44. [PubMed] [Google Scholar]
- 20.Jia SF, Worth LL, Densmore CL, Xu B, Zhou Z, Kleinerman ES. Eradication of osteosarcoma lung metastases following intranasal inter-leukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene Ther. 2002;9:260–6. doi: 10.1038/sj.cgt.7700432. [DOI] [PubMed] [Google Scholar]
- 21.Weber KL, Doucet M, Price JE, Baker C, Kim SJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling leads to inhibition of renal cell carcinoma growth in the bone of nude mice. Cancer Res. 2003;63:2940–7. [PubMed] [Google Scholar]
- 22.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 23.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 24.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 25.Han LY, Landen CN, Trevino JG, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66:8633–9. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakash O, Swamy OR, Peng X, et al. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood. 2005;105:3987–94. doi: 10.1182/blood-2004-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibbs ML, Tarlinton DM, Armes J, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 28.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 29.Katagiri K, Yokoyama KK, Yamamoto T, et al. Lyn and Fgr protein-tyrosine kinases prevent apoptosis during retinoic acid-induced granulocytic differentiation of HL-60 cells. J Biol Chem. 1996;271:11557–62. doi: 10.1074/jbc.271.19.11557. [DOI] [PubMed] [Google Scholar]
- 30.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8:61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 31.Hibbs ML, Harder KW. The duplicitous nature of the Lyn tyrosine kinase in growth factor signaling. Growth Factors. 2006;24:137–49. doi: 10.1080/08977190600581327. [DOI] [PubMed] [Google Scholar]
- 32.Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292:C1450–8. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- 33.Udell CM, Samayawardhena LA, Kawakami Y, et al. Fer and Fps/Fes participate in a Lyn-dependent pathway from FcεRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation. J Biol Chem. 2006;281:20949–57. doi: 10.1074/jbc.M604252200. [DOI] [PubMed] [Google Scholar]
- 34.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 35.Nakata Y, Tomkowicz B, Gewirtz AM, Ptasznik A. Integrin inhibition through Lyn-dependent cross talk from CXCR4 chemokine receptors in normal human CD34+ marrow cells. Blood. 2006;107:4234–9. doi: 10.1182/blood-2005-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 37.Boyce BF, Xing L, Yao Z, et al. SRC inhibitors in metastatic bone disease. Clin Cancer Res. 2006;12:s6291–5. doi: 10.1158/1078-0432.CCR-06-0991. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Iwakuma T, Harimaya K, Sato H, Iwamoto Y. EWS-Fli1 antisense oligodeoxynucleotide inhibits proliferation of human Ewing’s sarcoma and primitive neuroectodermal tumor cells. J Clin Invest. 1997;99:239–47. doi: 10.1172/JCI119152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovar H, Aryee DN, Jug G, et al. EWS/FLI-1 antagonists induce growth inhibition of Ewing tumor cells in vitro. Cell Growth Differ. 1996;7:429–37. [PubMed] [Google Scholar]
- 40.Ohno T, Rao VN, Reddy ES. EWS/Fli-1 chimeric protein is a transcriptional activator. Cancer Res. 1993;53:5859–63. [PubMed] [Google Scholar]
- 41.Welford SM, Hebert SP, Deneen B, Arvand A, Denny CT. DNA binding domain-independent pathways are involved in EWS/FLI1-mediated oncogenesis. J Biol Chem. 2001;276:41977–84. doi: 10.1074/jbc.M106757200. [DOI] [PubMed] [Google Scholar]