Abstract
In neutrophils activated to secrete with formyl-methionyl-leucyl-phenylalanine, intermediate filaments are phosphorylated transiently by cyclic guanosine monophosphate (cGMP)-dependent protein kinase (G-kinase). cGMP regulation of vimentin organization was investigated. During granule secretion, cGMP levels were elevated and intermediate filaments were transiently assembled at the pericortex to areas devoid of granules and microfilaments. Microtubule and microfilament inhibitors affected intermediate filament organization, granule secretion, and cGMP levels. Cytochalasin D and nocodazole caused intermediate filaments to assemble at the nucleus, rather than at the pericortex. cGMP levels were elevated in neutrophils by both inhibitors; however, with cytochalasin D, cGMP was elevated earlier and granule secretion was excessive. Nocodazole did not affect normal cGMP elevations, but specific granule secretion was delayed. LY83583, a guanylyl cyclase antagonist, inhibited granule secretion and intermediate filament organization, but not microtubule or microfilament organization. Intermediate filament assembly at the pericortex and secretion were partially restored by 8-bromo-cGMP in LY83583-treated neutrophils, suggesting that cGMP regulates these functions. G-kinase directly induced intermediate filament assembly in situ, and protein phosphatase 1 disassembled filaments. However, in intact cells stimulated with formyl-methionyl-leucyl-phenylalanine, intermediate filament assembly is focal and transient, suggesting that vimentin phosphorylation is compartmentalized. We propose that, in addition to changes in microfilament and microtubule organization, granule secretion is also accompanied by changes in intermediate filament organization, and that cGMP regulates vimentin filament organization via activation of G-kinase.
INTRODUCTION
The principal function of neutrophils is to digest foreign debris and microorganisms by an assortment of enzymes found in cytoplasmic granules. Neutrophils contain two major distinct categories of granules, the specific and azurophil granules, as well as a category of tertiary granules and secretory vesicles. Activation of neutrophils by soluble mediators such as chemoattractants or Ca2+ ionophores elicits secretion of lysosomal enzymes and mediators of inflammation into the extracellar space. One important mechanism for granule secretion involves changes in the organization of the cytoskeleton and cytoplasmic granules during neutrophil activation. The neutrophil cytoskeleton is a complex three-dimensional network composed of microfilaments, microtubules, and intermediate filaments (Pryzwansky et al., 1983). A filamentous network at the subplasmalemmal zone, composed primarily of microfilaments, is suggested to act as a barrier to prevent granule movement to the plasmalemma (Poste and Allison, 1973). This hypothesis is supported by the repeated observations that cytochalasins, inhibitors of actin polymerization (MacLean-Fletcher and Pollard, 1980), enhance granule secretion of agonist-stimulated neutrophils (Becker and Henson, 1973). Microtubules may be important in granule organization and transport in neutrophils (Hoffstein et al., 1977; Hoffstein, 1980; Ryder et al., 1988; Rothwell et al., 1989). Complexes of microtubules associated with granules significantly increase when neutrophils are stimulated to secrete with formyl-methionyl-leucyl-phenylalanine (fMLP) (Rothwell et al., 1989), and an increase in microtubule numbers and length is observed during neutrophil activation (Hoffstein, 1980; Schliwa et al., 1982b; Pryzwansky et al., 1985). However, other studies do not support an active role for microtubules in granule secretion because depolymerization of microtubules does not completely inhibit granule secretion (Becker and Showell, 1974; Hoffstein et al., 1977; Hoffstein and Weissmann, 1978). Presently, the function of intermediate filaments in neutrophils is unknown. In neutrophils, intermediate filaments are composed of vimentin subunits that may originate from an organizing center near the nucleus (Parysek and Eckert, 1984). Ten-nanometer filaments were described near the centriole in oriented neutrophils during chemotaxis (Malech et al., 1977) and in the pericortical cytoplasm and uropod of A23187 and fMLP-activated neutrophils (Hoffstein and Weissmann, 1978; Hoffstein et al., 1982; Parysek and Eckert, 1984; Wyatt et al., 1991; Pryzwansky et al., 1995). The physiological importance of these cytoskeletal structures during neutrophil activation is not understood.
More than 20 yr ago, cGMP was proposed as a positive regulator of neutrophil granule secretion (Becker and Henson, 1973; Ignarro, 1975; Weissmann et al., 1975). We reported (Wyatt et al., 1993) that cGMP levels were elevated in adherent neutrophils activated with fMLP or A23187 at the time of secretion of lactoferrin (LF, specific granule marker) and myeloperoxidase (MPO, azurophil granule marker). The mechanism(s) for cyclic nucleotide regulation of granule secretion remains an enigma (Coffey, 1992). In our pursuit to elucidate the role of cGMP as a positive regulator of neutrophil granule secretion, we reported that Type I cGMP-dependent protein kinase (G-kinase) is expressed in human neutrophils in small amounts, and that it is transiently colocalized with vimentin in neutrophils activated with fMLP or A23187 (Pryzwansky et al., 1990, 1995; Wyatt et al., 1991, 1993). We found that vimentin was transiently phosphorylated by G-kinase in fMLP and A23187-stimulated neutrophils only at the time that G-kinase was colocalized with vimentin, cGMP levels were elevated, and granule secretion took place. The lowering of cGMP levels with the guanylyl cyclase inhibitor, LY83583, inhibited targeting of G-kinase to intermediate filaments, phosphorylation of vimentin by G-kinase, and granule secretion.
In this report, the structural organization of vimentin was investigated in fMLP-stimulated neutrophils. We found that during the time of granule secretion, intermediate filaments assembled in regions of the cell excluding granules and microfilaments. These changes in the cytomatrix were dependent upon cGMP elevations and intact microfilaments and microtubules. G-kinase directly induced vimentin filament assembly in situ in detergent-extracted neutrophils. We propose that, in addition to changes in microfilament and microtubule organization, orderly granule secretion is also accompanied by changes in intermediate filament organization, and that cGMP regulates vimentin filament organization via activation of G-kinase.
MATERIALS AND METHODS
Neutrophil Isolation and Stimulation
Neutrophils were isolated from human peripheral blood collected in 0.38% sodium citrate by density gradient centrifugation in Polymorphprep (Nycomed, Oslo, Norway). The cells were resuspended at 2.5 × 106 cells/ml in Gey’s balanced salts containing 1.5 mM CaCl2, 1 mM MgCl2, 0.3 mM MgSO4 (GBSS), and supplemented with 10% human AB serum. The cells were layered onto glass coverslips or 60-mm Petri dishes for 15 min at 37°C. The nonadherent cells were removed and the monolayer was washed 2 times with GBSS to remove the serum. Cells were >98% viable by trypan blue exclusion and consisted of >95% neutrophils.
Neutrophil monolayers were stimulated with 0.1 μM fMLP (Peninsula, Belmont, CA) in the presence and absence of drugs from 30 s to 10 min at 37°C. Monolayers were preincubated with 100 μM LY83583 (Calbiochem, San Diego, CA) for 30 min and with 1 μM 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-Br-cGMP) (Biolog Life Science Institute, La Jolla, CA), 5 μg/ml cytochalasin D (Sigma Chemical, St. Louis, MO), or 2.5 μg/ml nocodazole (Sigma) for 5 min. Cells were then incubated in the presence of the drugs with 0.1 μM fMLP. For recovery experiments, cells were incubated with 100 μM LY83583 for 30 min, washed briefly with GBSS, and incubated with GBSS containing 10% human AB serum for 30 min. The cells were washed free of serum, incubated for 5 min with various concentrations of 8-Br-cGMP, and then stimulated in the absence of 8-Br-cGMP with 0.1 μM fMLP. All drugs were dissolved in DMSO, and controls included incubation of cells with vehical alone.
Measurements of Neutrophil Granule Secretion and cGMP Levels
Neutrophils adhered to 60-mm dishes were stimulated with 0.1 μM fMLP in the presence and absence of drugs from 1 to 10 min at 37°C. The culture media were removed and assayed by enzyme-linked immunosorbent assay for the release of lactoferrin (LF) and MPO to identify specific and azurophil granule contents, respectively (Wyatt et al., 1993). The monolayer was extracted with 0.1 N HCl, the samples acetylated, and the intracellular levels of cGMP measured by RIA using 125I-cGMP (Linco, St. Charles, MO) and rabbit anti-cGMP (Bethyl Labs, Montgomery, TX) according to the method of Harper and Brooker (Harper and Brooker, 1975). Protein levels were measured by the BCA method (Pierce Chemical, Rockford, IL).
Immunofluorescence Microscopy
Neutrophil monolayers were fixed at room temperature in 1% paraformaldehyde in 0.075 M cacodylate buffer containing 0.72% sucrose, pH 7.5, for 10 min, followed by 3.7% formaldehyde in PBS, pH 7.4, for 10 min, −20°C methanol for 4 min, and −20°C acetone for 1 min. Cells were washed in PBS after formaldehyde and acetone. The cells were stained for 30 min with mouse anti-porcine lens vimentin (Dako, Carpinteria, CA), washed in PBS, and stained for 30 min with either FITC goat anti-mouse immunoglobulin G and TRITC rabbit anti-LF, or with TRITC goat anti-mouse immunoglobulin G and FITC rabbit anti-MPO. Cells were also stained simultaneously with TRITC anti-LF and FITC anti-MPO. The specificity of the antibodies for LF and MPO has been published (Pryzwansky et al., 1979), and the antibodies are a gift from Dr. John Spitznagel (Emory University, Atlanta, GA). In some instances, cells were stained with mouse anti-actin (Amersham, Arlington Heights, IL).
For dual immunofluorescence staining of tubulin and vimentin, cells were washed briefly with Gey’s supplemented with 2 mM MgCl2 and lysed for 20 s with 0.5% Triton X-100 in PHEM buffer (60 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 6.9). Cells were then fixed for 10 min in 2% formaldehyde and 0.1% glutaraldehyde in PHEM buffer, washed in PBS, and fixed for 6 min in −20°C methanol and 6 min in −20°C acetone. Cells were washed in PBS and then stained with mouse anti-tubulin (clone DM 1α, Sigma) and rabbit anti-vimentin (ICN Biochemicals, Costa Mesa, CA). For dual staining of F-actin and vimentin, cells were fixed with 1% paraformaldehyde in 0.075 M cacodylate buffer for 10 min, washed in PBS, and permeabilized for 10 min with 100 μg/ml lysophosphatidylcholine in 3.7% formalin in PBS. Cells were then stained for F-actin by incubating cells for 15 min in the dark with 2.5 U/ml rhodamine-phalloidin (Molecular Probes, Eugene OR) and then stained for vimentin as above.
Cells were mounted in polyvinyl-alcohol and viewed on a Leitz fluorescence microscope. Photomicrographs were recorded with a Leitz Orthomat Camera on TMAX-400 film, or images were captured on a Sony Optronics DEI-470 video monitor and printed on a Sony video color printer.
G-Kinase in Situ Assay
Neutrophils attached to glass coverslips were lysed for 1 min at room temperature with 0.2% Triton X-100 in PHEM buffer (pH 6.9) containing a cocktail of phosphatase and protease inhibitors (100 mM NaF, 20 mM Na pyrophosphate, 1.2 mM p-aminobenzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml microcystin). Cells were washed briefly with 20 mM magnesium acetate in 20 mM Tris, pH 7.4, to remove the detergent, and then incubated for 15 min at 30°C with and without 0.01 μM G-kinase (gift of Dr. T. Lincoln, University of Alabama, Birmingham, AL) in a reaction mixture consisting of 200 μM ATP, 1.5 μM cGMP, 20 mM Tris, 20 mM magnesium acetate, 100 μM isobutylmethylxanthine, 0.7 μM cAMP-dependent protein kinase peptide inhibitor (PKI; Calbiochem, San Diego, CA), and 0.9 mg/ml BSA. In some instances, cells were also incubated in the presence of 1 μM Taxol (Calbiochem). After the phosphorylation reaction, some cells were incubated with 0.1 U of recombinant catalytic subunit of the α-isoform of type 1 protein phosphatase 1 (PP1, New England BioLabs, Beverly, MA) for 30 min at 30°C in 50 mM Tris-HCl (pH 7), 0.1 mM Na2EDTA, 5 mM dithiothreitol, 1 mM MnCl2, and 0.01% Brij 35. Cells were washed briefly with 20 mM magnesium acetate in 20 mM Tris, fixed, and stained for vimentin as above.
RESULTS
Intermediate Filament and Granule Organization
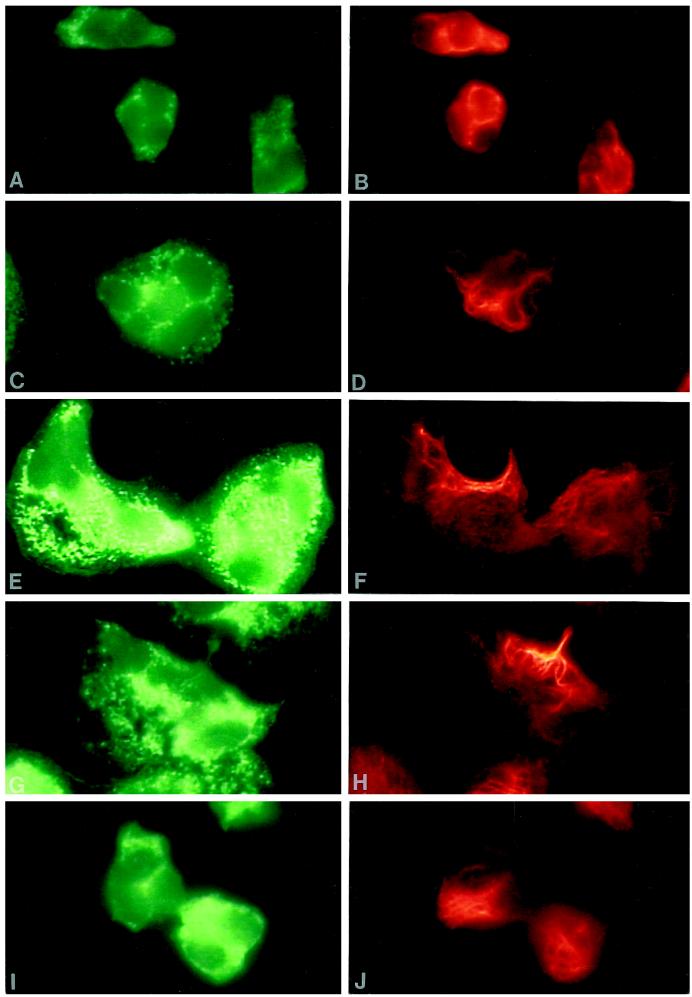
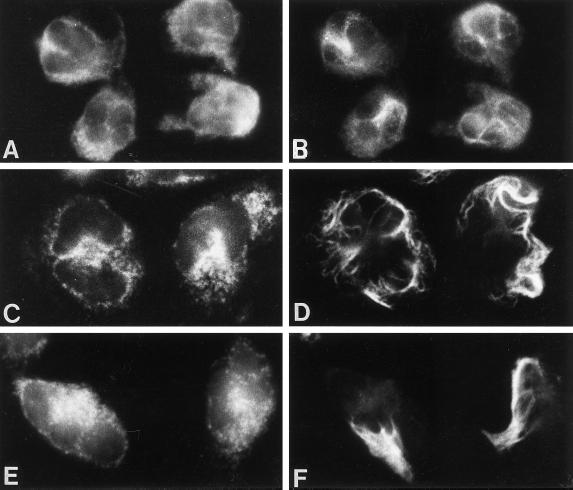
To investigate the structural organization of intermediate filaments and granules during fMLP stimulation, neutrophils were stained simultaneously for vimentin and either MPO or LF, azurophil, and specific granule markers, respectively. Unstimulated neutrophils were small and round, and MPO and vimentin were localized throughout the cytoplasm, with some staining of vimentin near the nuclear lobes (Figure 1, A and B). Filamentous staining of vimentin was not prominent in unstimulated cells, as most of the staining was particulate or diffuse. Neutrophils spread rapidly after 30 s stimulation with 0.1 μM fMLP, polarized within 2.5 min, and returned to a somewhat round appearance resembling that of unstimulated cells by 5 min. Examination of specific and azurophil granule distribution during fMLP stimulation demonstrated that the majority of both granule classes were localized similarly, with subtle differences near the cell margin. fMLP induced a transient change in vimentin organization. Bundles of vimentin filaments formed at 30 s (Figure 1D) and were focally localized at 1 and 2.5 min in the cytoplasm at the pericortex (Figure 1F) and uropod (Figure 1H). During this time, the vimentin filaments were clearly removed from the areas of the cytoplasm containing granules (Figure 1, E–H). After 5 min treatment with fMLP, neutrophils were no longer well spread, and vimentin was distributed throughout the granular areas of the cytoplasm (Figure 1, I and J). Similar findings were observed when neutrophils were stained for LF and vimentin. No staining was observed with preimmune sera or with secondary antibodies alone, and vimentin localization was similar using monoclonal or polyclonal antibodies against vimentin.
Figure 1.
Organization of azurophil granules and vimentin in fMLP-stimulated neutrophils. Dual-label immunofluorescence localization of MPO (left panel) and vimentin (right panel) is shown in control neutrophils (A and B) and cells stimulated with 0.1 μM fMLP for 30 s (C, and D), 1 min (E and F), 2.5 min (G and H), and 5 min (I and J). fMLP induced a transient change in vimentin organization. Intermediate filaments organized at 30 s and were localized within areas of the cell devoid of granules at 1 min and 2.5 min. By 5 min the cells were smaller and vimentin filaments were distributed throughout the cytoplasm with the granules. Magnification, 2500×.
Intermediate Filament, Microfilament, and Microtubule Organization
In unstimulated neutrophils, both actin and vimentin were distributed throughout the cytoplasm (Figure 2, A and B). However, after fMLP stimulation from 1 to 2.5 min, changes were observed in vimentin and microfilament organization (Figure 2, C and D). Vimentin was confined to one zone in the cytoplasm at the pericortex. In contrast, actin was distributed at the cell margin, at sites of adherence, and throughout the cytoplasm. A similar distribution of F actin and vimentin was also observed in fMLP-stimulated cells.
Figure 2.
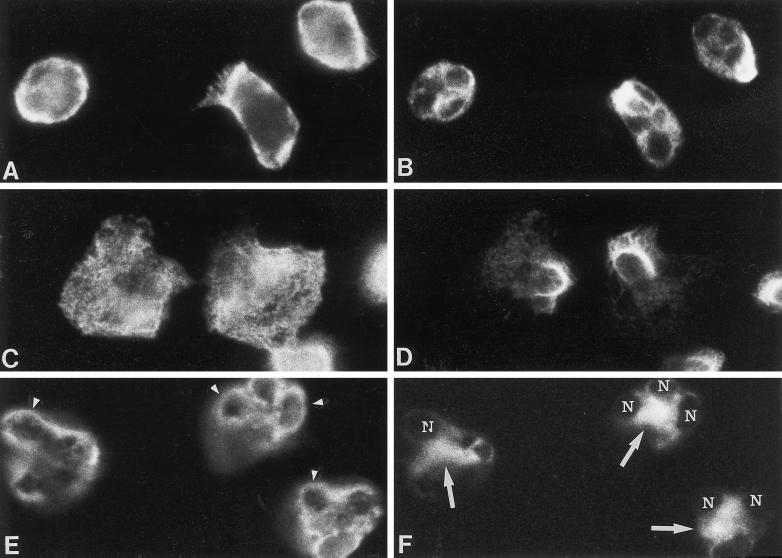
Effect of cytochalasin D on intermediate filament and microfilament organization in neutrophils stimulated with fMLP for 2.5 min. Dual-label immunofluorescence localization of actin (left panel) and vimentin (right panel) is shown in unstimulated neutrophils (A and B) and cells stimulated with fMLP in the absence (C and D) and presence of cytochalasin D (E and F). Vimentin filaments are removed from areas containing microfilaments after fMLP stimulation (C and D). The localization of actin and vimentin in neutrophils treated with cytochalasin D alone (our unpublished results) was similar to that of control neutrophils (A and B). However, fMLP with cytochalasin D (E and F) induced the accumulation of actin around the nuclear lobes (arrowheads) and vimentin at the cell center (arrows) within the nuclear clef (nucleus, N). Another plane of focus demonstrated that vimentin was organized as filaments within the nuclear clef and that aggregates of actin were distributed at the cell cortex. Magnification, 1800×.
A radial distribution of microtubules originating from the microtubule organizing center was observed in unstimulated neutrophils, and vimentin was found throughout the cytoplasm and/or in the vicinity of the nuclear lobes. Upon stimulation with fMLP for 1 or 2.5 min, vimentin filaments were localized at the pericortex, and microtubules were extended into the areas containing vimentin filaments as well as throughout the cell body. As we previously reported, an increase in microtubule numbers was evident after fMLP stimulation (Schliwa et al., 1982b).
Cytoskeletal inhibitors have been used to assess the importance of microtubules and microfilaments in neutrophil granule secretion. The effects of these inhibitors on intermediate filament organization are not known. Therefore, we investigated whether microtubule or microfilament organization influences intermediate filament organization by treating cells with cytoskeletal inhibitors. Cytochalasin D alone did not affect the distribution of actin and vimentin. However, cytochalasin D inhibited the typical changes in cell shape that are induced by fMLP. When cells were stimulated from 1 to 2.5 min with fMLP in the presence of cytochalasin D, both actin and vimentin were organized near the nucleus. Vimentin filaments were predominantly found within the nuclear clef, and actin was localized around the nuclear lobes (Figure 2, E and F). Actin was also localized in the cytoplasm and at the cell margin. Aggregates of F-actin accumulated near the nucleus and cell margin in cells stimulated with fMLP in the presence of cytochalasin D. Cytoplasmic granules were no longer prominent at 2.5 min, as most of the granule contents were exocytosed (Figure 4). Thus, cytochalasin D affects the organization of intermediate filaments and microfilaments in fMLP-stimulated cells.
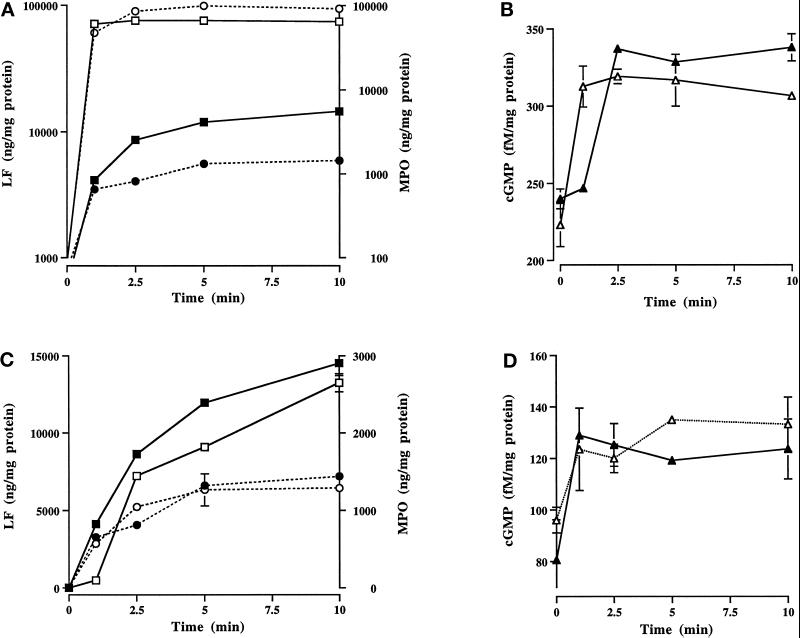
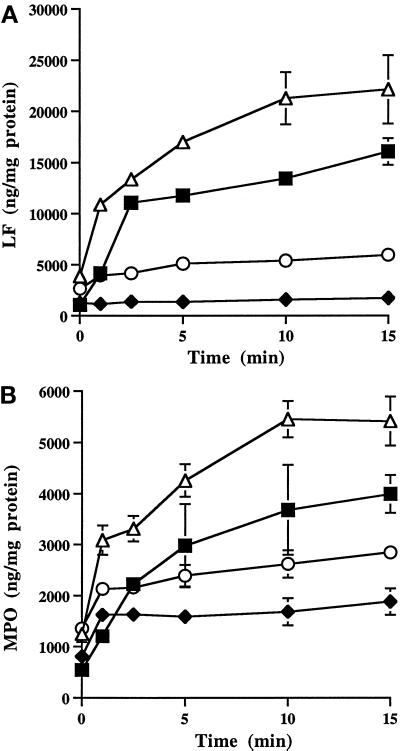
Figure 4.
Effect of cytochalasin D and nocodazole on granule secretion and cGMP levels in fMLP-stimulated neutrophils. Secretion of LF (▪,□) and MPO (•,○), and cGMP levels (▴,▵) in neutrophils stimulated for various times with fMLP in the absence (▪,•,▴) and presence (□,○,▵) of cytochalasin D (A and B) or nocodazole (C and D). Cytochalasin D accelerated granule secretion (A) and cGMP synthesis (B). Analysis of variance utilizing Student’s paired t test showed significance with and without cytochalasin D for secretion of LF and MPO at each time point between 1 and 10 min (p < 0.002). cGMP levels were significantly elevated at 2.5, 5, and 10 min in control cells (p < 0.001), and at all time points in the presence of cytochalasin D (1 min, p < 0.01; 2.5–10 min, p < 0.004). With nocodazole there were no significant differences in cGMP levels over control cells (D). Nocodazole (C) did not affect MPO secretion, but it significantly attenuated the amount of LF secreted at all time points except 10 min (1 min, p < 0.0001; 2.5 min, p < 0.01; 5 min, p < 0.0001). Data are from one of three experiments performed on a single blood donor. All three experiments demonstrated the same trends. Note that the scales are different for cytochalasin D and nocodazole treatment.
As observed by others, nocodazole caused microtubule depolymerization in neutrophils. Nocodazole did not inhibit shape changes induced by fMLP; however, it altered vimentin filament organization (Figure 3). Vimentin filaments were no longer localized at the pericortex or uropod, but were consistently found around the nuclear lobes, and removed from the granular areas of the cell body (Figure 3, C–F). The distribution of actin in cells preincubated with nocodazole and stimulated with fMLP was similar to that of control cells. Thus, microtubule depolymerization affected the site of vimentin filament organization, but not microfilament organization or the shape changes induced by fMLP.
Figure 3.
Effect of nocodazole on the distribution of MPO and vimentin in fMLP-stimulated neutrophils. Dual-localization of MPO (left panel) and vimentin (right panel) is shown in neutrophils preincubated with nocodazole (A and B) and stimulated with 0.1 μM fMLP in the presence of nocodazole for 1 min (C and D), 2.5 min (E and F), and 5 min (G and H). With nocodazole alone, vimentin was localized predominantly near the cell center or around the nuclear lobes (A and B). Nocodazole did not inhibit fMLP-induced shape changes; however, vimentin filaments were transiently localized around or on top of the nucleus at 1 (C and D) and 2.5 (E and F) min. During that time, vimentin was removed from the granular containing areas of the cytoplasm. At 5 min (G and H), vimentin localization was similar to that of cells treated with nocodazole alone. Magnification, 1500×.
Effect of Cytoskeletal Inhibitors on Granule Secretion and cGMP Levels
In adherent neutrophils stimulated with 0.1 μM fMLP, cGMP levels are elevated and granule secretion occurs at the time of intermediate filament assembly at the pericortex (Wyatt et al., 1993). Since cytoskeletal inhibitors affect intermediate filament organization and granule secretion (Smolen, 1992), we investigated their effects on cGMP levels during granule release. Cytochalasin D accelerated granule secretion and increased the amount of LF and MPO exocytosed in fMLP-stimulated cells (Figure 4A). Granule secretion was essentially complete at 1 min, with a 17-fold increase in LF and 72-fold increase in MPO exocytosed when compared with control cells at 1 min. This dramatic release of granule products at 1 min was accompanied by a 39% increase in cGMP levels (Figure 4B). The cGMP levels returned to levels of control-stimulated cells without cytochalasin D at 2.5 min. Thus, cytochalasin D accelerated the time of maximal granule release from 5 min to 1 min and significantly elevated cGMP levels at an earlier time (1 min vs. 2.5 min).
Nocodazole suppressed the rate of granule secretion and amount of LF, but not MPO, secreted from 1 to 5 min (Figure 4C). However, by 10 min the amount of LF secreted was similar to that of control cells. This attenuation of LF secretion by nocodazole suggests that intact microtubules are required for efficient specific granule secretion. Similar to control cells stimulated with fMLP, increased cGMP levels were observed with nocodazole (Figure 4D).
Effect of cGMP levels on Cytoskeletal and Granule Organization
Low concentrations of fMLP (1 nM) stimulate neutrophil chemotaxis, but not granule secretion, and cGMP levels are not elevated (Wyatt et al., 1993). To determine whether concentrations of fMLP that do not elevate cGMP levels or induce granule secretion affect intermediate filament and granule organization, neutrophils were stimulated from 1 to 5 min with 1 nM fMLP and stained for vimentin and MPO. Dramatic changes in cell shape did not take place with 1 nM fMLP, as cell spreading was minimal over the 5-min time course. While there was some vimentin filament bundling, vimentin filaments remained within the granular areas of the cytoplasm. Thus, intermediate filaments do not assemble at the pericortex in areas devoid of granules with concentrations of fMLP that do not induce granule secretion and elevate cGMP levels.
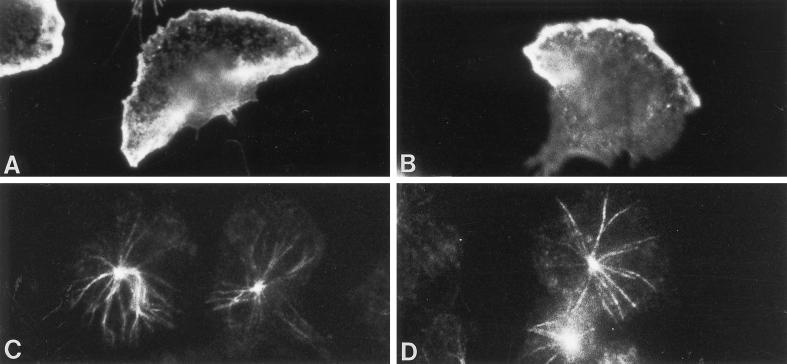
LY83583, an antagonist of guanylyl cyclase, inhibits cGMP elevations, targeting of G-kinase to intermediate filaments, G-kinase phosphorylation of vimentin, and granule secretion in fMLP-stimulated adherent neutrophils (Wyatt et al., 1993). To determine whether LY83583 affects intermediate filament organization, neutrophils were preincubated with 100 μM LY83583 and then stimulated with fMLP for various times. LY83583 did not affect vimentin organization in the absence of fMLP nor the shape changes induced by fMLP (Figure 5). However, LY83583 inhibited the focal assembly of vimentin filaments at the pericortex or uropod (Figure 5, C and D). At all time points, numerous vimentin filaments were distributed within the granular areas of the cytoplasm, at the cell cortex, and around the nucleus. LY83583 did not affect fMLP-induced F-actin distribution at the cell cortex and at sites of adhesion at 1 min (Figure 6, A and B) or microtubule organization at 2.5 min (Figure 6, C and D). Thus, lowering cGMP levels with LY83583 only affects vimentin filament organization.
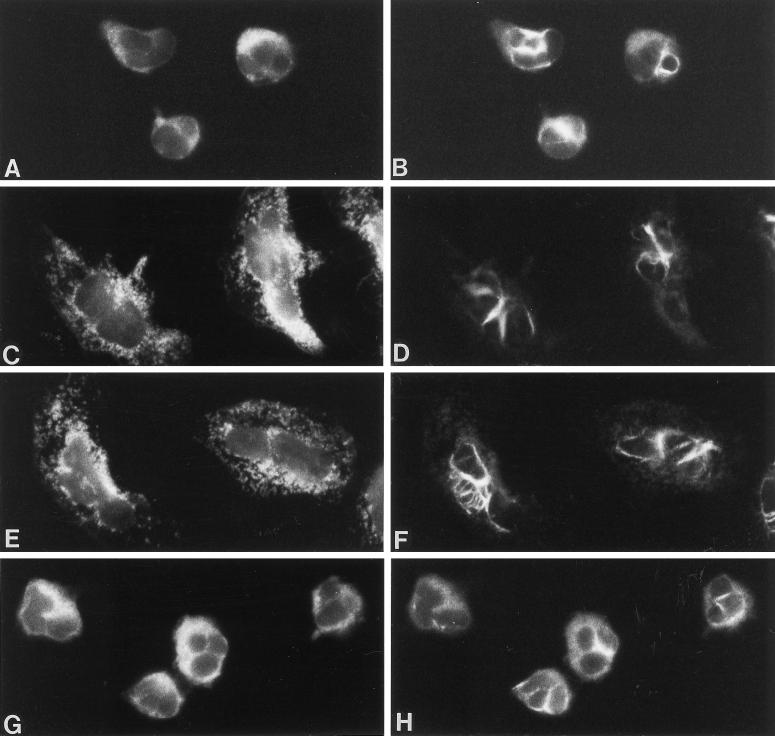
Figure 5.
The effect of the guanylyl cyclase inhibitor, LY83583, on granule and intermediate filament organization in fMLP-stimulated neutrophils, and the recovery of vimentin filament organization by 8-Br-cGMP. Dual-label immunofluorescence localization of MPO (left panel) and vimentin (right panel) is shown in neutrophils treated with 100 μM LY83583 alone (A and B) and stimulated with fMLP in the presence of LY83583 for 2.5 min (C and D). The localization of vimentin and MPO in cells treated with LY83583 alone (A and B) resembled that of unstimulated neutrophils (see Figure 1, A and B). LY83583 did not inhibit shape changes induced by fMLP. However, vimentin filaments assembled within the granular areas of the cytoplasm, at the cell cortex, and adjacent to the nucleus throughout the time course (C and D). The addition of 1 μM 8-Br-cGMP to the LY83583-treated cells caused vimentin filaments to assemble at the pericortex (E and F) in 38% of the cells when stimulated with fMLP for 2.5 min (see Figure 1, E–H for comparison). Magnification, 1800×.
Figure 6.
The effect of LY83583 on F-actin and microtubules in neutrophils stimulated with fMLP. LY83583 (100 μM) did not inhibit F-actin localization at the cell cortex and sites of adhesion at 1 min stimulation with fMLP (B), or microtubule organization at 2.5 min fMLP (D). Control cells are shown in panel A for F-actin and in panel C for microtubules. Magnification, 1800×.
Recovery of Granule Secretion and Vimentin Filament Organization by cGMP
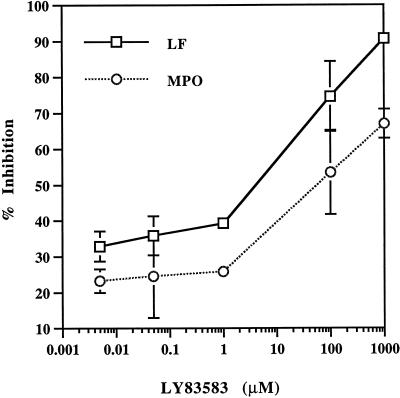
To demonstrate that cGMP is a positive regulator of neutrophil granule secretion, neutrophils were preincubated with 1 μM 8-Br-cGMP for 5 min and then stimulated with fMLP. A comparison of the effects of 8-Br-cGMP and LY83583 on LF and MPO secretion is shown in Figure 7. Secretion of both granule classes was accelerated by 8-Br-cGMP, and the amount of enzyme released was elevated at all time points. In contrast, lowering cGMP levels with LY83583 inhibited granule secretion. Secretion was not reversed by removing the drug and incubating cells with tissue culture medium for 30 min before fMLP stimulation. However, granule secretion of LF and MPO was restored 46 and 50%, respectively, with 1 μM 8-Br-cGMP (Figure 7). Recovery of granule secretion was restored within 2.5 min and was essentially complete by 5 min. Further recovery was not observed with higher concentrations or longer incubation times with 8-Br-cGMP. Inhibition of granule secretion by LY83583 was dose dependent (Figure 8). At 100 μM LY83583, secretion of LF and MPO was inhibited by ∼75 and 50%, respectively. Concentrations of 5 nM to 1 μM LY83583 inhibited ∼30 and 25% release of LF and MPO, respectively.
Figure 7.
The effects of LY83583 and 8-Br-cGMP on neutrophil granule secretion. Neutrophils were stimulated with fMLP for various times in the absence (▪) or presence of 1 μM 8-Br-cGMP (▵) or 100 μM LY83583 (♦). LY83583 inhibited secretion of LF (A) and MPO (B). Granule secretion was accelerated and augmented by 8-Br-cGMP. Analysis of variance utilizing Student’s paired t test showed that 8-Br-cGMP significantly enhanced LF and MPO secretion (p < 0.01), and that LY83583 significantly inhibited secretion at all time points (p < 0.001). Secretion was significantly restored by the addition of 1 μM 8-Br-cGMP to LY83583-treated cells (E) at 5 min for LF and MPO (p < 0.001). Data are from one of three experiments performed on a single blood donor. All three experiments demonstrated the same trends.
Figure 8.
Dose-dependent response of LY83583 on granule secretion. Neutrophils were preincubated with various concentrations of LY83583 for 30 min and then stimulated with fMLP for 5 min. The percent inhibition of LY83583 on secretion of LF and MPO is calculated from that of controls.
Similar to granule secretion, vimentin filament assembly at the pericortex was not reversed by replacing the drug with tissue culture medium for 30 min before fMLP stimulation. However, intermediate filament assembly at the pericortex was restored by 1 μM 8-Br-cGMP. In the presence of LY83583, 6% of the cells demonstrated a focal concentration of vimentin filaments at the pericortex at 2.5 min fMLP, as compared with 93% of control cells. In LY83583-treated cells, vimentin organization at the pericortex was restored in 38% of the cells with 1 μM 8-Br-cGMP at 2.5 min fMLP (Figure 5, E and F). These results suggest that cGMP regulates granule secretion and the structural organization of intermediate filaments in fMLP-stimulated neutrophils.
Intermediate Filament Assembly in Situ
We reported that G-kinase phosphorylates vimentin in vitro and in intact cells stimulated with fMLP (Wyatt et al., 1991). Neutrophils cannot be microinjected or transfected. Therefore, to further characterize the effects of G-kinase on intermediate filament organization, we used an in situ model to investigate whether G-kinase could directly induce changes in vimentin filament organization. Brief extraction of neutrophil monolayers with Triton-X-100 releases the bulk of soluble cellular proteins, including granules, and reveals the cytoskeleton (Pryzwansky et al., 1983). For these studies, neutrophil monolayers were briefly extracted with Triton X-100, and then incubated for 15 min at 30°C with purified G-kinase, cGMP, and ATP. PKI, an inhibitor of cAMP-dependent protein kinase (A-kinase), was included in all preparations to inhibit cross-activation of A-kinase by cGMP (Lincoln et al., 1995). The cells were fixed and vimentin distribution was analyzed by indirect immunofluorescence microscopy. Figure 9 shows the immunofluorescence staining pattern of vimentin filaments in extracted neutrophils incubated in the presence or absence of ATP and G-kinase. The extraction procedure alone did not alter the staining profile for vimentin in unstimulated neutrophils (Figure 9A), nor did the addition of cGMP and ATP for 10 min (Figure 9B). Diffuse and particulate staining for vimentin was observed in the cytoplasm. When cells were incubated with G-kinase in the presence of cGMP and ATP, numerous filaments were assembled adjacent to the nucleus and within the cytoplasm (Figure 9C). Unlike cells treated with fMLP, a preference for intermediate filament bundling at the cell cortex was not observed (Figure 1). To ensure that microtubule disassembly did not account for the sites of intermediate filament organization, the reaction mixture was conducted in the presence of taxol. A similar staining profile of vimentin filaments was observed with taxol. Vimentin filaments that were assembled in situ in the presence of G-kinase were disassembled when the cell extracts were treated with recombinant catalytic subunit of PP1 (Figure 9D). Thus, dephosphorylation of vimentin filaments induces vimentin filament disassembly. These data indicate that G-kinase is capable of directly inducing intermediate filament assembly.
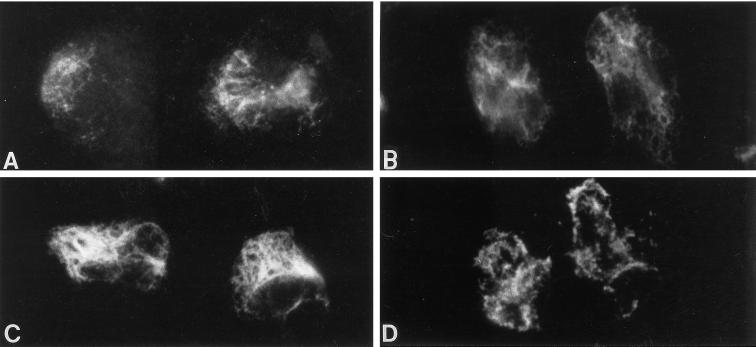
Figure 9.
Organization of vimentin filaments in situ of lysed neutrophil monolayers. Neutrophils were lysed with 0.2% Triton X-100 to extract cellular proteins and granules and then incubated in the presence and absence of purified G-kinase, cGMP, and ATP at 30°C for 15 min. PKI was included in all incubations to ensure that there was no cross-activation of A-kinase by cGMP (Lincoln et al., 1995). The cells were then washed and stained for vimentin. Shown are control extracted cells (A) and extracted cells incubated with ATP (B), or cGMP, G-kinase, and ATP (C). G-kinase caused a dramatic assembly of vimentin filaments throughout the cell body (C). Vimentin filaments were disassembled when cell extracts (incubated as shown in panel C) were further treated with the catalytic subunit of protein phosphatase 1 (D). Magnification, 1800×.
DISCUSSION
It is well established that fMLP induces changes in microfilament and microtubule organization in neutrophils. However, there are no comparable studies of intermediate filament organization. We report that in addition to actin polymerization and increases in microtubules, there are prominent changes in intermediate filament organization (Table 1). During granule secretion, intermediate filaments are assembled transiently to areas devoid of granules and microfilaments. Intermediate filament assembly is accompanied by cGMP elevations and G-kinase–mediated vimentin phosphorylation (Wyatt et al., 1991, 1993). We show that G-kinase, in and of itself, is capable of inducing extensive vimentin filament assembly in situ. However, in intact cells stimulated with fMLP, vimentin filament assembly is focal and transient, suggesting that vimentin phosphorylation is compartmentalized and is a consequence of G-kinase targeting to its substrate (Wyatt et al., 1991). Microtubule and microfilament inhibitors affected intermediate filament organization, granule secretion, and cGMP levels. The findings that both cytochalasin D and nocodazole changed intermediate filament organization indicate that these inhibitors are not specific for microfilaments or microtubules, respectively, in neutrophils. When cGMP levels were lowered by the guanylyl cyclase antagonist, LY83583, granule secretion and vimentin filament assembly at the pericortex were inhibited. These functions were partially restored by 8-Br-cGMP. Microtubule and microfilament organization were not affected by LY83583. Thus, both cGMP elevations and vimentin filament assembly at the pericortex in areas devoid of granules restored granule secretion. We propose that granule secretion is accompanied by changes in intermediate filament organization, and that cGMP serves as a second messenger to modulate vimentin filament assembly via activation of G-kinase.
Table 1.
Effects of inhibitors on neutrophil morphology, granule secretion, and cGMP levels in fMLP-stimulated neutrophils
| Condition | Shape change | Granule secretion | cGMP levels | Cytoskeletal changes at 1 min
|
||
|---|---|---|---|---|---|---|
| Vimentin | F-actin | Microtubules | ||||
| fMLP | + | Normal | Increase, 2.5 min | Focal at pericortex | Cortex | Increase |
| fMLP + cytochalasin D | − | Excessive, rapid | Increase, 1 min | Nucleus | Absent | Increase |
| fMLP + nocodazole | + | Delayed for specific granules | Increase, 2.5 min | Nucleus | Cortex | Absent |
| fMLP + LY83583 | + | Decreased | No change | Cytoplasm | Cortex | Increase |
Organization of Intermediate Filaments
In unstimulated neutrophils, vimentin is localized in the cytoplasm and around the nuclear lobes. FMLP promotes neutrophil spreading and rapid vimentin filament assembly at the pericortex juxtaposed to the nucleus in areas devoid of granules. The most notable structural change was the consistent organization of vimentin filaments to areas of the cell body lacking granules. At that time, cGMP levels were elevated and granule secretion occurred.
FMLP initiates actin polymerization at 45 s with subsequent depolymerization (Howard and Orseajo, 1985). Redistribution of F-actin during fMLP stimulation is a critical determinant of cell shape, and actin depolymerization at the cell cortex is required for granule secretion (Howard and Orseajo, 1985; Coates et al., 1992). In unstimulated neutrophils, F-actin was distributed at the cell margin and sites of adhesion. In response to fMLP, F-actin was rapidly distributed to the cell cortex at 1 min. At that time, vimentin was completely removed from areas of the cell containing actin or F-actin. In the presence of cytochalasin D, perinuclear staining for actin was prominent at 1 min fMLP, with some staining for actin in the cell body as well. Essentially all of the vimentin was distributed as filaments within the nuclear clef. Cytochalasin D alone was insufficient to cause vimentin or actin to organize at the nucleus.
Cytochalasin D does not inhibit vimentin phosphorylation (Huang et al., 1984) or microtubule assembly (Hoffstein et al., 1977) in fMLP-stimulated neutrophils. It has been proposed that cytochalasin inhibition of actin assembly augments secretion by facilitating granule access to the plasmalemma (Aunis and Bader, 1988). Proteins such as fodrin, caldesmon, or α-actinin may link actin filaments to granule membranes (Aunis and Bader, 1988). Electron microscopy shows an association between microfilaments and granules (Moore et al., 1976). The results reported here suggest that actin dissolution is accompanied by elevated cGMP levels and changes in intermediate filament organization. Thus, the redistribution of both intermediate filaments and microfilaments to the nucleus by cytochalasin D may provide unrestricted transport of granules to the plasma membrane. However, the cytochalasin D data must be interpreted carefully, because neutrophils do not spontaneously release all of their granule contents in vivo, and this drug is not physiological. We suggest that in the absence of cytochalasin D, a transient polymerization/depolymerization of actin at the cell cortex and assembly of intermediate filaments to areas devoid of granules allows unrestricted granule movement to the plasmalemma. In support of this hypothesis, recent studies tracking single particles in mouse fibroblasts with an inducible vimentin transgene demonstrated that the diffusion of vesicle-sized particles is severely restricted, in part, by intermediate filaments (Jones et al., 1997). It is interesting to note that mast cell granule secretion (Izushi et al., 1992) and fibroblast secretion of glycoconjugate (Bertrand et al., 1994) also involve changes in intermediate filament organization.
In fMLP-treated neutrophils, microtubules radiate from the microtubule organizing center, with some microtubules also extending into areas containing vimentin filaments at the pericortex. As we reported previously, fMLP increases microtubule numbers (Schliwa et al., 1982b). Nocodazole did not inhibit cGMP elevations nor the transient changes in cell shape accompanying granule secretion or actin localization. However, nocodazole altered the site of vimentin filament organization in fMLP-treated neutrophils. Vimentin filaments were no longer distributed at the pericortex but encircled the nucleus. These data suggest that the site of vimentin filament organization is dependent upon microtubule organization and not changes in cell shape.
Intact microtubules are required for efficient granule secretion, as nocodazole delayed the amount of specific granule contents exocytosed. Interestingly, azurophil granule secretion was normal in the presence of nocodazole. Kinesin, an enzyme that mediates organelle movement along microtubules, is associated with microtubules and granules in neutrophils, suggesting a close interaction between these structures (Ryder et al., 1988; Rothwell et al., 1989).
An interdependence between intermediate filaments and microtubules is reported in fibroblasts (Bershadsky and Vasiliev, 1988). Similar to neutrophils, fibroblasts incubated with microtubule-disrupting agents elicit a change in intermediate filaments from the cell periphery to filament bundles encircling the nucleus. In fibroblasts, intermediate filaments may maintain the integrity of the cytoplasm by stabilizing cytoskeletal interactions and cell shape (Goldman et al., 1996). Microinjection of fibroblasts with peptides that disassemble vimentin filaments in vitro is accompanied by dramatic alterations in cell shape and destabilization of microtubules and intermediate filaments (Goldman et al., 1996). In neutrophils, intermediate filament organization at the pericortex juxtaposed to the nucleus may modulate the architecture and cytoplasmic organization, as well as stabilize the plasma membrane or nucleus against stress while the cell is changing shape. This proposal of intermediate filament function in neutrophils is consistent with the mechanical and organizational role of intermediate filaments in other cell types (Klymkowsky et al., 1989; Skalli and Goldman, 1991; Georgatos and Maison, 1996).
In fMLP-stimulated cells, vimentin filaments organize juxtaposed to the nucleus, and near the nuclear clef with cytochalasin D or nocodazole. These data suggest that an intermediate filament organizing center is localized near the nucleus in neutrophils. These results are consistent with other cell types (Klymkowsky et al., 1989; Skalli and Goldman, 1991; Georgatos and Maison, 1996). Interestingly, neither cytochalasin D nor nocodazole alone was sufficient to alter vimentin filament organization in the absence of fMLP.
Vimentin filaments are probably linked to both microfilaments and microtubules, as drugs that inhibit these structures alter the site of vimentin filament assembly. Plectin sidearms link the interaction of vimentin with microtubules in fibroblasts (Svitkina et al., 1996). We observed 3-nm structures between intermediate filaments and microtubules, and intermediate filaments and actin filaments in whole-mount neutrophils by high-voltage electron microscopy (Schliwa et al., 1982a). In addition, cell fractionation and electron microscopy studies of purified granules suggest an interaction between microtubules and granules (Rothwell et al., 1989). Microfilaments and microtubules are important cytoskeletal structures involved in transporting granules to the cytoplasmic face during neutrophil activation (Hoffstein et al., 1977). Further investigation is required to determine whether the structural organization of intermediate filaments with microtubules, microfilaments, and granules is similar to that of fibroblasts.
Regulation of Intermediate Filament Organization by cGMP
cGMP is a positive regulator of neutrophil granule secretion. In fMLP-stimulated neutrophils, secretion of both granule classes was accelerated by 8-Br-cGMP, and the amount of enzyme exocytosed was elevated at all time points. Lowering cGMP levels with LY83583 inhibited granule secretion and primarily affected vimentin filament but not microfilament or microtubule organization. Similarly, concentrations of fMLP that do not elevate cGMP levels or induce granule secretion did not promote intermediate filament assembly at the cell cortex. The effects of LY83583 were not reversible by removing the drug. However, the membrane-permeable analog 8-Br-cGMP partially restored both granule secretion and vimentin filament organization at the pericortex in LY83583-treated cells. This recovery suggests that cGMP has a role in regulating neutrophil granule secretion. The finding that recovery was not complete by cGMP suggests that other signaling pathways are involved (Sklar, 1986), and/or conditions are not optimal for achieving 100% recovery.
Since G-kinase phosphorylates vimentin in fMLP-stimulated neutrophils, and LY83583 inhibits phosphorylation, we suggest that G-kinase–mediated vimentin phosphorylation is an important step in the sequence of events leading to granule secretion (Wyatt et al., 1991, 1993). In unstimulated neutrophils, G-kinase is localized at the microtubule organizing center and throughout the cytoplasm (Pryzwansky et al., 1990). However, G-kinase is transiently colocalized with vimentin in fMLP-stimulated cells (Wyatt et al., 1991). Vimentin was phosphorylated only at the time when enzyme and substrate were colocalized. We do not know whether vimentin filament phosphorylation causes or results from granule secretion. Unfortunately, neutrophils cannot be microinjected or transfected to investigate the direct effects of G-kinase on intermediate filament organization in intact cells. Therefore, we used an in situ model to determine whether G-kinase could directly affect vimentin organization in the absence of fMLP. Vimentin filaments were rare in cells not exposed to G-kinase. However, the addition of G-kinase induced vimentin filament assembly throughout the cell body. These filaments were disassembled by protein phosphatase 1, indicating that phosphorylation induced filament assembly. In intact unstimulated cells or fMLP-treated cells, this extensive network of vimentin filaments was not observed. Vimentin filaments were organized as bundles at the pericortex only after treatment with fMLP. Therefore, the in situ data suggest that fMLP signaling does not induce maximal vimentin phosphorylation by G-kinase. Since G-kinase is not present in neutrophils in high concentrations and phosphorylation is transient, compartmentalization of enzyme and substrate is a reasonable hypothesis. The mechanisms for intermediate filament assembly and organization in eukaryotic cells are based largely upon studies of mitotic cells where intermediate filaments are highly phosphorylated. The in situ studies suggest that only a small fraction of vimentin is phosphorylated in fMLP-stimulated neutrophils. Thus, differences in the phosphorylation state of cells may account for vimentin filament organization.
G-kinase binds vimentin with high affinity (Kd = 50 nM) and specificity (MacMillian-Crow et al., 1994). However, there are no consensus phosphorylation sites for G-kinase in vimentin. Vimentin is phosphorylated in vitro with the G-kinase dimer (holoenzyme), but not with the monomeric catalytic fragment of G-kinase (MacMillian-Crow et al., 1994). The monomeric catalytic fragment is catalytically active toward other substrates (histone F2b), but not with vimentin. This site of high-affinity interaction between G-kinase and vimentin allows for the interaction between the phosphorylation site of vimentin and the catalytic site of G-kinase to take place. The localization data suggest that targeting of G-kinase to its substrate is compartmentalized and may be regulated by localized pools of cGMP (Pryzwansky et al., 1990). Since there are multiple kinases that phosphorylate vimentin, compartmentalization of G-kinase in the vicinity of its substrate may serve as an efficient mechanism for cellular regulation.
Vimentin filament reorganization by G-kinase in neutrophils differs from in situ studies of lysed fibroblasts incubated with A-kinase (Lamb et al., 1989). In lysed or microinjected fibroblasts, A-kinase caused intermediate filaments to collapse around the nucleus. Vimentin localization at the nucleus was observed also in astrocytes introduced with a constitutively active form of protein kinase C or calmodulin kinase II (Ogawara et al., 1995). In neutrophils activated with fMLP, only disruption of actin or microtubules by cytoskeletal inhibitors caused intermediate filaments to collapse around the nucleus, and in both cases granule secretion was modestly (nocodazole) or severely (cytochalasin) affected.
In summary, the organization of all three major cytoskeletal components is important for orderly granule secretion, including actin polymerization/depolymerization at the cell cortex, microtubule assembly, and vimentin filament assembly (Figure 10). Thus, in addition to F-actin dissolution at the cell cortex, the transient assembly of vimentin filaments within areas devoid of granules may allow unrestricted granule movements directed by microtubules to the plasmalemma. We reported that l-arginine, the precursor for NO synthesis, augments granule secretion and G-kinase–mediated vimentin phosphorylation in fMLP and A23187-stimulated adherent neutrophils (Wyatt et al., 1991, 1993; Pryzwansky et al., 1995). The signaling mechanism for elevating cGMP levels by fMLP may involve NO generation, an activator of guanylyl cyclase (Arnold et al., 1977; Schmidt et al., 1989). We propose that fMLP induces Ca2+ mobilization and NOS activation, leading to NO synthesis and guanylate cyclase activation. Granule secretion, vimentin phosphorylation, and intermediate filament assembly may be transient because NO generation is transient. Therefore, NO may be an important signal upstream in the signal transduction pathway controlling neutrophil granule secretion.
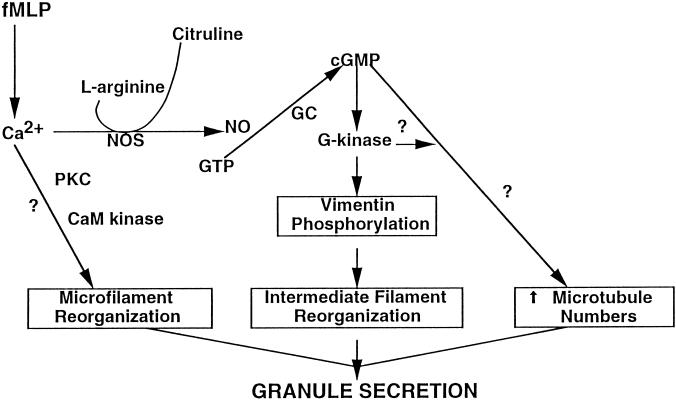
Figure 10.
Signal transduction of cGMP in fMLP-stimulated neutrophils. The signals that transduce granule secretion cause structural changes in microfilament, intermediate filament, and microtubule organization. FMLP induces calcium mobilization and activation of nitric oxide synthase (NOS). The generation of nitric oxide (NO) activates guanylyl cyclase (GC) to synthesize cGMP and activate G-kinase. Phosphorylation of vimentin by G-kinase results in intermediate filament assembly. The increase in microtubule numbers may be regulated by G-kinase, since G-kinase is targeted to the microtubule organizing center in neutrophils (Pryzwansky et al., 1990), and cGMP elevations have been correlated with increased microtubule numbers (Hoffstein, 1980). Actin polymerization and depolymerization are regulated by calcium and may involve phosphorylation of actin-binding proteins by protein kinase C or calmodulin kinase (Omann et al., 1987).
ACKNOWLEDGMENTS
This work was supported by a grant from National Science Foundation (MCB-9421731).
REFERENCES
- Arnold WP, Mittal C, Katsaki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunis D, Bader M. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Becker EL, Henson PM. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Becker EL, Showell HJ. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of the release. J Immunol. 1974;112:2055–2062. [PubMed] [Google Scholar]
- Bershadsky AD, Vasiliev JI. Cytoskeleton. P. Siekevitz, New York: Plenum Press; 1988. Cytoskeleton and internal organization of the cell; pp. 167–201. [Google Scholar]
- Bertrand F, Veissiere D, Hermelin B, Paul A, Capeau J, Picard J, Cherqui G. Phosphorylation of vimentin is an intermediate step in protein kinase C-mediated glycoconjugate seretion. Am J Physiol. 1994;266:C611–C621. doi: 10.1152/ajpcell.1994.266.3.C611. [DOI] [PubMed] [Google Scholar]
- Coates TD, Watts RG, Hartman R, Howard TH. Relationship of F-actin distribution to development of polar shape in human polymorphonuclear neutrophils. J Cell Biol. 1992;117:765–774. doi: 10.1083/jcb.117.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey RG. Granulocyte Responses to Cytokines: Basic and Clinical Research. R.G. Coffey, New York: Marcel Dekker; 1992. Effects of cyclic nucleotides on granulocytes; pp. 301–338. [Google Scholar]
- Georgatos SD, Maison C. Integration of intermediate filaments into cellular organelles. Int Rev Cytol. 1996;164:91–138. doi: 10.1016/s0074-7696(08)62385-2. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2′0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1:207–218. [PubMed] [Google Scholar]
- Hoffstein S, Goldstein IM, Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977;73:242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S, Weissmann G. Microfilaments and microtubules in calcium ionophore-induced secretion of lysosomal enzymes from human polymorphonuclear leukocytes. J Cell Biol. 1978;78:769–781. doi: 10.1083/jcb.78.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein ST. Intra-and extra-cellular secretion from polymorphonuclear leukocytes. In: Weissmann G, editor. Cell Biology of Inflammation. Amsterdam: Elsevier North-Holland; 1980. pp. 387–435. [Google Scholar]
- Hoffstein ST, Friedman RS, Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982;95:234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard TH, Orseajo CO. The kinetics of chemotactic peptide-induced change in f-actin content, f-actin distribution, and the shape of neutrophils. J Cell Biol. 1985;101:1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CK, Hill JM, Bormann BJ, Mackin WM, Becker EL. Chemotactic factors induced vimentin phosphorylation in rabbit peritoneal neutrophils. J Biol Chem. 1984;259:1386–1389. [PubMed] [Google Scholar]
- Ignarro LJ. Lysosomes in Biology and Pathology. J.T. Dingle and R.T. Dean, New York: American Elsevier; 1975. Regulation of lysosomal enzyme release by prostaglandins, autonomic neurohormones and cyclic nucleotides; pp. 481–522. [PubMed] [Google Scholar]
- Izushi K, Fujiwara Y, Tasaka K. Identification of vimentin in rat peritoneal mast cells and its phosphorylation in association with histamine release. Immunopharmacology. 1992;23:153–161. doi: 10.1016/0162-3109(92)90021-4. [DOI] [PubMed] [Google Scholar]
- Jones JD, Ragsdale GK, Rozelle A, Yin HL, Luby-Phelps K. Diffusion of vesicle-sized particles in living cells is restricted by intermediate filaments. Mol Biol Cell. 1997;8:174a. [Google Scholar]
- Klymkowsky MW, Bachant JB, Domingo A. Functions of intermediate filaments. Cell Motil Cytoskeleton. 1989;14:309–331. doi: 10.1002/cm.970140302. [DOI] [PubMed] [Google Scholar]
- Lamb NJC, Fernandez A, Feramisco JR, Welch WJ. Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the cAMP-dependent protein kinase. J Cell Biol. 1989;108:2409–2422. doi: 10.1083/jcb.108.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Komalavilas P, Boerth NJ, MacMillan-Crow LA, Cornwell TL. cGMP signaling through cAMP- and cGMP-dependent protein kinases. Adv Pharmacol. 1995;34:305–322. doi: 10.1016/s1054-3589(08)61094-7. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S, Pollard TD. Mechanism of action of cytochalasin B on actin. Cell. 1980;20:329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- MacMillian-Crow LA, Murphy-Ullrich JA, Lincoln TM. High affinity binding and localization of the cyclic GMP-dependent protein kinase with the intermediate filament protein vimentin. Biochemistry. 1994;33:8035–8043. doi: 10.1021/bi00192a007. [DOI] [PubMed] [Google Scholar]
- Malech HL, Root RK, Gallin JI. Structural analysis of human neutrophil migration. J Cell Biol. 1977;75:666–693. doi: 10.1083/jcb.75.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PL, Bank HL, Brissie NT, Spicer SS. The association of microfilament bundles with lysosomes in polymorphonuclear leukocytes. J Cell Biol. 1976;71:659–666. doi: 10.1083/jcb.71.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara M, et al. Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J Cell Biol. 1995;131:1055–1066. doi: 10.1083/jcb.131.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omann GM, Allen RA, Bokoch GM, Painter RG, Traynor AE, Sklar LA. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987;67:285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Parysek LM, Eckert BS. Vimentin filaments in spreading, randomly locomoting, and f-met-leu-phe-treated neutrophils. Cell Tissue Res. 1984;235:575–81. doi: 10.1007/BF00226955. [DOI] [PubMed] [Google Scholar]
- Poste G, Allison AC. Membrane fusion. Biochim Biophys Acta. 1973;300:421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Pryzwansky KB, MacRae EK, Spitznagel JK, Cooney M. Early degranulation of human neutrophils: immunocytochemical studies of surface and intracellular phagocytic events. Cell. 1979;18:1025–1033. doi: 10.1016/0092-8674(79)90215-0. [DOI] [PubMed] [Google Scholar]
- Pryzwansky KB, Schliwa M, Boxer LA. Microtubule organization of unstimulated and stimulated adherent human neutrophils in Chediak-Higashi syndrome. Blood. 1985;66:1398–1403. [PubMed] [Google Scholar]
- Pryzwansky KB, Schliwa M, Porter KR. Comparison of the three-dimensional organization of unextracted and Triton-extracted human neutrophilic polymorphonuclear leukocytes. Eur J Cell Biol. 1983;30:112–125. [PubMed] [Google Scholar]
- Pryzwansky KB, Wyatt TA, Lincoln TM. Cyclic guanosine monophosphate-dependent protein kinase is targeted to intermediate filaments and phosphorylates vimentin in A23187-stimulated human neutrophils. Blood. 1995;85:222–230. [PubMed] [Google Scholar]
- Pryzwansky KB, Wyatt TA, Nichols H, Lincoln TM. Compartmentalization of cyclic GMP-dependent protein kinase in formyl-peptide stimulated neutrophils. Blood. 1990;76:612–618. [PubMed] [Google Scholar]
- Rothwell SW, Nath J, Wright DG. Interactions of cytoplasmic granules with microtubules in human neutrophils. J Cell Biol. 1989;108:2313–2326. doi: 10.1083/jcb.108.6.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder MI, Weinreb RN, Niederman R. Microtubule-granule relationships in motile human polymorphonuclear leukocytes. Anat Rec. 1988;221:679–686. doi: 10.1002/ar.1092210302. [DOI] [PubMed] [Google Scholar]
- Schliwa M, Pryzwansky KB, van Blerkom J. Implications of cytoskeletal interactions for cellular architecture and behaviour. Phil Trans R Soc Lond. 1982a;299:199–205. doi: 10.1098/rstb.1982.0126. [DOI] [PubMed] [Google Scholar]
- Schliwa M, Pryzwansky KB, Euteneuer U. Centrosome splitting in neutrophils: an unusual phenomenon related to cell activation and motility. Cell. 1982b;31:705–717. doi: 10.1016/0092-8674(82)90325-7. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Seifert R, Bohme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor, and leukotriene B4. FEBS Lett. 1989;244:357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Skalli O, Goldman RD. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19:67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Sklar LK. Ligand-receptor dynamics and signal amplification in the neutrophil. Adv Immunol. 1986;39:95–143. doi: 10.1016/s0065-2776(08)60349-1. [DOI] [PubMed] [Google Scholar]
- Smolen JE. Neutrophil signal transduction: calcium, kinases, and fusion. J Lab Clin Med. 1992;120:527–532. [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G, Goldstein I, Hoffstein S, Chauvet G, Robineaux R. Yin/yang modulation of lysosomal enzyme release from polymorphonuclear leukocytes by cyclic nucleotides. Ann NY Acad Sci. 1975;256:222–232. doi: 10.1111/j.1749-6632.1975.tb36049.x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Lincoln TM, Pryzwansky KB. Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formyl-peptide stimulated neutrophils. J Biol Chem. 1991;266:21274–21280. [PubMed] [Google Scholar]
- Wyatt TA, Lincoln TM, Pryzwansky KB. Regulation of human neutrophil degranulation by LY83583 and L-arginine: role of cGMP-dependent protein kinase. Am J Physiol. 1993;34:C201–C211. doi: 10.1152/ajpcell.1993.265.1.C201. [DOI] [PubMed] [Google Scholar]