Abstract
We previously demonstrated that a significant proportion of interphotoreceptor retinoid-binding protein (IRBP)-specific uveitogenic T cells in the C57BL/6 mouse and Lewis rat express CD8. The aims of this study were to determine whether some of the IRBP-specific T cells in the B10RIII mouse also express CD8 and whether CD8 and CD4 IRBP-specific T cells in the B10RIII mouse recognize a different or the same antigenic epitope. Our results show that autoreactive CD8 T cells were abundant in B10RIII mice immunized with the uveitogenic peptide IRBP161-180. Using multimers of recombinant H-2Dr molecules, we also showed that the binding of the H-2Dr fusion protein to IRBP161-180-expanded CD8 T cells was dependent on the peptide complexed with the recombinant molecules. The use of a panel of truncated peptides showed that the truncated 10-mer peptide, IRBP168-177, retained the ability to bind to, and stimulate, IRBP161-180-specific CD8 T cells after complexing with a dimeric MHC class I (H-2Dr) molecule. Finally, adoptive transfer of IRBP161-180-specific T cells stimulated with IRBP168-177 consistently induced mild, but significant, EAU in naïve B10RIII mice.
Keywords: antigenic epitope, autoimmunity, CD8+ autoreactive T cell, EAU, uveitis
Introduction
Uveitis is a common cause of human visual disability and blindness. To investigate the pathogenesis of this disease, experimental models have been generated in rodents, in which immunization of recipients with characterized ocular antigens or transfer of isolated T cells specific for the antigen induces ocular inflammation that resembles human uveitis. One of the well-characterized ocular antigens that consistently induces ocular inflammation in both rat and mouse is interphotoreceptor retinal-binding protein (IRBP) (Fox et al., 1987,Silver et al., 1995,Avichezer et al., 2000b).
Early studies on autoreactive T cells in mouse or rat experimental autoimmune uveitis (EAU) focused on CD4 autoreactive T cells (Rizzo et al., 1996,Rozenszajn et al., 1986,Gregerson et al., 1986). However, we have recently demonstrated that, in both the Lewis rat (Shao et al., 2004) and B6 mouse (Shao et al., 2005a);(Peng et al., 2007), many CD8 autoreactive T cells are activated and actively participate in disease pathogenesis. To determine whether this observation applies to other EAU models, we tested the B10RIII mouse, which is the mouse strain most susceptible to EAU (Hankey et al., 2001,Karabekian et al., 2005). Since our previous studies demonstrated that CD4 and CD8 IRBP-specific T cells in the B6 mouse both responded to the same antigenic epitope (Shao et al., 2005a), we were also interested in testing whether this was also true in the B10RIII mouse. Here, we show that CD8 IRBP-specific T cells were abundant in the B10RIII mouse immunized with the uveitogenic peptide IRBP161-180. We also show that peptide 168–177 was the shortest peptide retaining the ability to bind to, and stimulate, CD8 IRBP161-180-specific T cells when complexed with a recombinant class I (H-2Dr) molecule in the absence or presence of co-stimulatory molecules. Finally, adoptive transfer of IRBP161-180-specific T cells stimulated in vitro with IRBP168-177 induced mild, but significant, EAU in the naïve B10RIII mouse. The determination of this core region of an antigenic epitope for CD8 autoreactive T cells in EAU should facilitate our efforts at characterizing the pathogenic T cell repertoire in this disease.
Methods
Animals and reagents
Pathogen-free female B10RIII mice (10- to 14-weeks-old) were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Louisville. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed. The sequences of IRBP161-180 and the truncated peptides are listed in Table 1. All were synthesized by Pepscan Systems (Lelystad, Netherlands) and were >85% pure.
Table 1.
Truncated peptides derived from IRBP161-180
| Peptide | Amino acid sequence |
|---|---|
| IRBP161-180 | SGIPYIISYLHPGNTILHVD |
| IRBP165-177 | YIISYLHPGNTIL |
| IRBP165-176 | YIISYLHPGNTI |
| IRBP165-175 | YIISYLHPGNT |
| IRBP165-174 | YIISYLHPGNT |
| IRBP165-173 | YIISYLHPGN |
| IRBP166-177 | IISYLHPGNTIL |
| IRBP167-177 | ISYLHPGNTIL |
| IRBP168-177 | SYLHPGNTIL |
| IRBP169-177 | YLHPGNTIL |
Animal model of experimental autoimmune uveitis (EAU)
EAU was induced in B10RIII mice either by immunization with IRBP161-180 (active induction) or by adoptive transfer of isolated IRBP-specific T cells. For active induction, mice were immunized subcutaneously with 200 μl of an emulsion containing 100 μg of IRBP161-180 (or truncated peptides) and 500 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in incomplete Freund’s adjuvant (Sigma, St Louis), distributed over six spots on the tail base and flank. For adoptive transfer studies, naïve B10RIII mice underwent adoptive transfer of 5 × 106 IRBP161-180-specific T cells as described previously (Shao et al., 2003b,Shao et al., 2003c,Shao et al., 2003a). The animals were examined three times a week for clinical signs of uveitis by fundoscopy, starting at week 2 post-transfer. Fundoscopic evaluation for longitudinal follow-up of disease was performed using a binocular microscope after pupil dilation using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions. The incidence and severity of EAU were graded on a scale of 0 to 4 in half-point increments using criteria described previously (Thurau et al., 1997), which are based on the type, number, and size of lesions present.
Pathological examination
Inflammation in the eye was confirmed by histopathology. Whole eyes were collected, immersed for 1 h in 4% phosphate-buffered glutaraldehyde, and transferred to 10% phosphate-buffered formaldehyde until processed. The fixed and dehydrated tissue was embedded in methacrylate and 5 μm sections cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin. Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Severity of EAU was scored on a scale of 0 (no disease) to 4 (maximum disease) in half-point increments, as described previously (Shao et al., 2003a).
Purification of CD4+ and CD8+ T cells using StemSep columns
CD4+- and CD8+-enriched T cell populations were isolated from freshly prepared draining lymph node and spleen cells using negative selection StemSep kits (Stem Cell Technology, Vancouver, Canada). The mouse lymph node and spleen cells were incubated with mixtures of bispecific antibody complexes, each of which binds both to dextran beads and to CD4 or CD8 and to a cell surface antigen on mouse hematopoietic cells (CD11b;Mac-1), NK cells (DX5), B cells (CD45R; B220), erythroid cells (TER119), or poymorphonuclear leukocytes (Ly-6G; Gr-1). The cells were then incubated for 15 min at 4°C with StepSep™ Magnetic Colloid, loaded into a magnetic column, and washed with 15 ml of medium according to the manufacturer’s protocol. The flow-through fraction containing either CD4+ or CD8+-enriched cells was collected and the purity of the isolated cell fraction determined by flow cytometry using fluorescein isothiocyanate (FITC)-conjugated anti-TCR antibodies and phycoerythrin (PE)-conjugated anti-CD4 or anti-CD8 antibodies.
Proliferation assay
T cells from IRBP161-180-immunized mice were prepared and seeded at 4 × 105 cells/well in 96-well plates, then cultured at 37° C for 48 h in a total volume of 200 μl of medium with or without IRBP-derived peptides in the presence of 2 × 105 irradiated (2000 Rad) syngeneic spleen cells (antigen-presenting cells; APCs), and [3H] thymidine incorporation during the last 8 h was assessed using a microplate scintillation counter (Packard). The proliferative response was expressed as the mean cpm ± standard deviation (SD) of triplicate determinations.
CFSE staining
T cells from the draining lymph nodes and spleen from immunized mice were prepared by passage through a nylon wool column and stained with the vital dye, CFSE (carboxy-fluorescein diacetate, succinimidyl ester, Molecular Probes, Eugene, OR) as described previously (Lyons and Parish, 1994). Briefly, the cells were washed and resuspended at 50 × 106 cells/ml in serum-free RPMI medium, then incubated at 37°C for 10 min with gentle shaking with a final concentration of 10 μM CFSE, washed twice with, and resuspended in, RPMI medium containing 10% FCS, stimulated with peptides and irradiated APCs for 2 days and analyzed by flow cytometry.
Preparation of a recombinant B10RIII mouse MHC class I molecule fusion protein
A modified mouse H-2Dr molecule fused to the Fc region of a human IgG1 to facilitate the secretion of the expressed protein was prepared. Total RNA was prepared from B10RIII mouse T cells and full length H-2Dr cDNA prepared by RT-PCR using the α-chain specific primers ATG GGG GCG ATG GCT CCG and CGC TTT ACA ATC TTG GAG AG. Based on the predicted transmembrane domain spanning sequence of residues 308–330 (http://www.cbs.dtu.dk/CBS prediction servers), a truncated cDNA depleted of the transmembrane sequence (residue 311 to the carboxyl terminal) was prepared to facilitate secretion. cDNA coding for the Fc region of a human IgG1 (hIgG-Fc) was prepared similarly. The BD Pharmingen baculovirus expression system and transfer cloning vector pVL1393 were used. Codons coding for eight additional amino acids (EFLQPGGS) were inserted as a linker between the cloned H-2Dr and hIgG-Fc cDNAs. Starting from the same total RNA used in H-2Dr construction, full length human β2 microglobulin cDNA was prepared from the products of RT-PCR using the primers ATGGCT CGC TCG GTG ACC CT and A TGC TTA TCA CAT GTC TCG. After verification of the cloned cDNAs by sequencing, SF9 cells were co-infected with recombinant viruses expressing either H-Dr–Fc or β2-microglobulin cDNA. Culture supernatants were collected and subjected to Western blot analysis using anti-human IgG antibody. The recombinant protein was purified using a protein A affinity column and concentrated using a Centricon (Plus-20, PL-30) (Amicon).
Flow cytometric detection of T cells binding IRBP peptides complexed with recombinant MHC class I (H-2Dr) dimers
The H-2Dr fusion protein was used to detect antigen-specific CD8 T cells. To produce the dimeric form, the protein was incubated at 4°C for 12–24 h with human β2-microglobulin (both at a final concentration of 0.15 mg/ml) and an excess of the test peptide (1 mg/ml). Double staining was performed by incubating 5 × 105 cells with 0.5 μg of peptide/dimer complexes at 4°C for 30 minutes in a volume of 0.5 ml, then washing them twice in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% sodium azide, and staining them with PE-labeled anti-human IgG1 antibody, followed by a FITC-conjugated antibody against either CD4 or CD8. The results are presented as PE staining versus FITC staining.
Immunofluorescence flow cytometry
Aliquots of 2 × 105 cells were double-stained with combinations of FITC- or PE-conjugated monoclonal antibodies against mouse αβTCR (H57), CD4, or CD8. All antibodies were purchased from BD Bioscience (La Jolla, CA). Data collection and analysis were performed on a FACScaliber flow cytometer using CellQuest software.
In vitro stimulation of CD8 IRBP-specific T cells with peptide/dimeric H-2Dr complexes
96-well flat-bottom plates were pre-coated for 18 h at 4°C with 5 μg/ml of recombinant B7 molecules (R&D) and thoroughly washed with PBS. Responder CD8 T cells (4 × 105/well) prepared by magnetic purification from IRBP161-180-immunized mice were added to each well, followed by dimeric H-2Dr molecules (10 μg/well) mixed with the indicated IRBP peptide (1 μg/ml). The cells were then incubated at 37°C for 48h and [3H] thymidine incorporation during the last 8 h assessed using a microplate scintillation counter (Packard). The proliferative response was expressed as the mean cpm ± standard deviation (SD) of triplicate determinations.
ELISA
IFN-γ were measured using commercially available ELISA kits (R&D Systems).
Statistical analysis
The data are expressed as the mean ± SD. Each experiment was performed at least three times.
Results
Detection of CD8± IRBP161-180-specific T cells in the immunized B10RIII mouse
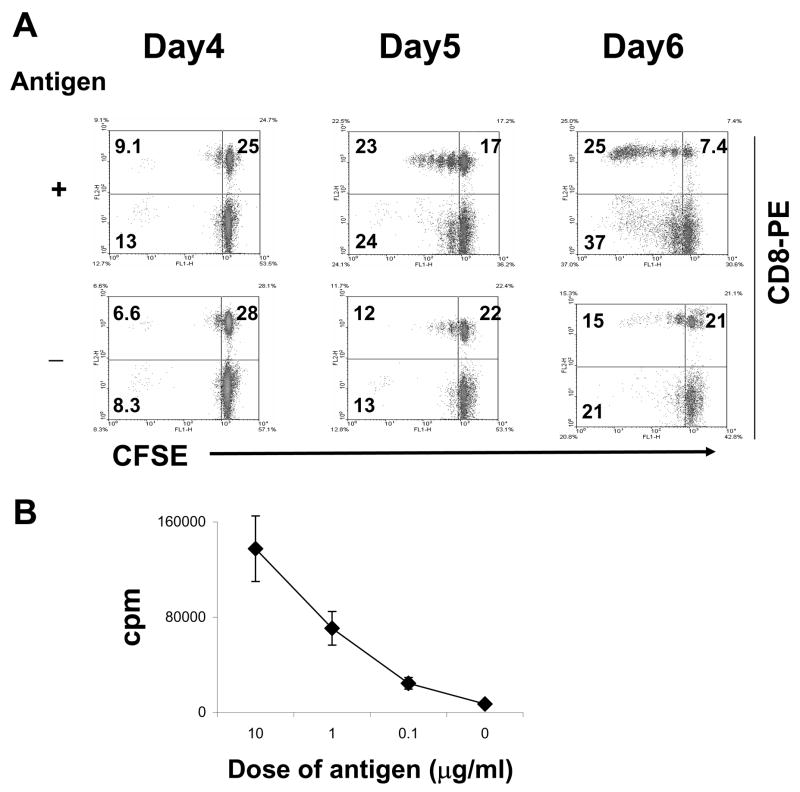
To determine whether autoreactive CD8 T cells were induced by the uveitogenic peptide IRBP161-180 (Silver et al., 1995,Hankey et al., 2001);(Shao et al., 2005b) in the EAU-susceptible B10RIII mouse, we immunized B10RIII mice with IRBP161-180, then, at 13 days post-immunization (p.i.), prepared T cells from the pooled draining lymph nodes and spleens using nylon wool. The T cells were first labeled with CFSE, then stimulated for 2 days with IRBP161-180 in the presence of irradiated syngeneic APCs. The activated T cell blasts were then separated by Ficoll gradient centrifugation and cultured in IL-2-containing medium for the indicated number of days (2 days stimulation with antigen, then the remaining days in culture with IL-2), then were stained with PE-labeled anti-CD8 antibodies, and subjected to FACS analysis. As shown in Fig. 1A, a large proportion of the CD8 T cells proliferated in the presence of immunizing peptide. This T cell response was antigen-specific, as the thymidine proliferation test showed that a strong response was only seen in the presence of immunizing peptide (Fig. 1B). Using the CFSE assay, proliferation was first seen at 5 days after the start of in vitro stimulation (Fig. 1A).
Fig. 1. Detection of CD8+ IRBP161-180-specific T cells using CFSE staining.
(A): Nylon wool-enriched T cells, prepared from IRBP161-180-immunized B10RIII mice on day 13 p.i., were stained with CFSE, then stimulated in vitro with or without IRBP161-180 for 2 days, and the activated T cell blasts separated on a Ficoll gradient and re-cultured in IL-2-containing medium. For FACS analysis, the T cells were stained separately with PE-labeled antibodies against mouse CD8. The days indicated include 2 in culture with antigen and the remainder in IL-2-containing medium. (B): Antigen-dependent proliferative response to IRBP161-180. Nylon wool-enriched T cells from IRBP161-180-immunized B10RIII mice were cultured at 37° C for 48 h in 96-well microtiter plates with syngeneic APC with graded doses of IRBP161-180, and [3H] thymidine incorporation during the last 8 h assessed. The proliferative response is expressed as the mean cpm ± SD for triplicate wells.
Response of in vivo IRBP161-180-primed T cells to truncated IRBP peptides
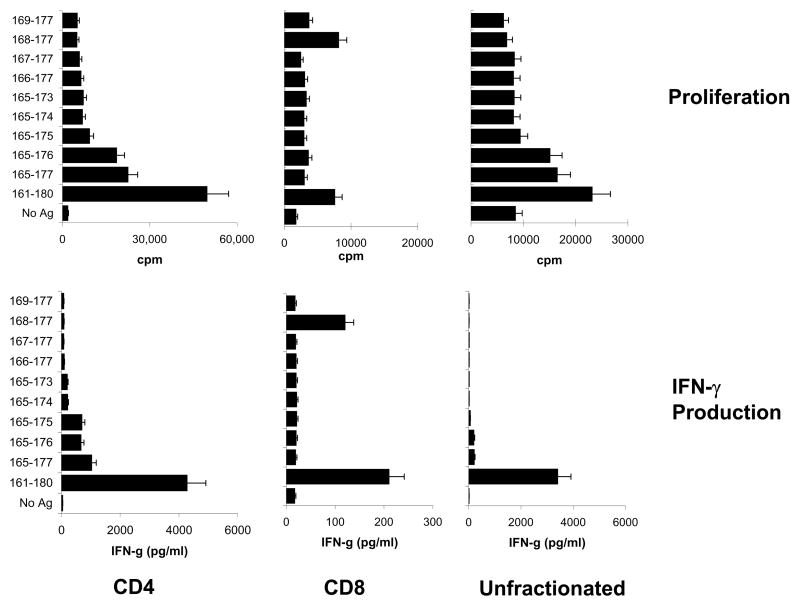
Since the uveitogenic peptide IRBP161-180 contains 20 amino acid residues, we then determined whether the CD4 and CD8 IRBP-specific T cells in the B10RIII mouse recognized the same or different antigenic epitopes. We prepared a panel of 10 truncated peptides by truncation of IRBP161-180 at the amino- and/or carboxyl terminus (Table 1) and prepared highly purified CD8 and CD4 T cells from the immunized mice at 13 days p.i. using magnetic beads. The purity of the separated CD8 and CD4 T cells was verified by staining with FITC-labeled antibodies followed by FACS analysis (Peng et al., 2007,Shao et al., 2005a) and the cells were tested for antigen-specific proliferation in the thymidine incorporation test by exposure to the truncated peptides (10 μg/ml) in the presence of syngeneic APCs. Unfractionated T cells containing both CD4 and CD8 T cells were also included for comparison. A representative proliferation result is shown in the upper panels of Fig. 2. The CD4 T cells responded to the non-truncated peptide IRBP161-180 and to a lesser, but significant, extent to the minimally truncated peptides IRBP165-176 and IRBP165-177, whereas the CD8 responders responded only to the full length peptide and the 10-mer peptide IRBP168-177. The results also showed that the response to IRBP168-177 can not be easily shown if unfractionated T cells were used as responder cells; conceivably, the dominant CD4 response has shadowed the CD8 response. These results were supported by IFN-γ assays (Fig. 2, lower panel).
Fig. 2. Response of in vivo IRBP161-180-primed T cells to truncated IRBP peptides.
Purified CD4+ and CD8+ T cells were prepared from the spleens of immunized B10RIII mice at day 13 p.i. using StemSep columns (see Materials and Methods) and were subjected to FACS analysis using FITC-labeled antibodies against mouse CD4 or CD8 as described previously (Shao et al., 2005a). Unfractionated splenic T cells were also prepared by passage through a nylon wool column. The proliferative response (upper panel) and IFN-γ production (lower panel) of purified CD4+ and CD8+ IRBP-specific T cells or unfractionated T cells from IRBP161-180-immunized mice to a panel of truncated IRBP peptides were tested. The results of the proliferation assay are shown are the mean cpm and are representative of those for 5 separate experiments, each involving pooled T cells from 8–10 IRBP161-180-immunized B10RIII mice; the SD was always less than 15%. IFN-γ was evaluated by ELISA and the results are representative of those for 3 assays.
Binding of MHC class I (H-2Dr) molecules complexed with IRBP-derived peptides to CD8± IRBP-specific T cells
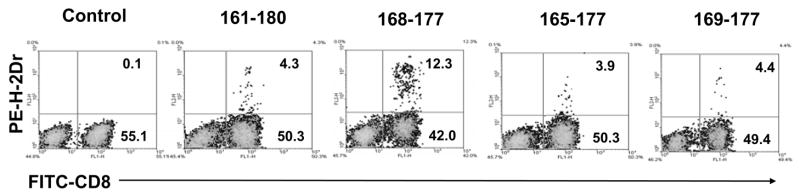
To further demonstrate the presence of IRBP-specific CD8 T cells, an H-2Dr-hIgFc fusion protein was prepared and tested for its ability to bind to CD8 IRBP161-180-specific T cells after complexing with varying truncated IRBP peptides. IRBP161-180-specific T cells were prepared from the spleens and lymph nodes of IRBP161-180-immunized mice at day 13 p.i. using nylon wool, then were stimulated with immunizing peptide for 2 days and expanded in IL-2-containing medium for 7 days. The separated T cells were incubated with the H-2Dr-hIgFc fusion protein complexed with various truncated peptides, then with a PE-labeled anti-human IgG1 antibody (see Materials and Methods), followed by a FITC-labeled anti-mouse CD8 antibody, then all the samples were subjected to FACS analysis. Figure 3 shows that the binding of the H-2Dr fusion protein to IRBP-expanded CD8 T cells was dependent on the nature of the peptide in the complex. The greatest binding was seen with complexes of H-2Dr and IRBP168-177, which bound to 12.3% of the T cells or 23% of the CD8 T cells, whereas naked H-2Dr molecules were barely (< 0.1%) able to bind to the T cells and other peptides showed only marginal binding. Binding of the peptide-conjugated H-2Dr molecules was very sensitive to the length of the peptide under test; for example, deletion of a single amino acid at the amino-terminal of P168-177 (P169-177) or addition of a few amino acids to the amino and/or carboxyl terminus greatly reduced binding.
Fig. 3. Binding of complexes containing H-2Dr and various IRBP161-180-derived peptides to IRBP161-180-specific T cells.
IRBP161-180-specific T cells, prepared from immunized B6 mice at day 13 p.i., were stimulated in vitro with 20 μg/ml of IRBP161-180 and APCs, then T cell blasts were separated by Ficoll gradient centrifugation and cultured in IL-2 containing medium for a week. The cells were then incubated with complexes containing H-2Dr and the indicated IRBP-derived peptide or no peptide (control), then were incubated with PE-labeled anti-human IgG1 antibody (detecting bound H-2Dr fusion protein, see Methods) (y axis) and FITC-labeled anti-mouse CD8 antibody (x axis).
In vitro stimulation of CD8 IRBP161-180-specific T cells using MHC class I (H-2Dr) molecules complexed with IRBP-derived peptides
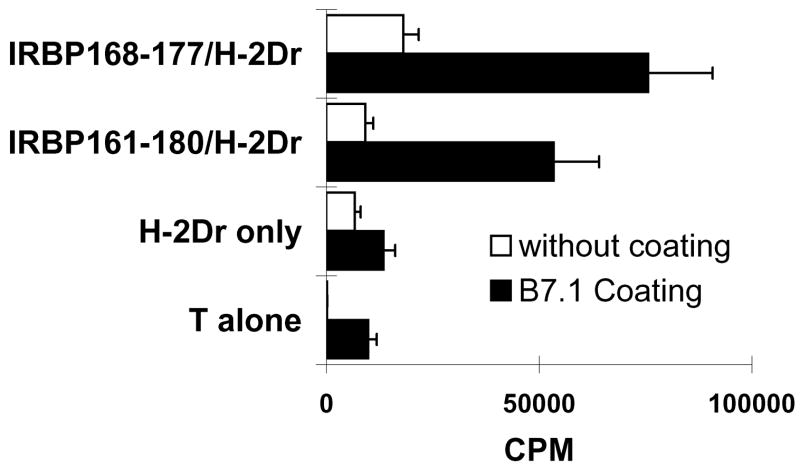
We then examined whether the H-2Dr fusion protein/IRBP peptide complexes had an in vitro stimulatory effect on in vivo primed CD8 T cells. To test this, CD8 T cells (4 ×105/well) separated from IRBP161-180-immunized mice were seeded in 96-well plates pre-coated or not pre-coated with a mouse B7-Ig fusion protein (10 μg/ml), then dimeric recombinant H-2Dr molecules [10 μg/ml] complexed with truncated or non-truncated IRBP peptides [1 μg/ml] were added. As shown in Fig. 4, H-2Dr fusion protein complexed with IRBP161-180 or 168–177 significantly stimulated the proliferation of the responder CD8 T cells in the presence of B7 molecules. Importantly, the H-2Dr molecules alone did not have a significant stimulatory effect. Again, the results indicated that IRBP168-177 contained an essential sequence for the activation of CD8+ IRBP-specific T cells.
Fig. 4. Response of CD8+ IRBP161-180-specific T cells to H-2Dr/IRBP-derived peptide complexes in vitro.
The proliferative response of 4 × 105 in vivo primed CD8+ IRBP161-180-specific T cells to IRBP-derived peptide-complexed or non-complexed recombinant H-2Dr molecules (10 μg/ml) was tested in the absence or presence of B7 molecules in the thymidine incorporation assay. The results shown are representative of those for 4 separate tests.
Assessment of the pathogenic activity of the truncated IRBP168-177 peptide
We also tested the pathogenic activity of the truncated peptides. We first immunized naïve B10RIII mice with IRBP peptides of various lengths, including the truncated peptide IRBP168-177 and the full-length peptide IRBP161-180. The results showed that, while the full-length peptide induced severe disease, the truncated peptides, including IRBP168-177, failed to induced appreciable EAU (data not shown).
Since our previous studies suggested that adoptive transfer induced stronger EAU in the B6 mouse (Shao et al., 2006,Shao et al., 2003c), we then tested the pathogenic activity of the truncated peptide using adoptive transfer. B10RIII mice were immunized with the uveitogenic full-length peptide IRBP161-180, then T cells from the immunized mice were subjected to in vitro stimulation with various peptides before transfer to recipient mice. As shown in Fig. 5, IRBP168-177-activated T cells induced significant EAU in naïve B10RIII recipients, even though disease severity was modest when compared to that in mice injected with the same IRBP-specific T cells stimulated in vitro with the full-length IRBP161-180 peptide.
Fig. 5. Uveitogenic activity of IRBP168-177-activated IRBP161-180-specific T cells.
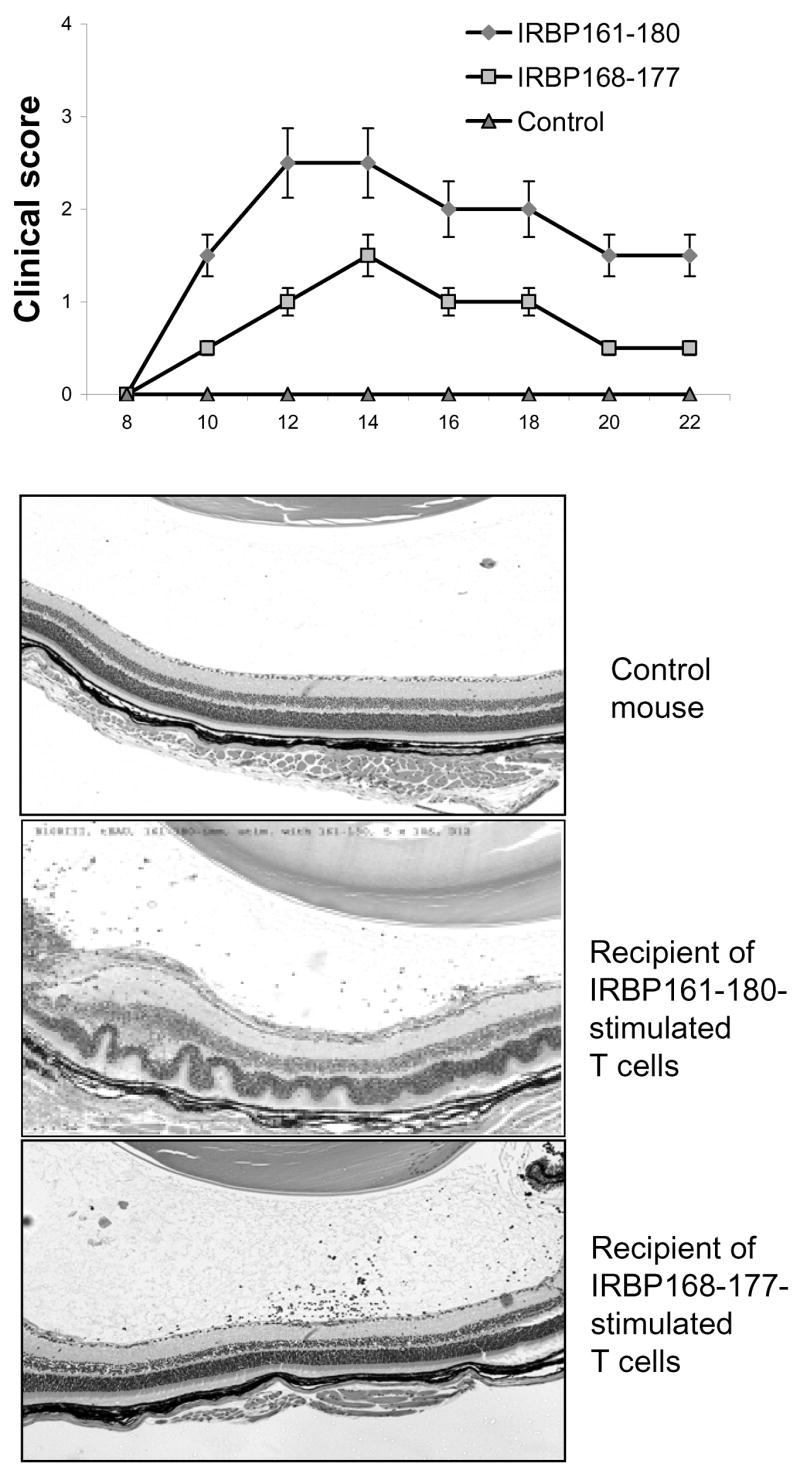
Nylon wool-enriched splenic T cells, prepared from IRBP161-180-immunized B10RIII mice at day 13 p.i., were subjected to in vitro stimulation with 20 μg/ml of IRBP161-180 or IRBP168-177 and APCs (the control group was exposed to APC alone in the absence of peptide), then 5 ×106 T cell blasts, separated by Ficoll gradient centrifugation, were transferred to each groups (n=4) of recipient mouse. The clinical score was monitored by fundoscopy and the eyes of the recipient mice subjected to pathological examination 15 days later. Eye histology showed that IRBP168-177-stimulated T cells induced modest, but significant, EAU upon adoptive transfer to naïve B10RIII mice.
Discussion
Initially, CD4+ autoreactive T cells were believed to be the major pathogenic T cells in autoimmune diseases, such as experimental autoimmune encephalitis (EAE) and EAU (Gregerson et al., 1986,Jones et al., 1997,Karabekian et al., 2005). This conclusion was chiefly supported by the observations that cultured autoreactive T cells in such diseases (Sun et al., 1988) (Tuohy et al., 1988,Reddy et al., 2003);(Rizzo et al., 1996,Rozenszajn et al., 1986,Gregerson et al., 1986) are exclusively CD4+αβTCR+ and that, upon transfer to syngeneic naive animals, these CD4+ autoreactive T cell lines are capable of causing the related autoimmune disease (Gregerson et al., 1986,Rizzo et al., 1996,Rozenszajn et al., 1986). However, a pathogenic role of autoreactive CD8+ T cells has also been recently demonstrated in a number of autoimmune diseases, such as diabetes (Wang et al., 1996,Nagata et al., 1994), arthritis (Tada et al., 1996), and EAE (Biddison et al., 1998). Laboratories (Perchellet et al., 2004,Huseby et al., 2001,Zeng et al., 2005), including our own (Sun et al., 2003,Sun et al., 2001), have demonstrated that CD8+ encephalitogenic T cells play a major role in Myelin/Oligodendrocyte Glycoprotein (MOG)-induced EAE in the B6 mouse. Our recent studies have also shown that co-activation of CD8+ uveitogenic T cells can be readily demonstrated in IRBP-induced uveitis in the rat (Shao et al., 2004) and B6 mouse (Peng et al., 2006,Shao et al., 2005a).
The B10RIII mouse provides an attractive EAU model (Jiang et al., 1999,Silver et al., 1999), as it is much more susceptible to actively induced disease than the C57BL/6 mouse (Avichezer et al., 2000a,Avichezer et al., 2000b). To determine whether CD8+ uveitogenic T cells could be demonstrated in mouse EAU models other than that in the B6 strain, we examined the B10RIII mouse and found that autoreactive CD8 T cells were more readily demonstrated than in the B6 mouse. In the B6 mouse, if CD4 and CD8 cells are not separated, the dominant proliferating cells commonly express CD4 after long-term (weeks) culture or after repeated re-stimulation in vitro. However, in the B10RIII mouse under similar culture conditions, CD8 T cells often became dominant. It is unclear whether this difference is related to the increased EAU susceptibility of the B10RIII mouse. The CSFE assay demonstrated that, if CD4 and CD8 cells from the B10RIII mouse were not separated before exposure to immunizing antigen, the CD8 cells proliferated and expanded more vigorously and rapidly than their CD4 counterparts (not shown).
Proliferation and cytokine-producing assays (Fig. 2) using highly purified CD4 and CD8 T cells prepared from IRBP161-180-immunized mice showed that the strongest response of CD4 T cells was seen during in vitro stimulation of the T cells with the full-length 20-mer peptide or minimally truncated peptides, such as IRBP165-176 or IRBP165-177, which agrees with the disease-inducing effect of the peptides (data not shown). However, of the truncated peptides tested, CD8 T cells reacted only to the 10-mer IRBP168-177 and the non-truncated IRBP161-180. A binding assay using complexes of IRBP peptides and H-2Dr molecules (Fig. 3) further supported the idea that IRBP168-177 showed the strongest binding to CD8 IRBP161-180-specific T cells.
We found that complexes of peptide 168–177 and the H-2Dr fusion protein had a strong stimulatory effect on CD8 IRBP-specific T cells (Fig. 4). The intensity of the response was higher than when the same T cells responded to peptides and APCs (Fig. 2). It is likely that this is due to the dimeric nature of the recombinant H-2Dr molecules. Conceivably, autoreactive CD8 T cells will be better activated by ligands that can crosslink the antigen receptors (T cell receptors). A previous study has shown that while the truncated IRBP165-177 peptide minimally retained pathogenic activity, the peptide IRBP168-180 was non-pathogenic (Silver et al., 1995). Our studies showed that IRBP168-177 was able to activate IRBP161-180-specific T cells, which were able to induce EAU upon transfer to naïve B10RIII mice. The higher disease-inducing effect of the truncated peptide of our study could be attributed to the different way of disease induction, in which we adoptively transfer disease by injection to naïve recipients with autoreactive T cells in vivo primed with the non-truncated IRBP161-180 peptide, but restimulated in vitro with the truncated IRBP168-177 peptide. Conceivably, the secondary activation of in vivo primed T cells is less stringent in antigen requirement than the primary activation of the T cells in vivo.
Our results show that, in the B10RIII mouse, autoreactive T cells contain both CD4 and CD8 cells, as in the B6 mouse. Importantly, the uveitogenic 20-mer peptide stimulated responses of both CD4 and CD8 T cells. In the absence of CD4 T cells, the CD8 T cell response was significantly weaker, supporting our previous observation in the B6 EAU model that CD8 T cells show increased activation when CD4 T cells are also activated (Peng et al., 2006,Shao et al., 2005a). Moreover, the CD8 response is greatly amplified in the presence of cytokines produced by CD4 T cells (Peng et al., 2006). However, the responses of CD8 T cells in the B10RIII mouse did not completely mirror those in the B6 mouse. For example, autoreactive CD4 and CD8 T cells in the B6 mouse recognize the same IRBP antigenic epitope (Peng et al., 2006,Shao et al., 2005a); while, in the B10RIII mouse, only one of the truncated peptides retained the ability to stimulate CD8 autoreactive T cells and was different from those that stimulated the CD4+ autoreactive T cells.
A typical CD8+ T cell antigenic epitope is composed of 9-10 amino acids (Rötzschke et al., 1990,Rammensee et al., 1993). However, in our study IRBP peptides longer than a 9-mer (up to 13-mer) had the ability to bind to, and stimulate, IRBP161-180-specific CD8 T cells, even though they were less effective than the 20-mer IRBP161-180. It remains to be determined whether the longer peptides fit into the MHC class I groove by inserting both ends into the groove, leaving the rest of the peptide as a “bulge” outside the groove, as suggested by previous reports (Macdonald et al., 2003,Zernich et al., 2004).
In summary, our study demonstrates that involvement of autoreactive CD8 T cells in the pathogenesis of EAU can also be demonstrated in the B10RIII mouse and that peptide IRBP168-177 is a major epitope for IRBP161-180-specific CD8 T cells, but not CD4 T cells. We now plan to test whether the synergistic effect of multiple pathogenic T cell subsets may cause increased disease not only via synergistic actions, but also by reciprocal enhancement of activation and expansion of pathogenic T cell subsets.
Acknowledgments
Supported in part by NIH grants EY014366 and NEI-EY017373, Vision Research Infrastructure Development (grant R24 EY015636), and the Commonwealth of Kentucky Research Challenge Trust Fund.
Abbreviations
- CFSE
carboxy-fluorescein diacetate, succinimidyl ester
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid-binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avichezer D, Chan CC, Silver PB, Wiggert B, Caspi RR. Residues 1-20 of IRBP and whole IRBP elicit different uveitogenic and immunological responses in interferon gamma deficient mice. Experimental Eye Research. 2000a;71:111–118. doi: 10.1006/exer.2000.0860. [DOI] [PubMed] [Google Scholar]
- Avichezer D, Silver PB, Chan CC, Wiggert B, Caspi RR. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest Ophthalmol Vis Sci. 2000b;41:127–131. [PubMed] [Google Scholar]
- Biddison WE, Cruikshank WW, Center DM, Pelfrey CM, Taub DD, Turner RV. CD8+ myelin peptide-specific T cells can chemoattract CD4+ myelin peptide-specific T cells: importance of IFN-inducible protein 10. J Immunol. 1998;160:444–448. [PubMed] [Google Scholar]
- Fox GM, Kuwabara T, Wiggert B, Redmond TM, Hess HH, Chader GJ, Gery I. Experimental autoimmune uveoretinitis (EAU) induced by retinal interphotoreceptor retinoid-binding protein (IRBP): differences between EAU induced by IRBP and by S-antigen. Clin Immunol Immunopathol. 1987;43:256–264. doi: 10.1016/0090-1229(87)90133-4. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Obritsch WF, Fling SP, Cameron JD. S-antigen-specific rat T cell lines recognize peptide fragments of S-antigen and mediate experimental autoimmune uveoretinitis and pinealitis. J Immunol. 1986;136:2875–2882. [PubMed] [Google Scholar]
- Hankey DJ, Lightman SL, Baker D. Interphotoreceptor retinoid binding protein peptide-induced uveitis in B10.RIII mice: characterization of disease parameters and immunomodulation. Exp Eye Res. 2001;72:341–350. doi: 10.1006/exer.2000.0957. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HR, Lumsden L, Forrester JV. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Invest Ophthalmol Vis Sci. 1999;40:3177–3185. [PubMed] [Google Scholar]
- Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. Journal Of Immunology. 1997;158:5997–6005. [PubMed] [Google Scholar]
- Karabekian Z, Lytton SD, Silver PB, Sergeev YV, Schneck JP, Caspi RR. Antigen/MHC Class II/Ig Dimers for Study of Uveitogenic T Cells: IRBP p161-180 Presented by both IA and IE Molecules. Invest Ophthalmol Vis Sci. 2005;46:3769–3776. doi: 10.1167/iovs.05-0187. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Macdonald WA, Purcell AW, Mifsud NA, Ely LK, Williams DS, Chang L, Gorman JJ, Clements CS, Kjer-Nielsen L, Koelle DM, Burrows SR, Tait BD, Holdsworth R, Brooks AG, Lovrecz GO, Lu L, Rossjohn J, McCluskey J. A Naturally Selected Dimorphism within the HLA-B44 Supertype Alters Class I Structure, Peptide Repertoire, and T Cell Recognition. J Exp Med. 2003;198:679–691. doi: 10.1084/jem.20030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta-cells in nonobese diabetic mice. J Immunol. 1994;152:2042–2050. [PubMed] [Google Scholar]
- Peng Y, Shao H, Ke Y, Zhang P, Han G, Kaplan H, Sun D. Minimally Activated CD8 Autoreactive T Cells Specific for IRBP Express a High Level of Foxp3 and Are Functionally Suppressive. Invest Ophthalmol Vis Sci. 2007;48:2178–2184. doi: 10.1167/iovs.06-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Shao H, Ke Y, Zhang P, Xiang J, Kaplan H, Sun D. In Vitro Activation of CD8 Interphotoreceptor Retinoid-Binding Protein-Specific T Cells Requires not only Antigenic Stimulation but also Exogenous Growth Factors. J Immunol. 2006;176:5006–5014. doi: 10.4049/jimmunol.176.8.5006. [DOI] [PubMed] [Google Scholar]
- Perchellet A, Stromnes I, Pang J, Goverman J. CD8+ T cells maintain tolerance to myelin basic protein by ‘epitope theft’. Nat Immunol. 2004;5:606–614. doi: 10.1038/ni1073. [DOI] [PubMed] [Google Scholar]
- Rammensee HG, Falk K, Rötzschke O. Peptides naturally presented by MHC class I molecules. Ann Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Reddy J, Bettelli E, Nicholson L, Waldner H, Jang MH, Wucherpfennig KW, Kuchroo VK. Detection of Autoreactive Myelin Proteolipid Protein 139-151-Specific T Cells by Using MHC II (IAs) Tetramers. J Immunol. 2003;170:870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan CC, Caspi RR. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156:1654–1660. [PubMed] [Google Scholar]
- Rötzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee HG. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Rozenszajn LA, Muellenberg-Coulombre C, Gery I, El-Saied M, Kuwabara T, Mochizuki M, Lando Z, Nussenblatt RB. Induction of experimental autoimmune uveoretinitis by T-cell lines. Immunology. 1986;57:559–565. [PMC free article] [PubMed] [Google Scholar]
- Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1-20-specific T cells. 2006:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Shao H, Peng Y, Liao T, Wang M, Song M, Kaplan HJ, Sun D. A shared epitope of the interphotoreceptor retinoid-binding protein (IRBP) recognized by the CD4+ and CD8+ autoreactive T cells. J Immunol. 2005a;175:1851–1857. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Sun SL, Kaplan HJ, Sun D. Characterization of Rat CD8+ Uveitogenic T Cells Specific for Interphotoreceptor Retinal-Binding Protein 1177-1191. J Immunol. 2004;173:2849–2854. doi: 10.4049/jimmunol.173.4.2849. [DOI] [PubMed] [Google Scholar]
- Shao H, Fu Y-X, Song L, Sun S, Kaplan HJ, Sun D. LTβR-Ig treatment blocks actively induced, but not adoptively transferred, uveitis in Lewis rats. Eur J Immunol. 2003a;33:1743. doi: 10.1002/eji.200323745. [DOI] [PubMed] [Google Scholar]
- Shao H, Lei S, Sun S, Xiang J, Kaplan HJ, Sun D. CpG-ODN1826 converts the weak uveitogenic rat IRBP1181-91 peptide into a strong uveitogen. J Immunol. 2003b;171:4780–4785. doi: 10.4049/jimmunol.171.9.4780. [DOI] [PubMed] [Google Scholar]
- Shao H, Song L, Sun SL, Kaplan HJ, Sun D. Conversion of monophasic to recurrent autoimmune disease by autoreactive T cell subsets. J Immunol. 2003c;171:5624–5630. doi: 10.4049/jimmunol.171.10.5624. [DOI] [PubMed] [Google Scholar]
- Shao H, Fu Y, Liao T, Peng Y, Chen L, Kaplan HJ, Sun D. Anti-CD137 mAb Treatment Inhibits Experimental Autoimmune Uveitis by Limiting Expansion and Increasing Apoptotic Death of Uveitogenic T Cells. Invest Ophthalmol Vis Sci. 2005b;46:596–603. doi: 10.1167/iovs.04-0835. [DOI] [PubMed] [Google Scholar]
- Silver PB, Chan CC, Wiggert B, Caspi RR. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest Ophthalmol Vis Sci. 1999;40:2898–2905. [PubMed] [Google Scholar]
- Silver PB, Rizzo LV, Chan CC, Donoso LA, Wiggert B, Caspi RR. Identification of a major pathogenic epitope in the human IRBP molecule recognized by mice of the H-2r haplotype. Invest Ophthalmol Vis Sci. 1995;36:946–954. [PubMed] [Google Scholar]
- Sun D, Qin Y, Chluba J, Epplen JT, Wekerle H. Suppression of experimentally-induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988;332:843–846. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin Antigen-Specific CD8+ T cells are Encephalitogenic and Produce Severe Disease In C57BL/6 Mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhang Y, Wei B, Peiper SC, Shao H, Kaplan HJ. Encephalitogenic activity of truncated myelin oligodendrocyte glycoprotein (MOG) peptides and their recognition by CD8+ MOG-specific T cells on oligomeric MHC class I molecules. Int Immunol. 2003;15:261–268. doi: 10.1093/intimm/dxg023. [DOI] [PubMed] [Google Scholar]
- Tada Y, Ho A, Koh DR, Mak TW. Collagen-induced arthritis in CD4- or CD8-deficient mice - CD8+ T cells play a role in initiation and regulate recovery phase of collagen-induced arthritis. J Immunol. 1996;156:4520–4526. [PubMed] [Google Scholar]
- Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997;109:370–376. doi: 10.1046/j.1365-2249.1997.4571356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. A synthetic peptide from myelin proteolipid protein induces experimental allergic encephalomyelitis. J Immunol. 1988;141:1126–1130. [PubMed] [Google Scholar]
- Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernich D, Purcell AW, Macdonald WA, Kjer-Nielsen L, Ely LK, Laham N, Crockford T, Mifsud NA, Bharadwaj M, Chang L, Tait BD, Holdsworth R, Brooks AG, Bottomley SP, Beddoe T, Peh CA, Rossjohn J, McCluskey J. Natural HLA Class I Polymorphism Controls the Pathway of Antigen Presentation and Susceptibility to Viral Evasion. J Exp Med. 2004;200:13–24. doi: 10.1084/jem.20031680. [DOI] [PMC free article] [PubMed] [Google Scholar]