Abstract
Many excitable cells express L-type Ca2+ channels (LTCCs), which participate in physiological and pathophysiological processes ranging from memory, secretion, and contraction to epilepsy, heart failure, and hypertension. Clusters of LTCCs can operate in a PKCα-dependent, high open probability mode that generates sites of sustained Ca2+ influx called “persistent Ca2+ sparklets.” Although increased LTCC activity is necessary for the development of vascular dysfunction during hypertension, the mechanisms leading to increased LTCC function are unclear. Here, we tested the hypothesis that increased PKCα and persistent Ca2+ sparklet activity contributes to arterial dysfunction during hypertension. We found that PKCα and persistent Ca2+ sparklet activity is indeed increased in arterial myocytes during hypertension. Furthermore, in human arterial myocytes, PKCα-dependent persistent Ca2+ sparklets activated the prohypertensive calcineurin/NFATc3 signaling cascade. These events culminated in three hallmark signs of hypertension-associated vascular dysfunction: increased Ca2+ entry, elevated arterial [Ca2+]i, and enhanced myogenic tone. Consistent with these observations, we show that PKCα ablation is protective against the development of angiotensin II-induced hypertension. These data support a model in which persistent Ca2+ sparklets, PKCα, and calcineurin form a subcellular signaling triad controlling NFATc3-dependent gene expression, arterial function, and blood pressure. Because of the ubiquity of these proteins, this model may represent a general signaling pathway controlling gene expression and cellular function.
Keywords: angiotensin II, myogenic tone, sparklets, transcription factors, voltage-gated calcium channels
Arterial tone is elevated during hypertension, increasing the probability of stroke, coronary artery disease, cardiac hypertrophy, and renal failure (1, 2). Although the etiology of arterial dysfunction during hypertension is unclear, multiple studies suggest that increased L-type Ca2+ channel (LTCC) activity in arterial smooth muscle is a major contributor to this pathological change (3, 4). However, the mechanisms and functional implications of increased Ca2+ influx via LTCCs remain unclear.
In normotensive arterial smooth muscle, the opening of LTCCs produces local elevations in [Ca2+]i called “Ca2+ sparklets” (5, 6). Two modes of Ca2+ sparklet activity have been identified (6–8). Low-activity Ca2+ sparklets are produced by brief random openings of LTCCs that result in limited Ca2+ influx. In contrast, long openings of LTCCs associated with PKCα produce high activity, persistent Ca2+ sparklets that create regions of sustained Ca2+ influx. Low- and high-activity, persistent Ca2+ sparklets are produced by the opening of a single or a small cluster of LTCCs. PKCα is required for persistent, but not for low-activity, Ca2+ sparklets. Under physiological conditions, Ca2+ entry and global [Ca2+]i and, thus, contraction are regulated by low-activity and PKCα-dependent persistent Ca2+ sparklets.
At present, the role of PKCα and Ca2+ sparklets in the chain of events leading to arterial dysfunction during hypertension is unknown. Several lines of evidence suggest that they may be important in this process. (i) LTCC function is increased in hypertensive arterial smooth muscle (3, 4). (ii) The vasoconstrictor angiotensin II (AngII), an activator of PKC, is a likely contributor to vascular dysfunction in human (9) and model hypertension (10). Accordingly, AngII-converting enzyme inhibitors and AngII receptor antagonists are used extensively for the treatment of hypertension in humans. (iii) AngII activates the Ca2+-sensitive phosphatase calcineurin via LTCC Ca2+ entry in hypertension. Calcineurin in turn dephosphorylates and hence activates the transcription factor NFATc3, decreasing the expression of voltage-gated K+ (Kv) channels in hypertensive arterial smooth muscle (11). Decreased Kv channel function depolarizes arterial smooth muscle, which indirectly increases LTCC function (12). The mechanism by which these channels activate calcineurin in smooth muscle is unclear (11, 13).
In this study, we examined the role of PKCα and Ca2+ sparklet activity in the development of arterial dysfunction during hypertension. Our data suggest that increased Ca2+ influx via persistent Ca2+ sparklet sites underlies increased arterial [Ca2+]i and myogenic tone during hypertension. Furthermore, PKCα-dependent persistent Ca2+ sparklets specifically activated NFATc3 in human arterial myocytes, resulting in decreased Kv2.1 channel expression. Importantly, loss of PKCα eliminated persistent Ca2+ sparklets and protected against the development of hypertension. These data support the concept that NFATc3, Kv channel expression, arterial [Ca2+]i, myogenic tone, and hypertension are locally controlled by Ca2+ sparklet activity.
Results
We examined the mechanisms controlling Ca2+ influx via LTCCs and calcineurin/NFATc3 signaling in arterial smooth muscle during genetic and induced hypertension. This required investigation of the spatial organization of functional LTCCs and PKCα as well as NFATc3 activity in living cells. To establish the broad implications of our findings, experiments were performed with human arterial smooth muscle and two established models of hypertension.
AngII Increases Ca2+ Sparklet Activity in Arterial Smooth Muscle.
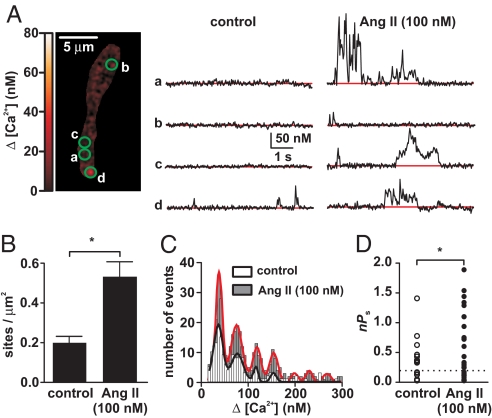
First, we tested the hypothesis that AngII increases Ca2+ sparklet activity in arterial myocytes from mesenteric and cerebral arteries (Fig. 1). The membrane potential of these cells was controlled by using the patch-clamp technique. Application of AngII (100 nM) increased Ca2+ sparklet activity (i.e., Ca2+ influx) by augmenting the activity of previously active Ca2+ sparklet sites and activating new sites (Fig. 1A). Indeed, at the physiological membrane potential of −40 mV and external Ca2+ of 2 mM, AngII increased Ca2+ sparklet site density ≈2.3-fold (Fig. 1B). We investigated whether this increase in Ca2+ sparklet activity was accompanied by an increase in the amplitude of elementary Ca2+ sparklet events. Fig. 1C shows an amplitude histogram of Ca2+ sparklets before and after the application of AngII. This histogram illustrates that AngII increased the number of Ca2+ sparklets observed. Note, however, that both histograms have clearly discernable peaks that could be fit with a multi-Gaussian function with a quantal unit of Ca2+ influx of 38 nM (control; χ2 = 1.1; AngII; χ2 = 1.9), suggesting that AngII did not change the amplitude of elementary Ca2+ sparklets.
Fig. 1.
AngII increases Ca2+ sparklet activity. (A) Total internal reflection fluorescence (TIRF) image of an arterial myocyte (Left). Traces (Right) are time courses of [Ca2+]i in the sites indicated by the green circles before and after application of 100 nM AngII. (B) Ca2+ sparklet density before and after application of AngII. (C) Amplitude histogram of Ca2+ sparklets before and after AngII application. The black and red lines are the best fit to the control and AngII data, respectively, by using the Gaussian function. (D) nPs values for individual Ca2+ sparklet sites under control conditions and after application of AngII. The dashed line defines the threshold between low and high nPs sites.
AngII did not increase Ca2+ sparklet activity homogeneously throughout the sarcolemma. Rather, AngII increased Ca2+ influx at specific sites by increasing the activity [quantified as nPs, where n is number of quantal levels and Ps is the probability of Ca2+ sparklet occurrence; see supporting information (SI) Text for details of this analysis] of previously active sites and activating new Ca2+ sparklet sites (Fig. 1 A and D). As previously reported (6), Ca2+ sparklet activity was bimodal, with sites of low activity (nPs = 0.07 ± 0.02) and sites of high activity (nPs = 0.54 ± 0.10; also referred as “persistent Ca2+ sparklet sites”). Acute application of AngII (100 nM) increased total Ca2+ sparklet activity nearly 12-fold (P < 0.05; Fig. 1D).
To quantify Ca2+ influx (ΔQCa; in coulombs) associated with each Ca2+ sparklet event, we determined the “signal mass” of Ca2+ sparklets before and after the application of AngII. The ΔQCa of Ca2+ sparklet events in low and high activity, persistent sites increased after AngII (Fig. S1A). However, we found that the duration of individual low- and high-activity, persistent Ca2+ sparklet events was similar before and after AngII (Fig. S1B). This indicates that AngII increases Ca2+ influx in arterial myocytes by increasing the probability of Ca2+ sparklet occurrence (Ps) and/or the number of functional channels (i.e., n), without increasing the duration of individual Ca2+ influx events or the amplitude of quantal Ca2+ sparklets.
Regulation of Ca2+ Sparklets, Arterial [Ca2+]i, and Tone by AngII Requires PKCα.
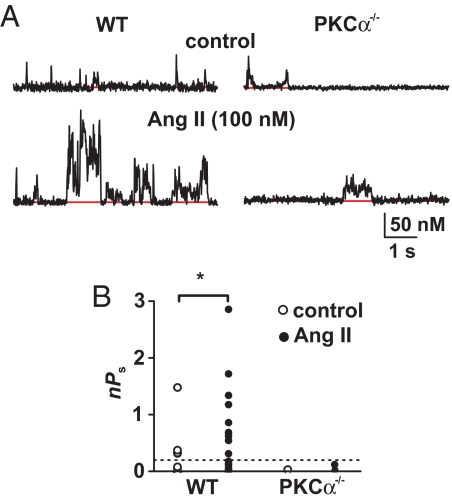
AngII activates PKCα in arterial smooth muscle. Because PKCα is necessary for persistent Ca2+ sparklet activity in arterial myocytes (6, 8), we examined the effects of AngII on Ca2+ sparklets in WT and PKCα null (PKCα−/−) myocytes (Fig. 2). Ca2+ sparklet activity was lower in PKCα−/− than in WT myocytes (Fig. 2 A and B). This was due to the absence of high-activity, persistent Ca2+ sparklets in PKCα−/− myocytes (Fig. 2B). AngII increased the number and activity of Ca2+ sparklets in WT myocytes but not in PKCα−/− myocytes (Fig. 2 A and B; P < 0.05). Thus, PKCα is necessary for AngII-dependent stimulation of Ca2+ sparklets.
Fig. 2.
AngII increases Ca2+ sparklet activity through the activation of PKCα. (A) Representative time course of [Ca2+]i in Ca2+ sparklet sites in WT and PKCα−/− cells before and after AngII (100 nM) treatment. (B) Scatter plot of nPs values for individual Ca2+ sparklet sites in WT and PKCα−/− cells under control conditions and after application of AngII.
We measured arterial wall [Ca2+]i in pressurized (80 mmHg) WT and PKCα−/− mesenteric arteries (Fig. S2 A and B). Consistent with our Ca2+ sparklet data, arterial wall [Ca2+]i was lower in PKCα−/− than in WT arteries under control conditions and after application of AngII (n = 7, P < 0.05). Indeed, the AngII-induced increase in arterial wall [Ca2+]i was ≈4-fold smaller in PKCα−/− than in WT arteries. Note that application of nifedipine (1 μM) decreased [Ca2+]i in WT and PKCα−/− arteries to a similar level (P > 0.05), indicating that PKCα−/− arteries have a lower dihydropyridine-sensitive (control [Ca2+]i − [Ca2+]i with nifedipine) [Ca2+]i than WT arteries.
To examine the physiological consequences of reduced Ca2+ sparklet activity and [Ca2+]i in PKCα−/− arterial smooth muscle, we evaluated the effect of pressure on the diameter of WT and PKCα−/− arteries (Fig. S2 C and D). Consistent with our Ca2+ sparklet and arterial wall [Ca2+]i data, PKCα−/− arteries were significantly less constricted than WT arteries at all intravascular pressures examined (Fig. S2C). Furthermore, AngII evoked a smaller constriction in PKCα−/− than in WT arteries (Fig. S2D).
PKCα Is Required for Increased Persistent Ca2+ Sparklets During Hypertension.
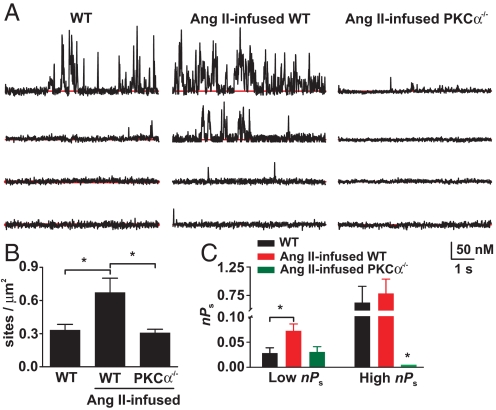
We investigated the role of PKCα in the chain of events leading to an increase in Ca2+ entry into arterial myocytes during hypertension. In these experiments, Ca2+ sparklets were recorded in myocytes from saline (control) and AngII-infused WT and PKCα−/− mice in the absence of externally applied AngII (Fig. 3A). AngII infusion induced hypertension in WT mice, increasing mean arterial pressure (MAP) from 114 ± 3 to 149 ± 3 mmHg (see Fig. 6A; n = 5, P < 0.05). We found that Ca2+ sparklet density and activity was higher in myocytes isolated from AngII than in saline-infused WT mice (Fig. 3 B and C; P < 0.05). Importantly, note that chronic AngII infusion did not increase Ca2+ sparklet density and activity in PKCα−/− myocytes. These findings suggest that PKCα is required for increased Ca2+ sparklet activity during AngII-induced hypertension.
Fig. 3.
Ca2+ sparklet activity is increased during AngII-induced hypertension. (A) Time courses of [Ca2+]i in a smooth muscle cell from saline and AngII-infused WT and PKCα−/− mice. (B) Bar plot of Ca2+ sparklet density. (C) Bar plot of nPs values for individual Ca2+ sparklet sites in smooth muscle cell from saline- and AngII-infused WT and PKCα−/− mice.
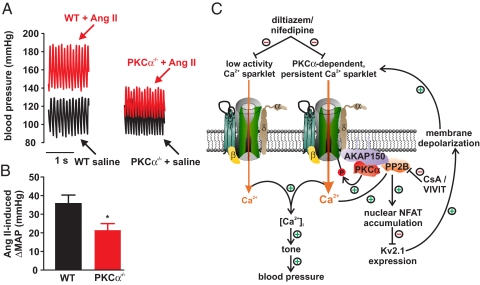
Fig. 6.
PKCα is required for AngII-induced hypertension. (A) Representative pressure waveforms from WT and PKCα−/− mice before and after AngII infusion. (B) Bar plot of the change in mean arterial pressure (ΔMAP) induced by AngII in WT and PKCα−/− mice. (C) Proposed mechanism by which persistent Ca2+ sparklet activity increase tone and blood pressure during hypertension (see Discussion for details).
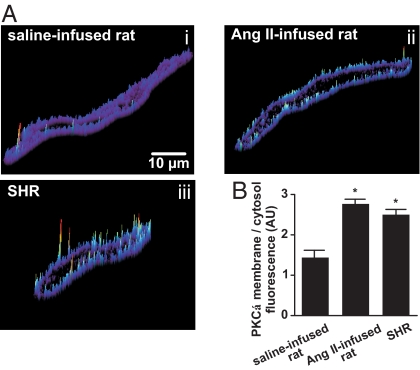
We examined PKCα activity, Ca2+ sparklets, and LTCC function in another widely used model of hypertension, the spontaneously hypertensive rat (SHR). For comparison, we also performed experiments using AngII-infused rats. Increased AngII signaling is implicated in the development of hypertension in SHRs (10). We used an immunofluorescence approach to establish the level of sarcolemmal PKCα translocation (i.e., activation) in SHR and AngII-infused cells. This approach has been used to determine PKC activity in smooth muscle (14). As shown in Fig. 4, PKCα localization at the sarcolemma was higher in cerebral and mesenteric artery myocytes from AngII-infused rats and SHR than in normotensive myocytes. Consistent with this, and as in the mouse and rat model of AngII-induced hypertension, the density of Ca2+ sparklet sites was ≈3-fold higher (P < 0.05) in SHR than in normotensive myocytes (Fig. S3A). This resulted from an increase in the number of low and high nPs Ca2+ sparklet sites in SHR cells (Fig. S3B).
Fig. 4.
Increased PKCα activity during acquired and genetic hypertension. (A) Representative surface-plot of PKCα-associated fluorescence in cerebral artery myocytes from saline-infused rats, (Ai), AngII-infused rats, (Aii), and SHR (Aiii). (B) Bar plot of the PKCα membrane-to-cytosol fluorescence ratio from saline- and AngII-infused and SHR myocytes.
We investigated if, as in cells from AngII-infused animals, PKC activity contributes to higher basal Ca2+ channel activity in SHR than in normotensive myocytes (Fig. S3C). Consistent with our SHR Ca2+ sparklet data—and previous reports (3, 15, 16)—LTCC currents were ≈3-fold larger in SHR than in normotensive cells. Interestingly, application of the PKC inhibitor Gö6976 (100 μM) decreased this difference in Ca2+ currents between SHR and normotensive cells, indicating that increased basal PKC activity contributes to higher LTCC activity in SHR than in normotensive cells (Fig. S3 C and D). In addition, and consistent with other reports (15), Cav1.2 expression was increased in SHR arterial smooth muscle (mesenteric and cerebral arteries) (Fig. S3E). These data support the view that increased PKC activity and Cav1.2 expression contributes to higher persistent Ca2+ sparklet activity in arterial myocytes during hypertension.
PKC-Induced Local Ca2+ Signals via LTCCs Activate NFATc3 and Decrease Kv2.1 Expression in Human Arterial Smooth Muscle.
In arterial smooth muscle, Ca2+ sparklet activity is regulated locally by PKCα and calcineurin via a targeting mechanism involving the scaffolding protein AKAP150 (8). Because persistent Ca2+ sparklet activity (see above) and calcineurin/NFATc3 activity (13) are increased during hypertension, we investigated the possibility that PKCα-dependent persistent Ca2+ sparklets activate calcineurin/NFATc3 signaling in arterial myocytes. To test this hypothesis, we measured calcineurin activity in saline- and AngII-infused WT and PKCα−/− mesenteric arteries. We found that calcineurin activity was decreased in arteries from saline-infused WT and PKCα−/− mice (Fig. S4). Infusion of AngII increased calcineurin activity in WT, but not in PKCα−/−, arteries, suggesting that PKCα is required for calcineurin activation during chronic AngII signaling activation.
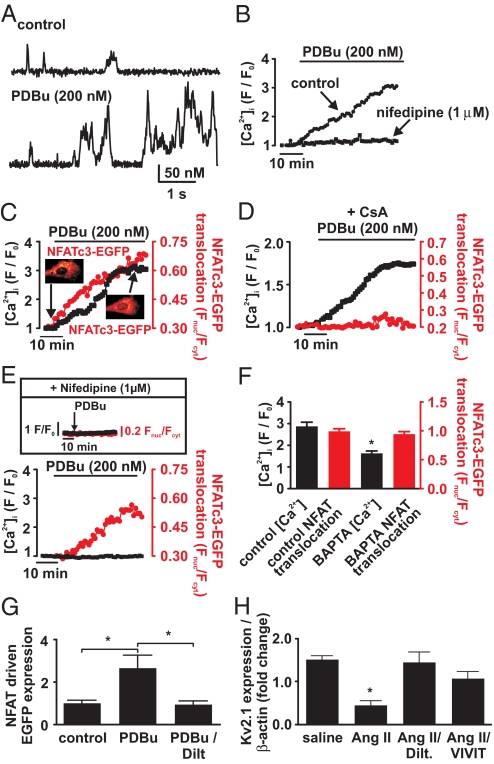
We recorded Ca2+ sparklets, [Ca2+]i, and nuclear NFATc3 translocation in primary cultured human aortic smooth muscle cells expressing NFATc3 tagged with EGFP (17, 18) (Fig. 5). These cells were used to expand on our findings in rodent models of hypertension and gain insights into the mechanisms of Ca2+ and NFATc3 signaling in humans. As anticipated, Ca2+ sparklets are present in human arterial myocytes (Fig. 5A). Similar to murine smooth muscle, Ca2+ sparklets were abolished in human arterial myocytes by superfusion of Ca2+-free solution (data not shown). Furthermore, activation of PKC with PDBu (200 nM) increased Ca2+ sparklet activity and global [Ca2+]i in human myocytes (Fig. 5 A, B, and D). Nifedipine (1 μM) prevented the increase in global [Ca2+]i evoked by PDBu, suggesting that activation of LTCCs is required for the increase in [Ca2+]i induced by PKC activation.
Fig. 5.
Activation of NFATc3 signaling by PKC-dependent local Ca2+ signals via LTCCs in human aortic myocytes. (A) Ca2+ sparklet records from a representative cell before and after 200 nM PDBu. (B) Global [Ca2+]i response to the application of PDBu during control conditions and in the presence of nifedipine (1 μM). (C and D) The time course of [Ca2+]i and nuclear NFATc3-EGFP translocation in response to PDBu or PDBu + CsA (1 μM), respectively. Insets in C show two NFATc3-EGFP images from this cell before and after application of PDBu. (E) Time course of [Ca2+]i and NFATc3-EGFP nuclear translocation in a cell loaded with BAPTA-AM after application of PDBu. The Inset shows global [Ca2+]i (black trace and left y axis; F/F0 units) and NFATc3-EGFP nuclear translocation (red trace and y axis; Fnuc/Fcyt units) in a cell exposed to PDBu (arrow) in the presence of nifedipine. (F) The bar plot of the peak [Ca2+]i and NFATc3-EGFP nuclear translocation during control conditions and in cells loaded with BAPTA-AM. (G) Bar plot of relative EGFP expression in human aortic smooth muscle cells cultured for 48 h under control conditions in the presence of PDBu (200 nM) and PDBu + diltiazem (10 μM). (H) Bar plot of the normalized (to β-actin) Kv2.1 transcript level in human aortic smooth muscle cells cultured for 48 h under control conditions with angiotensin II (100 nM), angiotensin II + diltiazem (10 μM), or angiotensin II + VIVIT (1 μM). Kv2.1 transcript levels were determined by real-time RT-PCR. *, P < 0.05.
To provide a real-time examination of the relationship between [Ca2+]i and nuclear translocation of NFATc3 in living human arterial smooth muscle cells, we expressed NFATc3 fused to EGFP (NFATc3-EGFP) in these cells (19). [Ca2+]i and NFATc3-EGFP translocation were simultaneously recorded by using confocal microscopy. We found that activation of PKC with PDBu increased global [Ca2+]i and induced nuclear translocation of NFATc3-EGFP in human aortic smooth muscle cells (Fig. 5 C and D). Consistent with the calcineurin activity data presented above, application of the calcineurin inhibitor cyclosporine (CsA, 1 μM) prevented nuclear NFATc3-EGFP translocation but not the rise in [Ca2+]i evoked by PDBu (Fig. 5D, n = 5).
We tested the hypothesis that calcineurin and NFATc3 signaling is activated by local Ca2+ signals in human aortic smooth muscle cells (Fig. 5E). To do this, we examined [Ca2+]i and NFATc3-EGFP translocation during application of PDBu in human smooth muscle cells loaded with the fast Ca2+ buffer BAPTA-AM to prevent a global increase in [Ca2+]i. We recorded Ca2+ sparklet activity in arterial myocytes loaded with BAPTA and found their amplitude and gating modalities were similar to those recorded with EGTA in the patch-pipette (Fig. S5). The area of Ca2+ sparklets was smaller in cells loaded with BAPTA than with EGTA, likely because of BAPTA's faster Ca2+-binding kinetics.
We found that application of PDBu resulted in nuclear translocation of NFATc3-EGFP in the absence of a change in global [Ca2+]i. Indeed, PDBu-induced NFATc3-EGFP translocation was similar in control (i.e., no exogenous Ca2+ buffer) and BAPTA-loaded cells (Fig. 5F; P > 0.05). Furthermore, in the presence of nifedipine (1 μM), application of PDBu failed to induce nuclear NFATc3-EGFP translocation (Fig. 5E Inset; n = 5). Together, these data support the view that NFATc3 translocation in human arterial smooth muscle is activated by PKC-dependent, local Ca2+ signals via LTCCs (presumably persistent Ca2+ sparklets) and not global changes in [Ca2+]i.
We examined the effects of PKC and LTCC activation on NFAT-dependent gene expression in human arterial myocytes expressing an NFAT reporter construct expressing EGFP in response to NFAT activation (19). In these experiments, EGFP expression (i.e., fluorescence) was used as an indicator of NFAT transcriptional activity. EGFP fluorescence was examined in cells under three experimental conditions: control, with 200 nM PDBu, and PDBu plus the LTCC blocker diltiazem (10 μM). We used diltiazem rather than nifedipine in these relatively long experiments because of its greater stability.
PDBu induced a ≈2.5-fold increase in NFAT transcriptional activity (Fig. 5G). Diltiazem prevented the increase in NFAT activity induced by PDBu, indicating that LTCC activity (i.e., Ca2+ sparklets) is required for PKC-induced NFAT activation in human arterial myocytes. We also examined the effects of the physiological PKC activator AngII on the expression of Kv2.1 transcript in human arterial myocytes. Kv2.1 channels are important regulators of membrane potential and, consequently, [Ca2+]i and tone in arterial myocytes (20). Furthermore, the Kv2.1 gene has putative NFAT binding sites in its promoter region and could be down-regulated by NFATc3 (11). We found that sustained AngII exposure (48 h) decreased Kv2.1 transcript levels in control human aortic smooth muscle cells, but not in the presence of the LTCC blocker diltiazem (10 μM) or the NFAT inhibitor VIVIT (1 μM) (21) Fig. 5H).
PKCα Contributes to AngII-Induced Hypertension.
To relate our in vitro findings to the intact animal, we measured blood pressure in PKCα−/− and WT mice infused with saline or AngII (Fig. 6). PKCα−/− mice allowed us to investigate the role of this kinase in the development of AngII-induced hypertension. MAP was similar in control PKCα−/− (n = 5) and WT mice (n = 6; P > 0.05). However, AngII infusion increased MAP in PKCα−/− mice to a lower extent (≈40% lower) than in WT mice (P < 0.05; Fig. 6B), suggesting that PKCα contributes to the development of hypertension.
Discussion
Our findings provide a mechanistic framework for how Ca2+ influx increases during hypertension. We also provide the first direct demonstration that PKCα contributes to the development of AngII-induced hypertension. We show that increased Ca2+ influx during hypertension is not simply the result of random activation of LTCCs as previously implied (3, 4). Rather, increased Ca2+ influx during hypertension results primarily from increased PKCα-dependent persistent Ca2+ sparklet activity. Our data suggest that an increase in number and probability of activation of Ca2+ sparklet sites—not an increase in the quantal unit of Ca2+ influx or the duration of Ca2+ entry events—underlies increased Ca2+ influx via LTCCs into arterial myocytes during hypertension. This is likely the result of a combinatorial increase in Cav1.2 channel expression (15) and PKCα activity in arterial smooth muscle during hypertension.
We performed the first examination of the relationship between [Ca2+]i and nuclear NFATc3 translocation in living human arterial smooth muscle cells. Interestingly, our data suggest that PKC-induced local Ca2+ signals via LTCCs (presumably persistent Ca2+ sparklets) activate NFATc3 via calcineurin, thereby modifying gene expression in human arterial myocytes. Multiple lines of evidence support this view. (i) Activation of PKC increases [Ca2+]i by increasing persistent Ca2+ sparklet activity and induced nuclear NFATc3-EGFP translocation in these cells. (ii) The LTCC blocker nifedipine prevented the rise in [Ca2+]i and nuclear NFATc3 translocation evoked by the activation of PKC. (iii) Activation of PKC induced NFATc3 translocation even in cells loaded with the fast Ca2+ buffer BAPTA. This observation is important because in the presence of BAPTA, Ca2+ signals are confined to a small region of the cell near the site of Ca2+ entry (22), suggesting that calcineurin/NFATc3 signaling is activated by a local PKC-dependent Ca2+ signal via LTCCs in human arterial smooth muscle. (iv) Ca2+ influx via LTCCs is required for NFAT-mediated transcriptional activity during PKC activation in these cells.
Recent work suggests a potential mechanism for local activation of NFATc3 by LTCCs in arterial myocytes. The scaffolding protein AKAP150 (the rodent ortholog of human AKAP79) is required for sarcolemmal PKCα expression and persistent Ca2+ sparklets in arterial myocytes (23). AKAP150/79 also targets calcineurin to the surface membrane (24). Indeed, we found that calcineurin opposes persistent Ca2+ sparklet activity in arterial myocytes (8, 23). Furthermore, consistent with AKAP150/79's role in targeting calcineurin, we found this scaffolding protein is required for calcineurin-dependent modulation of Ca2+ sparklets in smooth muscle (23). Collectively, these data suggest that AKAP150/79 targets PKCα and calcineurin to specific regions of the sarcolemma of arterial myocytes where they modulate nearby LTCCs. In combination with our Ca2+ sparklet, calcineurin, and NFATc3 data, we propose that AKAP150, PKCα, calcineurin, and LTCCs form a positive feedback loop that modulates Ca2+ influx and gene expression in smooth muscle (Fig. 6C).
In this model, PKCα, and calcineurin, are targeted by AKAP150/79 to specific regions of the sarcolemma of arterial myocytes. Activation of PKCα induces persistent Ca2+ sparklet activity in these sites. This increase in local Ca2+ influx activates nearby calcineurin, which dephosphorylates NFATc3. Dephosphorylated NFATc3 translocates into the nucleus of arterial myocytes where it modulates gene expression. Under physiological conditions, calcineurin and NFATc3 activities are low in arterial smooth muscle (11, 13) likely because of low levels of persistent Ca2+ sparklet activity and relatively high nuclear NFATc3 export rates (25). However, during genetic and AngII-induced hypertension, an increase in PKCα activity increases persistent Ca2+ sparklet activity, thus increasing calcineurin activity and consequently NFATc3 nuclear import rate, which, if coupled with a decrease in export rate (11, 13), would lead to high nuclear accumulation of this transcription factor. This, in turn, leads to nuclear accumulation of NFATc3 and down-regulation of Kv2.1 transcript expression (11). As suggested previously (11, 12, 26), down-regulation of Kv2.1 decreases Kv currents and contributes to arterial smooth muscle depolarization during hypertension, which indirectly increases Ca2+ sparklet activity, global [Ca2+]i, and tone. Initiation or continuation of this feedback loop could be prevented by inhibiting PKCα, calcineurin, or NFATc3 activation (13) or with LTCC blockers.
In normotensive smooth muscle, Ca2+ entry via persistent Ca2+ sparklet sites accounts for ≈50% of the total dihydropyridine-sensitive Ca2+ influx into these cells under physiological conditions (7). Our Ca2+ sparklet data from normotensive, SHR and AngII (chronic and acute exposure) myocytes suggest that Ca2+ influx is increased in hypertensive smooth muscle due to an increase in the density and activity (i.e., nPs) of low activity and persistent Ca2+ sparklet sites. At present, however, whether the relative contribution of low and high activity, persistent Ca2+ sparklet sites to Ca2+ influx in arterial myocytes is altered during hypertension is unclear. Future studies should address this issue.
Arterial smooth muscle is depolarized during hypertension (12). Membrane depolarization increases low-activity and persistent Ca2+ sparklet activity (7). Thus, voltage-independent (i.e., increased in Cav1.2 expression and PKCα activity) and -dependent mechanisms (i.e., depolarization) could synergize to increase Ca2+ sparklet activity in arterial myocytes during hypertension. Indeed, it is intriguing to speculate that loss of PKCα could protect against hypertension by decreasing voltage-dependent and -independent activation of Ca2+ sparklets.
To conclude, our data support the concept that Ca2+ influx, global [Ca2+]i, NFATc3-dependent gene expression, and myogenic tone are modulated locally by Ca2+ sparklet activity in arterial myocytes. Importantly, our data suggest that PKCα could be a novel target for the treatment of hypertension.
Materials and Methods Summary
A detailed version of this section can be found online in SI Text. Briefly, we used Sprague–Dawley and SHR as well as WT and PKCα−/− mice. Myocytes were dissociated from cerebral and mesenteric arteries as reported previously (11). AngII or saline were administered by using osmotic minipumps. Blood pressure was monitored by using telemetry (13). The patch-clamp technique was used to record Ca2+ currents. Ca2+ sparklets were recorded as described (6, 8). Real-time PCR was performed by using QuantiTect SYBR green PCR. Calcineurin activity was assessed by using a commercial kit as described (13). Nuclear NFATc3 translocation was assessed in cells transfected with NFATc3-EGFP. NFAT transcriptional activity in response to PKC activation was assessed using an NFAT-EGFP reporter construct. *, P < 0.05.
Supplementary Material
Acknowledgments.
We thank Dr. Y. M. Usachev (University of Iowa, Iowa City) for providing the NFAT-EGFP reporter construct. The NFATc3-EGFP plasmid was created by Dr. F. McKeon (Harvard University, Cambridge, MA) and provided by Dr. M. Iino (University of Tokyo, Tokyo, Japan). This work was supported by American Heart Association and National Institutes of Health grants.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808759105/DCSupplemental.
References
- 1.MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 2.Crowley SD, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusch NJ, Hermsmeyer K. Calcium currents are altered in the vascular muscle cell membrane of spontaneously hypertensive rats. Circ Res. 1988;63:997–1002. doi: 10.1161/01.res.63.6.997. [DOI] [PubMed] [Google Scholar]
- 4.Ohya Y, Abe I, Fujii K, Takata Y, Fujishima M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res. 1993;73:1090–1099. doi: 10.1161/01.res.73.6.1090. [DOI] [PubMed] [Google Scholar]
- 5.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 6.Navedo MF, Amberg G, Votaw SV, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Geel PP, et al. Angiotensin II type 1 receptor A1166C gene polymorphism is associated with an increased response to angiotensin II in human arteries. Hypertension. 2000;35:717–721. doi: 10.1161/01.hyp.35.3.717. [DOI] [PubMed] [Google Scholar]
- 10.Bunkenburg B, Schnell C, Baum HP, Cumin F, Wood JM. Prolonged angiotensin II antagonism in spontaneously hypertensive rats. Hemodynamic and biochemical consequences. Hypertension. 1991;18:278–288. doi: 10.1161/01.hyp.18.3.278. [DOI] [PubMed] [Google Scholar]
- 11.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv21 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 12.Wellman GC, et al. Membrane depolarization, elevated Ca2+ entry, and gene expression in cerebral arteries of hypertensive rats. Am J Physiol. 2001;281:H2559–H2567. doi: 10.1152/ajpheart.2001.281.6.H2559. [DOI] [PubMed] [Google Scholar]
- 13.Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the β1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 14.Khalil RA, Lajoie C, Morgan KG. In situ determination of [Ca2+]i threshold for translocation of the alpha-protein kinase C isoform. Am J Physiol. 1994;266:C1544–C1551. doi: 10.1152/ajpcell.1994.266.6.C1544. [DOI] [PubMed] [Google Scholar]
- 15.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 16.Wilde DW, Furspan PB, Szocik JF. Calcium current in smooth muscle cells from normotensive and genetically hypertensive rats. Hypertension. 1994;24:739–746. doi: 10.1161/01.hyp.24.6.739. [DOI] [PubMed] [Google Scholar]
- 17.Shibasaki F, Price ER, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 18.Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson JG, Usachev YM, Thayer SA. Bradykinin-induced nuclear factor of activated T-cells-dependent transcription in rat dorsal root ganglion neurons. Mol Pharmacol. 2007;72:303–310. doi: 10.1124/mol.107.035048. [DOI] [PubMed] [Google Scholar]
- 20.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol. 2006;291:C348–C356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 21.Aramburu J, et al. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 22.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navedo MF, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 24.Coghlan VM, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 25.Gomez MF, et al. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: Role of JNK2 and Crm-1. J Biol Chem. 2003;278:46847–46853. doi: 10.1074/jbc.M304765200. [DOI] [PubMed] [Google Scholar]
- 26.Cox RH. Comparison of K+ channel properties in freshly isolated myocytes from thoracic aorta of WKY and SHR. Am J Hypertens. 1996;9:884–894. doi: 10.1016/s0895-7061(96)00179-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.