Abstract
New neurons in the adult dentate gyrus are widely held to incorporate into hippocampal circuitry via a stereotypical sequence of morphological and physiological transitions, yet the molecular control over this process remains unclear. We studied the role of brain-derived neurotrophic factor (BDNF)/TrkB signaling in adult neurogenesis by deleting the full-length TrkB via Cre expression within adult progenitors in TrkBlox/lox mice. By 4 weeks after deletion, the growth of dendrites and spines is reduced in adult-born neurons demonstrating that TrkB is required to create the basic organization of synaptic connections. Later, when new neurons normally display facilitated synaptic plasticity and become preferentially recruited into functional networks, lack of TrkB results in impaired neurogenesis-dependent long-term potentiation and cell survival becomes compromised. Because of the specific lack of TrkB signaling in recently generated neurons a remarkably increased anxiety-like behavior was observed in mice carrying the mutation, emphasizing the contribution of adult neurogenesis in regulating mood-related behavior.
Keywords: BDNF, LTP, neurogenesis, plasticity, dendritogenesis
The life-long generation of new neurons is well documented in the subgranule zone of the dentate gyrus in the hippocampus (1–4). Dentate gyrus neurons result from local self-replicating radial glia-like stem cells (5). Once generated, the vast majority of neurons remain located on the hilar side of the granule layer and attempt to connect into the existing neuronal network, finally receiving afferent input from perforant path fibers (6, 7) and providing efferent output to CA3 cells (8, 9). While new neurons are held to incorporate into the preexisting circuitry via a stereotypical sequence of morphological transitions (10), the molecular mechanisms regulating the functional integration and/or survival of newborn neurons are not yet fully understood. There is growing evidence of the role of neuronal activity in this process. New neurons sense neuronal activity through ambient γ-aminobutyric acid (GABA) before receiving, in sequence, GABAergic and glutamatergic inputs. Defects in the GABA responsiveness of newborn neurons, such as that obtained by inducing the conversion of GABA-mediated depolarization into hyperpolarization, lead to marked deficits in dendritic arborization and synapse formation (11), suggesting that network activity controls key morphological transitions required for the connectivity of adult-born neurons. At the initiation of connectivity glutamatergic inputs control newborn neuron survival (12). Indeed, access to afferent inputs may be the key for their life and death decisions. A central hypothesis arising from this regulation is that adult-born neurons could contribute to the formation of new circuits in tune with network needs, which, in turn, relates to the functional incorporation of adult-born neurons into hippocampal circuits. Hence, around connectivity time and later adult-born neurons become preferentially recruited into functional networks, i.e., memory networks (13) and selectively express enhanced synaptic plasticity (14–16). As neurogenesis continuously proceeds, the integration of adult-born neurons may play a key role in the anatomical and functional plasticity of hippocampal circuitry, which has been thought to underlie complex cognitive (1, 2) and emotional (17) functions.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family reported to regulate the extent of neurogenesis in the dentate gyrus (18–21). BDNF may also be a good candidate for regulating the integration of adult-born neurons into functional circuits of the hippocampal network. The present study used a mouse line expressing inducible Cre in adult neural stem cells and retrovirus-mediated single-cell Cre expression to obtain the specific lack of BDNF receptor TrkB signaling in adult-born neurons of TrkBlox/lox mice. We demonstrated that the BDNF receptor TrkB is required to create the basic organization of synaptic connections like dendritic arbor complexity and spine density. Moreover, TrkB-deficient adult-born neurons show impaired synaptic plasticity, and a substantial population of these neurons die at the immature-to-mature neuronal transition. Although TrkB signaling was impaired only in the newly generated neurons, this resulted in a remarkably increased anxiety-like behavior in mice carrying the mutation.
Results
Deletion of TrkB Specifically in Adult-Born Neurons.
To examine the role of TrkB signaling specifically in adult neurogenesis we took advantage of a recently generated mouse line expressing the inducible form of Cre (CreERT2) in the locus of the astrocyte-specific glutamate transporter (GLAST) (22) to induce efficient Cre-recombination by tamoxifen in the radial glia-like stem cells of the dentate gyrus. This mouse line was crossed with homozygous TrkBlox/lox mice (23) in which the second exon of the TrkB tyrosine kinase region had been flanked by two loxP sites (for simplicity TrkBlox/lox-Cre). To visualize the recombined cells we additionally crossed TrkBlox/lox-Cre mice with transgenic mice expressing green fluorescent protein (GFP) (TrkBlox/lox-Cre Z/EG) or β-galactosidase (βgal) (TrkBlox/lox-Cre R26R) reporters [see supporting information (SI) Table S1]. Tamoxifen treatment in the resulting mice causes the CreERT2 fusion protein to translocate into the nucleus of GLAST-expressing cells, where it recombines paired loxP sites and removes the catalytically active TrkB (23) without interfering with truncated TrkB (24) (Fig. S1D). As expected from previous studies (22), mice induced by tamoxifen for 5 days and killed 28 days post-tamoxifen application (28 dptm) showed reporter-positive astrocytes throughout the brain parenchyma and radial glia-like stem cells and their neuronal progeny in the dentate gyrus of the hippocampus (see Fig. S1 A–C). However, as mature astrocytes express only truncated TrkB, but not the full-length receptor (Fig. S1D), the effect of full-length TrkB deletion was restricted to progenitors and differentiating neurons.
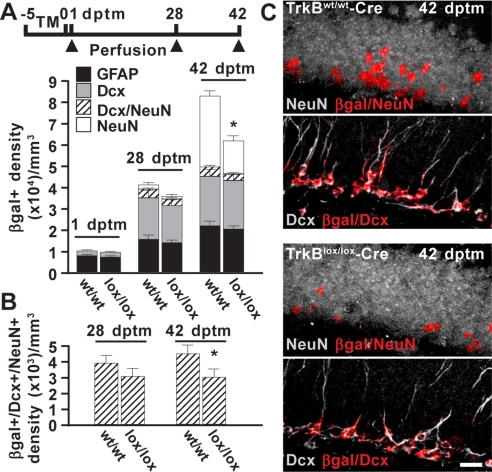
The temporal pattern of Cre activity was first investigated in control mice. Mice were induced by tamoxifen for 5 days and killed 1, 28, and 42 dptm (Fig. 1A). At each time point we quantified reporter-positive cells identified by cell type-specific antigens. At 1 dptm the majority of reporter-positive cells expressed the astroglial marker glial fibrillary acidic protein (GFAP), consistent with the recombination occurring specifically in cells with a glial identity. On the contrary, small amount of neuroblasts or immature neurons expressing doublecortin (Dcx) was detectable at this time. Only at later stages did the number of Dcx+ cells increase and reach a plateau at 28 dptm. At approximately this time, reporter-positive cells started to express the mature neuronal marker NeuN, which transiently overlapped with Dcx. At 42 dptm, most of the NeuN+ cells no longer coexpressed Dcx and by now contributed half of the reporter-positive cell population. Overall, these findings show that neurogenesis follows a typical pattern of progression (2), and manipulations such as Cre activity by tamoxifen treatment in these mice did not alter the temporal maturation stages of newborn neurons.
Fig. 1.
Deletion of TrkB affects the survival of newborn neurons. (A) Schematic diagram showing the experimental paradigm used for tamoxifen-induced Cre recombination in TrkBlox/lox-Cre R26R mice and control littermates. Histograms depict the density distribution of reporter-positive cells (βgal+) expressing GFAP, Dcx, or NeuN in TrkBwt/wt-Cre (wt/wt) and TrkBlox/lox-Cre (lox/lox) mice. (B) Density of reporter-positive cells coexpressing Dcx and NeuN as in A. Ten slices per hemisphere and two mice for each time point were analyzed (*, P < 0.05). (C) Representative confocal images depict the reporter marker βgal colocalized with either the neuronal marker NeuN (βgal/NeuN) or Dcx (βgal/Dcx). Colocalization signals (red) were superimposed on NeuN or Dcx immunoreactivity (gray). Labeling of each single marker from which colocalization was obtained is shown as SI Materials and Methods (see Fig. S2). (Scale bar, 20 μm.)
Reduced Long-Term Survival of TrkB-Deficient New Neurons.
To assess whether TrkB signaling influenced the lineage progression of the newly generated cells described above, we examined tamoxifen-induced TrkBlox/lox-Cre mice (Fig. 1 A and C). At all time points investigated, the fraction of reporter-positive cells expressing GFAP or Dcx was not significantly different from that in control littermates, indicating that the composition of progenitors and new neurons that exit the cell cycle was not altered by deletion of TrkB. However, at 42 dptm the number of reporter-positive cells expressing NeuN was markedly reduced in TrkBlox/lox-Cre mice. To further define the precise cell-maturation stage at the time of TrkB-dependent survival, we quantified neurons at an earlier developmental stage when they still coexpress both Dcx and NeuN. Notably, the density of reporter-positive cells double positive for these markers (Dcx+/NeuN+) decreased significantly at 42 dptm (Fig. 1B). Thus, the survival effect mediated by TrkB occurs at the immature-to-mature neuronal transition, corresponding to a critical time after 4 weeks in a neuron's life.
Reduced Dendritic Arbor and Spine Complexity of TrkB-Deficient New Neurons.
We next investigated whether the integration of adult-born neurons into the existing circuitry would be impaired in the absence of TrkB. As the ability of newborn neurons to integrate involves a progression through distinct morphological stages of maturation (7, 10) we focused on axon growth, dendritic arborization, spine shape and number, and synapse formation at 28 dptm, the time at which neuronal cell death was not yet apparent. As neurogenesis proceeds continuously, 28 dptm reporter-positive cells consist of a heterogeneous population including precursors and adult-born neurons of mixed age. To establish the exact age of reporter-positive neurons we performed retroviral-birthdating analysis on tamoxifen-induced TrkBlox/lox-Cre R26R mice. Mice were injected with retrovirus transducing GFP through stereotaxic delivery into the dentate gyrus of the hippocampus on the fifth day of tamoxifen treatment (Fig. 2A), the time immediately preceding the beginning of neurogenesis after Cre recombination.
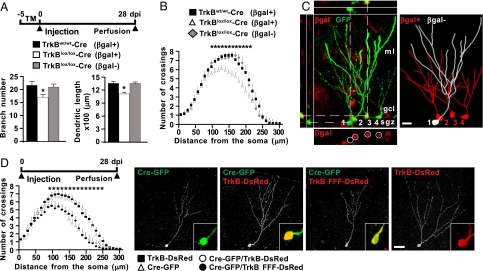
Fig. 2.
TrkB is required for dendritic arborization in adult-born neurons. (A) Schematic diagram showing the experimental paradigm used for retrovirus injection into tamoxifen-induced TrkBlox/lox-Cre R26R and control mice. Histograms show the total number of branches and the dendritic length of reporter-positive (βgal+) or -negative (βgal−) cells virally transduced with GFP (n = 20 neurons; 3 animals per genotype) (*, P < 0.05). (B) Graph depicts Sholl analysis of the dendritic arbors in the same neurons as in A (n = 20 neurons; 3 animals per genotype) (*, P < 0.05). (C) Representative confocal images depicting the dendritic morphology of reporter-positive (2–4; βgal+) or -negative (1; βgal−) cells transduced with GFP expressing retrovirus in TrkBlox/lox-Cre R26R mice. Two-dimensional projection of tridimensional reconstruction of the cells is shown. (Scale bar, 10 μm.) (D) Schematic diagram showing the experimental paradigm. Graph depicts Sholl analysis of the dendritic arbor of cells single transduced (Cre or TrkB) or cotransduced (Cre/TrkB or Cre/TrkB FFF) with the retrovirus (n = 10; 3 animals for each experimental group) (*, P < 0.05). Representative confocal images of transduced cells are presented. Insets show the expression of the reporter marker GFP (green) and/or DsRed (red). (Scale bar, 20 μm.)
Axonal Growth.
At day 28 postinjection (28 dpi) we observed axon fibers labeled by GFP at the inner margin of the CA3 area in the hippocampus (see Fig. S3A). Axonal length was scored by tracing axon fibers from the dentate to the end of the axons (10). We found that traced lengths were similar in knockout (1002 ± 34 μm; n = 11) and control (987 ± 29 μm; n = 11) mice, indicating that the elongation and direction of axons projecting to CA3 were independent of TrkB in these neurons.
Dendritic Growth.
In the same sections we analyzed dendritic growth on transduced neurons extending through the molecular layer of the dentate gyrus. Injection of the virus resulted in a sparse labeling of cells, enabling us to follow detailed changes in dendrite morphology of individual neurons. When confocal analysis followed by three-dimensional reconstruction and morphometric evaluation of the dendritic arbor was carried out in labeled neurons, it was evident that the total number of branches and dendritic length were significantly reduced in TrkB-deficient vs. control neurons (Fig. 2A). Moreover, Sholl analysis (25) revealed that the dendritic complexity of TrkB-deficient neurons was reduced at sites further than 90 μm from the soma compared with control neurons (Fig. 2B). As expected from a previous study (22), we observed that recombination occurred in most (∼90%) of the newly generated neurons in these mice, but few cells were reporter negative. This permitted us to compare the dendritic morphology of newborn neurons transduced by GFP virus that were recombined (βgal+) with those in which the recombination did not take place (βgal−) in the same section. Strikingly, while βgal− cells expressed features of control neurons the βgal+ displayed thin dendrites that were poorly arborized (Fig. 2 A–C).
We next examined the specific requirement of TrkB tyrosine kinase signaling on dendritic growth of single adult-born neurons. By injecting a retrovirus transducing both Cre recombinase and the reporter gene GFP (Cre-GFP) into the dentate gyrus of TrkBlox/lox mice, we first confirmed that dendritic growth was also impaired by this approach in newborn neurons at 14 and 28 dpi as revealed by Sholl analysis (see Fig. S3B). These morphological deficits were fully rescued by coinjection of retrovirus transducing functional TrkB (TrkB-DsRed) (see Fig. S4) together with that transducing Cre (Fig. 2D). When hippocampal sections stained with antibodies against GFP and DsRed were examined, single- and double-labeled neurons intermingled in the same sections disclosed a rather distinct dendritic complexity. Cells expressing GFP alone showed less complex dendritic arbors than cells expressing only DsRed or cells expressing both GFP and DsRed, indicating that dendritogenesis was rescued by TrkB. Notably, dendrite growth could not be restored when catalytically inactive TrkB (TrkB FFF-DsRed) was used for rescue. Taken together these data indicate that there is indeed a regulation of dendritic arbor complexity by TrkB kinase activity specifically acting in neurons born in the adult dentate gyrus of the hippocampus.
Spine Growth.
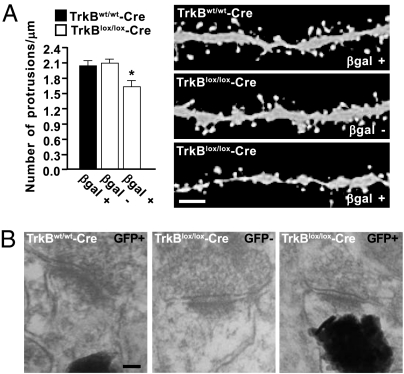
Injection of an improved retroviral vector (10) in tamoxifen-induced control mice resulted in a persistent expression of GFP and strong fluorescence intensity of the dendritic segments at 28 dpi (Fig. 3A). This permitted us to visualize dendritic spines of rather diverse shapes and morphology, most of which appeared to be thin spines with small heads. Consistent with findings in wild-type mice (10), total spine density was high and few spines with mushroom morphology were detected at this stage of development. However, spine density was significantly reduced upon TrkB deletion in newborn neurons of the same age. As an internal reference, we analyzed spines of the small fraction of βgal− neurons in these mice, showing that they possess a similar high density of spines to those in control littermates. Moreover, to assess whether spines could form excitatory and inhibitory synaptic contacts, we performed immunohistochemistry using glutamatergic (vGlut1) and GABAergic (vGAT) synaptic markers, respectively. Immuno reactivity for vGlut1 and vGAT was observed in both TrkBlox/lox-cre and control mice, and some of these immunoreactive dots were opposed to GFP+ spines, thereby suggesting synapse formation (see Fig. S3C).
Fig. 3.
Spine density is regulated by TrkB in adult-born neurons. (A) Graphs depict the quantification of protrusions in dendritic segments of newborn neurons transduced with retrovirus expressing GFP in tamoxifen-induced TrkBlox/lox-Cre R26R (n = 24 dendritic segments; 18 βgal+; 11 βgal−; 3 animals) and control mice (n = 60 dendritic segments; 26 βgal+; 3 animals) mice. The density of protrusions is expressed as the number of protrusions per micrometer of dendritic length (*, P < 0.01). Representative images show spine morphology in reporter-positive (βgal+) or -negative (βgal−) cells 28 dpi. (Scale bar, 2 μm.) (B) Representative electron micrographs showing synaptic contacts of newborn neurons in tamoxifen-induced TrkBlox/lox-Cre Z/EG and control mice. Reporter-positive cells (GFP+) show GFP fluorescence as photoconverted electron-dense signals at postsynaptic sites. A synapse from a reporter-negative cell (GFP−) is shown as a reference. (Scale bar, 50 nm.)
Synapse Formation.
To assess directly whether spines could still form axo/dendritic synapses after TrkB deletion, we examined the architecture of the synapses of newborn neurons at the ultrastructural level using photoconversion of GFP in hippocampal sections from TrkBlox/lox-Cre Z/EG mice 28 dptm followed by electron microscopy (26). This process converts GFP fluorescence into electron dense 3,3′-diaminobenzidine precipitates, allowing specific localization of the synaptic contacts. We focused on two hallmarks of mature synapses, the appearance of synaptic vesicles at presynaptic sites and the postsynaptic density. We observed that there are synapses formed by both TrkB-deficient and control newborn neurons, and their mature morphology matched that of reference synapses in the same preparations (Fig. 3B). Thus, TrkB-deficient neurons maintained the ability to form mature synaptic contacts.
Overall, these data suggest that the reduced complexity of the dendritic arbor and spines, rather than synaptic contact per se, are morphological features regulated by TrkB in adult-born neurons.
Deletion of TrkB Affects Neurogenesis-Dependent LTP in the Dentate Gyrus.
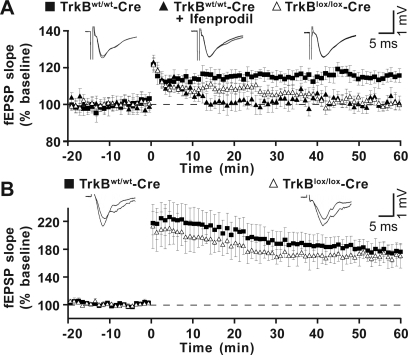
New neurons have been shown to exhibit a small but stable form of long-term potentiation (LTP) (27, 28), which was distinguished from that of the existing mature neurons in being insensitive to GABAergic inhibition and specific for the activation of the N-methyl-D-aspartic acid (NMDA) receptors subunit 2B (NR2B). We investigated whether this specific form of LTP was adversely affected in TrkBlox/lox-Cre vs. control mice. Slices derived from these mice were used for extracellular recordings between 28 and 42 dptm. Specifically, stimulation of medial perforant path (MPP) fibers evoked field excitatory postsynaptic potentials (fEPSPs), which were recorded by placing the extracellular recording electrode in the medial molecular layer of the upper blade of the dentate gyrus (Fig. 4A). The synaptic transmission was evaluated by testing input–output responses and paired-pulse depression in the dentate gyrus (see Fig. S5). One hundred-hertz stimulation elicited LTP that was stable during the recording time (>90 min) in slices from control mice and was prevented by pretreating slices with the NR2B inhibitor ifenprodil (3 μM) (Fig. 4A). On the contrary, slices from TrkBlox/lox-Cre mice displayed a potentiation that lasted for only 30–40 min after stimulation, before returning to baseline (Fig. 4A). To confirm that the short-term potentiation was specifically attributable to TrkB-deficient newborn neurons, experiments were performed in the presence of the GABA receptor blocker bicuculline (20 μM), which unmasks the synaptic potentiation of granule neurons (27, 28). Under this experimental condition, 100-Hz stimulation induced a large and persistent potentiation that was indistinguishable in slices from TrkBlox/lox-Cre and control mice (Fig. 4B). Thus, targeted deletion of TrkB in newborn neurons causes an impairment in the late-phase of the neurogenesis-dependent form of LTP without altering the synaptic plasticity of mature granule neurons in the same subfield.
Fig. 4.
TrkB is required for neurogenesis-dependent LTP. (A) LTP evoked in tamoxifen-induced TrkBlox/lox-Cre and control mice. Experiments were conducted in hippocampal slices of TrkBwt/wt-Cre mice in the absence (n = 8 slices, 4 animals) or presence (n = 6 slices, 2 animals) of ifenprodil (3 μM) and in slices of TrkBlox/lox-Cre mice (n = 7 slices, 3 animals). Each point in the graph shows the average fEPSP slope elicited in response to a test stimulus before (−20–0 min) and after (0–60 min) tetanic stimulation of the MPP. Representative fEPSP traces recorded before (−5 min) and after (60 min) tetanus are shown on the top. (B) LTP evoked as in A in the presence of bicuculline (20 μM) (n = 4 slices, 4 animals).
Deficits in Neurogenesis Correlated with Anxiety-Like Behavior.
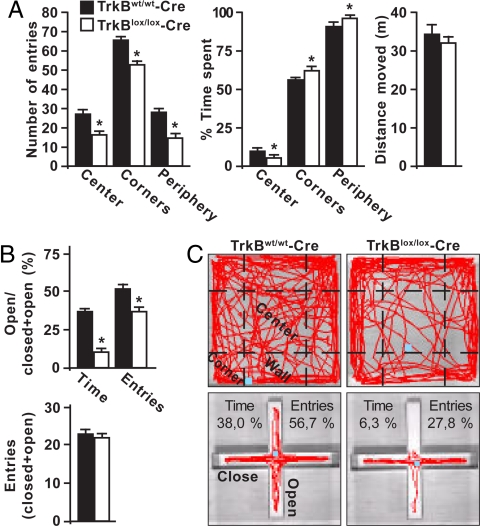
While the functions of newborn neurons in the adult are largely elusive, there is increasing evidence that adult neurogenesis (17) and BDNF expression (29) in the dentate gyrus are involved in mood regulation. Our genetic approach now offered us the possibility to examine the effect of TrkB deletion on anxiety-like behavior specifically in recently generated neurons. We first focused on the open-field behavioral test, a well established paradigm used to study anxiety. We observed that TrkBlox/lox-Cre mice between 28 and 42 dptm exhibited decreased spontaneous activity in the open-field arena compared with control littermates (Fig. 5 A and C). While the distance traveled was comparable in these mice, TrkB-deficient mice entered the center areas of the arena less frequently and spent less time there than controls. Moreover, in the periphery (including the corners) the number of entries was reduced but the time spent was significantly increased. To further confirm the defects in exploratory behavior, mice were examined in the elevated plus maze test (Fig. 5 B and C). Mice lacking TrkB specifically in the newborn neurons showed a significant decrease in the percentage of entries and time spent in the open arms vs. control littermates. The number of entries was comparable in these mice, consistent with the absence of any general activity defects. When the test was performed before TrkB-deficient newborn neurons had acquired morphological maturation, between 7 and 12 dptm, no differences in exploratory behavior were found between knockout and control mice (data not shown), indicating that alteration in anxiety-like behavior by TrkB does not take place before the morphological integration of adult-born neurons.
Fig. 5.
Deletion of TrkB in newborn neurons affects exploratory activity in mice exposed to a novel environment. (A) Exploratory behavior of tamoxifen-induced TrkBlox/lox-Cre and control mice in the open-field test. Histograms show the number of entries and the percentage of time spent in the center, corner, or the entire periphery of the arena (n = 20) (*, P < 0.05). The total distance moved is shown in the right panel. (B) Exploratory behavior in the elevated plus maze test. Histograms show the proportion of entries and time spent in the open arms (n = 20) (*, P < 0.05). The total number of entries in the four arms is shown in the lower panel. (C) Digital tracking of mice exposed to the open-field (upper panels) and the elevated plus maze (lower panels) behavioral tests. Representative traces of mice pattern activity (red) resulting from video tracking are depicted. Each panel is representative of individual mice activity.
Discussion
The number of surviving newborn neurons in the adult brain is thought to depend upon functional integration into the existing network, which in turn has been suggested to be experience dependent (2). The present study shows that the survival of newborn neurons in the adult dentate gyrus depends critically on the activation of the TrkB full-length receptor. The TrkB-dependent decision regarding life or death in these newborn neurons takes place right at the transition point where they mature from a Dcx+ to a NeuN+ stage, after 4 weeks of age. Two weeks before newborn neurons start to die, they exhibit a drastic reduction in dendritic complexity and spine density compared with wild-type newborn neurons, indicating that this receptor is required for the integration of adult-born neurons. Both the failure to become integrated and subsequent dying, and the loss of TrkB signaling lead to impaired LTP that has been shown to be specific for these young neurons (27, 28). Synaptic potentiation in newborn neurons when they are 4–6 weeks old depends on the activation of an NMDA receptor containing the NR2B subunit that is selectively expressed in these cells, but absent in mature neurons (16). Interestingly, the LTP mediated by this NMDA receptor can be elicited in the absence of GABA-A receptor antagonists (27, 28), suggesting that this LTP is exclusively confined to synapses on newborn neurons. The present study showed that while an ifenprodil-sensitive LTP can be induced in wild-type mice, LTP is drastically impaired in mice in which the vast majority of newborn neurons lack TrkB. While an early, albeit reduced, LTP could be observed, the late phase was totally abolished. The slight reduction in the early phase of LTP may already reflect the reduced number of surviving neurons, while the lack of a late phase of LTP is strikingly similar to the effect of the loss of TrkB or its ligand BDNF at the Schaffer collateral synapses. In any case, the apparent deficit in synaptic long-term plasticity is likely to have an important bearing on the participation of newborn neurons in functional network activity.
The present study shows that mice lacking functional full-length TrkB signaling specifically in the newborn neuron population exhibit a markedly enhanced anxiety-like behavior. Consistent with our results a recent study (30) demonstrated that mice expressing a BDNF variant (Val66Met) showing an impaired activity-dependent secretion of BDNF exhibit enhanced anxiety-like behavior. Remarkably, the behavioral phenotype correlated with a poor dendritic arborization, highly reminiscent of the dendritic phenotype observed here. However, this study could not determine the possible contribution of adult-generated neurons to this phenotype. Here, we show that lack of TrkB signaling in newborn neurons is sufficient to cause an enhanced anxiety-like behavior, suggesting that the mood state may be particularly sensitive to the functional recruitment of newborn neurons. The behavioral output we observed after TrkB deletion in adult-born neurons was restricted to anxiety as depression was not observed. The tail suspension test, a paradigm often used to study depression, failed to disclose apparent differences in the immobile state of TrkB-deficient mice (134.8 ± 20.2 s; n = 12) compared with wild-type mice (138.3 ± 13.1 s; n = 12), indicating that newborn neurons permit selected changes in adult brain function (17, 31). We also observed that TrkB-deficient newborn neurons did not affect cognitive behavior in mice carrying the deletion. Hence, spatial learning and memory retention investigated in the Morris water maze test was normal in TrkB-deficient and control mice (see Fig. S6). Overall, these data suggest that lack of TrkB signaling specifically in the newborn contributes to selectively regulate the anxiety state of the animal.
Materials and Methods
Immunohistochemistry.
Slices were permeabilized for 20 min in 1% Triton X-100 (Sigma) in PBS and incubated overnight in 3% BSA (Sigma) in PBS containing primary antibodies diluted 1:200 chicken anti-GFP (Aves Lab), guinea pig anti-Dcx (Chemicon), mouse anti-GFAP (Sigma), mouse anti-NeuN (Chemicon), rabbit anti-red fluorescent protein (Chemicon), rabbit anti-βgal (MP Biomedicals), mouse anti-Cre (Covance), anti-vGlut1 (Synaptic System), anti-vGAT (Synaptic System), anti-TrkB (Santa Cruz) and anti-TrkB (Chemicon). Slices were incubated for 2 h at room temperature with secondary antibodies conjugated with FITC, Cy3, Cy5 (Chemicon), Alexa 488, 546, and 647 (Invitrogen), mounted in Aqua Poly/Mount (Polysciences, Inc.), and analyzed by confocal microscopy (see SI Materials and Methods).
Electron Microscopy.
TrkBlox/lox-Cre and control mice induced for the expression of the recombination marker GFP were perfused with PBS followed by a solution of 4% paraformaldehyde in PBS. Photoconversion and electron microscopy were performed as previously reported (26) (see SI Materials and Methods).
Electrophysiology.
Electrophysiological recording was performed as previously described (32) (see SI Materials and Methods).
Open Field.
Mice were placed in the experimental room 1 h before the test. Open field activity was measured in a square arena (50 × 50 cm). At the beginning of a session, mice were placed in the central part of the arena. We scored the number of entries into the center, corners, and periphery (corners plus walls) and the time spent in the same areas by video-tracking recording for 20 min. Experiments were carried out under white light between 09:00 and 15:00 h and activity was videotaped and scored by EthoVision software (Noldus, The Netherland).
Elevated Plus Maze.
Mice were placed in the experimental room 1 h before the test. The apparatus consisted of two open arms, two enclosed arms of the same size (30 × 8 cm), and a central area (8 × 8 cm); placement was 50 cm above the floor. At the beginning of a session, mice were placed in the central part of the maze facing one of the open arms. The number of entries and the time spent in the open and close arms were recorded for 5 min. An entry was defined as the mice entering into an arm with all four paws. The test was run out between 09:00 and 15:00 and behavior was videotaped and scored by EthoVision software.
Supplementary Material
Acknowledgments.
We thank H. Thoenen, B. Berninger, L. Godinho, and R. Carr for insightful comments on the manuscript; R. Klein for providing TrkBlox/lox mice; F. H. Gage for providing the CAG-GFP viral vector; R. Jagasia for providing the IRES–DsRed-encoding vector; and A. Lepier for the retrovirus production. Research was supported by Research Program of National Interest (PRIN) to M.C., by Deutsche Forschungsgemeinschaft (DFG), Center for Integrated Protein Science, European Transcriptome, Regulome and Cellular Commitment Consortium, Bundesministerium für Bildung und Forschung, and the Bavarian Ministry to M.G. and by DFG (to R.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803702105/DCSupplemental.
References
- 1.Ming G-L, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 2.Lledo P-M, Alonso M, Grubb M-S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 3.Aimone J-B, Wiles J, Gage F-H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 4.Ninkovic J, Götz M. Signaling in adult neurogenesis: From stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17:338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Seri B, García-Verdugo J-M, McEwen B-S, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 8.Hastings N-B, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Markakis E-A, Gage F-H. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 10.Zhao C, Teng E-M, Summers R-G, Jr, Ming G-L, Gage F-H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tashiro A, Sandler V-M, Toni N, Zhao C, Gage F-H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 13.Kee N, Teixeira C-M, Wang A-H, Frankland P-W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Scott B-W, Wojtowicz J-M. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 15.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 16.Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H-A. Critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Duan W, Mattson M-P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 19.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 22.Mori T, et al. Inducible gene deletion in astroglia and radical glia–A valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 23.Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 25.Sholl D-A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 26.Grabenbauer M, et al. Correlative microscopy and electron tomography of GFP through photooxidation. Nat Methods. 2005;2:857–862. doi: 10.1038/nmeth806. [DOI] [PubMed] [Google Scholar]
- 27.Snyder J-S, Kee N, Wojtowicz J-M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 28.Saxe M-D, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z-Y, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Colino A, Malenka R-C. Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.