Abstract
Background and Purpose: This study was designed to evaluate the effects of competing ions and electroosmosis on the transdermal iontophoresis of dexamethasone phosphate (Dex-Phos) and to identify the optimal conditions for its delivery.
Methods: The experiments were performed using pig skin, in side-by-side diffusion cells (0.78 cm2), passing a constant current of 0.3 mA via Ag-AgCl electrodes. Dex-Phos transport was quantified for donor solutions (anodal and cathodal) containing different drug concentrations, with and without background electrolyte. Electrotransport of co-ion, citrate, and counterions Na+ and K+ also was quantified. The contribution of electroosmosis was evaluated by measuring the transport of the neutral marker (mannitol).
Results: Electromigration was the dominant mechanism of drug iontophoresis, and reduction in electroosmotic flow directed against the cathodic delivery of Dex-Phos did not improve drug delivery. The Dex-Phos flux from the cathode was found to be optimal (transport number of ∼0.012) when background electrolyte was excluded from the formulation. In this case, transport of the drug is limited principally by the competition with counterions (mainly Na+ with a transport number of ∼0.8) and the mobility of the drug in the membrane.
Discussion and Conclusion: Dex-Phos must be delivered from the cathode and formulated rationally, excluding mobile co-anions, to achieve optimal iontophoretic delivery.
The use of corticosteroids for the treatment of a variety of musculoskeletal conditions is common practice.1–6 Local administration of the drug by injection into the relevant subcutaneous compartment usually results in improved clinical outcome, at least temporarily.2–6 Furthermore, when depot formulations are injected intramuscularly, the systemic effects associated with oral delivery are reduced.2 However, although injections are generally safe, they present some disadvantages and risks (eg, postinjection pain and flare, infection, difficulty in accurately placing the needle, need for an experienced health care professional to perform the injection).2,3
Alternatively, iontophoresis also is used in clinical practice to deliver corticosteroids locally.7–9 Iontophoresis is a minimally invasive technique that enhances the transport of charged and highly polar molecules across the skin by the application of a small electrical current (with a current density <0.5 mA/cm2). The 2 main mechanisms of transport of this electrically enhanced method are electromigration and electroosmosis.8,10,11
Electromigration originates from the direct interaction of the electrical field and the ions present in the formulation and the skin and, therefore, will enhance the transport of cationic drugs from the anode and, inversely, negatively charged drugs from the cathode. Conservation of charge requires that the sum of the electrical current carried by each ion equal the total electrical current supplied by the power source. Practically, as far as drug delivery is concerned, this means that the drug competes with all the other ions present in the system.10,11 The efficiency of transport, that is, the fraction of the total charge transported by a given drug (its transport number, td), can be determined experimentally by measuring its flux (Jd) and applying the relation:
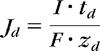 |
where I is the total current passed, F is the Faraday's constant, and zd is the valence of the drug.12
Electroosmosis has its origin in the fact that the skin is a negatively charged membrane at physiological pH. When an electrical potential is applied across a membrane containing a fixed charge, a bulk volume flow of solution occurs in the direction of the counterion movement.13 This means that for the negatively charged skin, the electroosmotic flow is in the anode-to-cathode direction. This assists the transport of cations and retards that of anions. This flow of solvent carries through the skin any dissolved solute and, therefore, is the mechanism enhancing transdermal delivery of neutral, polar molecules. Electroosmotic flow is the dominant mechanism of transport for the delivery of larger molecules.11 The drug flux due to the electroosmotic mechanism (JdEO) is proportional to the concentration (Cd) of the solute:
where v is the solvent volume flow.14 The formulation's pH and ionic strength are the main parameters that can be used to modulate electroosmosis by, respectively, modifying and screening the skin's charge.11,15,16
Dexamethasone phosphate (Dex-Phos, MW: 472.4), a phosphorylated prodrug of dexamethasone (Dex), undoubtedly has been the most studied corticosteroid for iontophoretic delivery.8 The disodium salt, dexamethasone sodium phosphate (Dex-Na2-Phos, MW: 516.4), is water soluble and at physiological pH is present mainly in its dianionic form (pKas: 1.9 and 6.4). Iontophoretic treatment of various musculoskeletal problems with Dex-Phos has been the subject of clinical studies,17–29 many of which have reported beneficial effects.17–27 However, improvement in the patient's condition is not always seen,28,29 perhaps due to the variety of iontophoretic conditions used8 and to the fact that the optimal conditions for Dex-Phos delivery have not been identified.
The delivery electrode and the formulation of the drug solution are controllable parameters that are expected to greatly influence the efficiency of the delivery. Earlier studies have used the anode to deliver Dex-Phos,17,22,30 but, given that Dex-Phos is negatively charged at physiological pH (z∼−2 at pH 7.4), delivery from the cathode (with electromigration as the main driving force) is more appropriate than that from the anode (electroosmosis being the only available driving force).9,11 Furthermore, many clinical studies evaluating the iontophoretic delivery of Dex-Phos have combined the drug with lidocaine hydrochloride.17–22,28 Again, this practice may have a detrimental effect on Dex-Phos delivery due to the introduction of exogenous ions that are competing with the drug for the transport of current through the skin.9,11 Thus, there is a need to optimize the conditions for Dex-Phos iontophoresis.
The primary objective of this research, therefore, was to identify the optimal iontophoretic conditions to deliver Dex-Phos. A further goal was to better understand the effects of: (1) the ions present in the formulation or in the subdermal compartment that are competing with Dex-Phos to transport the current and (2) the electroosmotic flow, which normally occurs in the anode-to-cathode direction, that is, against the electromigration of Dex-Phos delivered from the cathode.
Method
Materials
Dex (>98%) and Dex-Na2-Phos (>98%) were purchased from Sigma-Aldrich.* Solutions of injectable Dex-Na2-Phos were obtained from American Regent† (equivalent to 4 mg/mL Dex-Phos), Faulding‡ (again equivalent to 4 mg/mL Dex-Phos), and Organon Laboratories§ (equivalent to 4.6 mg/mL of Dex-Phos). Topical lidocaine hydrochloride 4% (Xylocaine Topical 4%) was obtained from AstraZeneca.‖ Sodium chloride, potassium chloride, sodium phosphate (monobasic), potassium phosphate (dibasic), mannitol, phosphoric acid (85%), and methanesulfonic acid (99%) were obtained from Acros Organics.# Potassium citrate, methanol (high-performance liquid chromatography grade), acetonitrile (far UV high-performance liquid chromatography grade), ethyl acetate, and hydrochloric acid 37% (weight-to-weight ratio) were provided by Fisher Scientific.** Sodium hydroxide 50% (ion chromatograpy eluent grade) was obtained from Fluka.†† Silver wire (>99.99% purity) and silver chloride (99.999%) were purchased from Sigma-Aldrich. All reagents were at least analytical grade, and all aqueous solutions were prepared using high-purity deionized water (18.2 MΩ·cm, Barnstead Nanopure Diamond‡‡). The concentrations of Dex-Na2-Phos solutions are expressed in terms of the Dex-Phos concentration. For example, a 0.4% Dex-Phos solution contains 4.4 mg/mL of Dex-Na2-Phos.
Skin Preparation
Porcine ears were obtained from a local abattoir. Ears were cleaned under running cold water, and the skin was dermatomed to a nominal thickness of 750 μm (Zimmer Electric Dermatome§§). The pieces of tissue obtained (∼9 cm2) were wrapped individually in Parafilm and stored for no more than 3 months at −20°C until use. The required pieces of skin then were thawed at room temperature for approximately 30 minutes before each iontophoresis experiment.
Preparation of Dex-Phos Solution at pH 4
In one experiment, a solution of Dex-Phos prepared in deionized water at pH 4 was used. To avoid the inclusion of extraneous anions inevitable when a strong acid is used to lower the pH of a Dex-Na2-Phos solution prepared in water (typical pH=7.5), an equimolar mixture of the diacid (dexamethasone-dihydrogen-phosphate [Dex-H2-Phos]) and its conjugate base (Dex-Na2-Phos) was dissolved in water to obtain a final concentration of 0.4% of Dex-Phos. Dex-H2-Phos was prepared from the disodium salt by adding one part of 37% weight-to-weight ratio HCl to 3 parts of a solution of 25 mM of Dex-Na2-Phos in water. The insoluble dihydrogen acid precipitated and was recovered by liquid extraction using ethyl acetate. The ethyl acetate was evaporated, leaving a powder of Dex-H2-Phos, which contained only trace amounts of extraneous ions when assayed by ion chromatography.
Iontophoresis
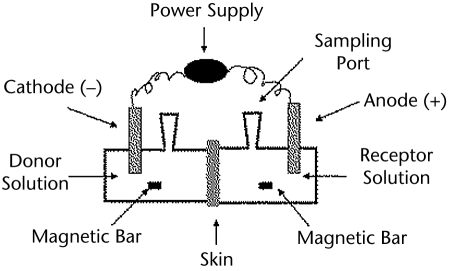
In vitro iontophoresis experiments were performed in side-by-side diffusion cells, as illustrated in Figure 1 (transport area=0.78 cm2). The skin was clamped between the 2 half cells, with the stratum corneum side facing the donor (drug-containing solution) chamber. In all experiments, the subdermal compartment was filled with phosphate-buffered saline (PBS; 170 mM sodium, 1.4 mM potassium, 137 mM chloride, and 18 mM phosphate) at pH 7.4. Both chambers held 3.5 mL of solution and were magnetically stirred. Thirty minutes prior to iontophoresis, the cathodal chamber was filled with 3.5 mL of deionized water and the anodal chamber was filled with 3.5 mL of PBS to check for leaks. Both chambers then were emptied and refilled with the appropriate donor and receptor solutions before the experiment was started. In all experiments, a 0.3-mA constant current intensity (current density=0.38 mA/cm2) was applied for 6 hours via Ag-AgCl electrodes connected to a power source (Yokogawa 7651 Programmable DC source‖‖). All experiments were performed at room temperature with a minimum of 4 replicates using the skin from at least 2 different pigs. To measure the delivery of Dex-Phos, 1 mL of the subdermal compartment was collected and replaced by the same volume of PBS every hour for the first 2 hours and every half hour thereafter. A 24-hour control experiment, with no electrical current applied, also was performed using Dex-Phos 0.4% in water as the donor solution.
Figure 1.
Schematic diagram of the experimental setup.
Effect of delivery electrode polarity.
First, the effect of the polarity of the delivery electrode was verified for 2 formulations containing sufficient Cl− to satisfy the electrochemistry at the anode: (1) Dex-Phos 0.4% in 0.9% NaCl and (2) a mixture containing one part of injectable Dex-Phos (Faulding) for 2 parts of lidocaine hydrochloride.
Effect of donor composition.
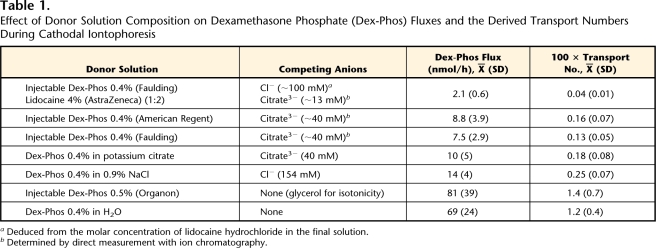
Dex-Phos delivery from the cathode was measured for a variety of donor solutions (Tab. 1). The effect of the concentration of the drug in the absence of a background electrolyte was verified for concentrations ranging from 0.2% to 0.8%. In one experiment, to ensure that the concentration of the Cl− released by the Ag-AgCl electrode remained an order of magnitude lower than that of the drug in the donor solution (Dex-Phos 0.4% in H2O), the latter was continuously perfused (15 mL/h), using a peristaltic pump, during the iontophoretic delivery.
Table 1.
Effect of Donor Solution Composition on Dexamethasone Phosphate (Dex-Phos) Fluxes and the Derived Transport Numbers During Cathodal Iontophoresis
aDeduced from the molar concentration of lidocaine hydrochloride in the final solution.
bDetermined by direct measurement with ion chromatography.
Effect of electroosmosis.
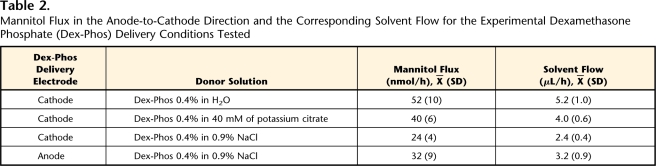
A strategy to reduce electroosmosis against the delivery of Dex-Phos was explored. The approach involved delivery of 0.4% Dex-Phos from a donor solution at pH 4. In the latter iontophoretic conditions and those described in Table 2, electroosmotic flow was assessed using mannitol (at 10 mM) as a marker. The flux of mannitol was evaluated in both anode-to-cathode and cathode-to-anode directions. In addition to sampling of the receptor chamber, 0.3 mL of the donor solution was collected hourly. The passive diffusion of mannitol across the skin also was measured as a control.
Table 2.
Mannitol Flux in the Anode-to-Cathode Direction and the Corresponding Solvent Flow for the Experimental Dexamethasone Phosphate (Dex-Phos) Delivery Conditions Tested
Contribution of competing ions.
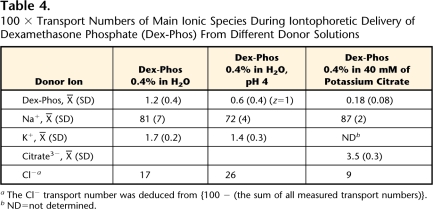
Finally, the donor solution samples obtained in the experiment with mannitol, in the 3 cases described in Table 4, were assayed for the major competing counterions sodium and potassium. The concentration of the co-ion citrate in the receptor also was measured when Dex-Phos was formulated with this anion. From these values, the contributions of the main competing ions to the total current were determined.
Table 4.
100 × Transport Numbers of Main Ionic Species During Iontophoretic Delivery of Dexamethasone Phosphate (Dex-Phos) From Different Donor Solutions
aThe Cl− transport number was deduced from {100 − (the sum of all measured transport numbers)}.
bND=not determined.
Sample Analysis
The concentrations of Dex-Phos and Dex in the receptor chamber were assayed by high-performance liquid chromatography (ASI-100 automated sample injector, P680 pump, TCC-100 thermostated column compartment, PDA-100 diode array detector, Chromeleon software)## under isocratic conditions. A mobile phase consisting of 30:70 (volume-to-volume ratio) acetonitrile:phosphate buffer (0.15 M, pH 2) was pumped (0.75 mL/min) through a Lichrosphee 100 RP-18 (4 × 125 mm) reverse-phase column*** fitted with its guard column and thermostated at 25°C. Dex and Dex-Phos concentrations were quantified via their UV absorbance at 240 nm using their respective calibration curves obtained from a minimum of 5 standard solutions (made from 500-ppm stock solutions in methanol appropriately diluted in PBS at pH 7.4) covering the entire range of experimental concentrations. The retention times for Dex-Phos and Dex were ∼3.5 and ∼10.5 minutes, respectively; the detection limit for both was 0.02 μg/mL for a 25-μL injection, and detection was linear up to 150 μg/mL (correlation coefficient >.999, relative standard deviation <5%).
Ion chromatography with suppressed conductivity detection (AS50 autosampler and thermal compartment, GP50 gradient pump, ED50 electrochemical detector)## was used to measure the concentrations of sodium, potassium, and citrate. For the quantification of cations, the 20 mM of methanesulfonic acid eluent was pumped under isocratic conditions (1 mL/min) through an IonPac CS12A column## (250 × 4 mm) thermostated at 30°C and a CSRS ULTRA II suppressor## (4 mm) set at a current of 62 mA. Concentrations of Na+ and K+ were measured against linear calibration curves obtained from standard solutions (at least 5 different concentrations, correlation coefficient >.998, relative standard deviation <6%) of their respective chloride salt. For the quantification of citrate anions in the receptor compartment, the 35 mM of NaOH mobile phase was pumped under isocratic conditions (1 mL/min) through an IonPac AS16 column## (250 × 4 mm) thermostated at 30°C and an ASRS ULTRA II suppressor## (4 mm) set at a current of 90 mA. At least 5 potassium citrate solutions were used as standards to generate the linear calibration curve (correlation coefficient >.999, relative standard deviation <4%).
Analysis of mannitol was performed using ion chromatography coupled with pulsed amperometric detection.## The mobile phase (380 mM of NaOH) was pumped (0.4 mL/min) through a CarboPac MA1 column## (250 × 4 mm) maintained at a temperature of 20°C. Again, the linear calibration curve was obtained from at least 5 standards of mannitol in PBS (correlation coefficient >.999, relative standard deviation <7%.)
Data Analysis
Linear regressions and statistical analyses were performed using GraphPad Prism V.4.00.††† Statistical differences within multiple data sets were assessed by one-way analysis of variance, followed by a Tukey multiple comparison test. The level of statistical significance was fixed at P<.05. The fluxes were obtained from the slope of the cumulative amount delivered as a function of time for each replicate and are expressed as mean (SD). The reported Dex-Phos flux is the sum of the Dex-Phos and Dex fluxes into the receptor phase to account for the partial dephosphorylation of the prodrug that occurred during transdermal passage or in the receptor compartment. Transport numbers were calculated using these fluxes and equation 1. The valence used for Dex-Phos in the calculations was 2, unless otherwise stated.
Results
Passive Diffusion Control
The passive diffusion flux of Dex-Phos from a 0.4% solution in pure water after 6 hours was below the detection limit and reached only 0.13 (0.11) nmol/h at 24 hours. Considering that all iontophoresis experiments lasted 6 hours, the contribution of passive diffusion could be assumed to be negligible compared with electrotransport.
Anodic Versus Cathodic Delivery
Dex-Phos delivery from the anode was compared directly with that from the cathode. Two donor solutions, which contained sufficient Cl− to ensure good functioning of the Ag-AgCl electrode during anodic delivery, were tested: (1) 0.4% drug in normal saline and (2) a mixture of commercially available citrate-buffered injectable Dex-Phos (Faulding, 0.4% weight-to-volume ratio) and lidocaine hydrochloride (4% weight-to-volume ratio). In the former case, cathodal and anodal fluxes were 14 (4) and 0.3 (0.2) nmol/h, respectively; for the latter, the corresponding transport rates were 2.1 (0.6) nmol/h from the cathode and below the level of detection from the anode.
Effect of Donor Composition on Cathodic Delivery
The next logical step was to optimize delivery from the cathode. In the case of the negatively charged Dex-Phos, the efficiency of iontophoretic delivery will be reduced by the presence of competing anions in the donor solution.11 Dex-Phos flux, therefore, was measured from different formulations, many of which had been used in clinical studies, containing a more or less constant concentration of the drug together with a range of background electrolytes. The Dex-Phos solutions tested, the drug's flux, and the corresponding transport numbers are reported in Table 1. The pH of all solutions tested, except the mixture of Dex-Phos and lidocaine, was between 7.2 and 7.9, meaning that >85% of the drug carried a charge of −2. For the Dex-Phos/lidocaine formulation, the pH was 6.8, and only ∼70% of the drug had a charge of −2. However, because in all cases the majority of the drug was doubly charged, the value for z in equation 1, which was used to determine the transport number from the flux, was assumed equal to 2.
Clearly, the absence of background electrolyte (Dex-Phos in pure water and the injectable Dex-Phos formulation from Organon Laboratories containing a neutral molecule, glycerol, for isotonicity) greatly improved drug delivery. These 2 solutions resulted in fluxes that were not significantly different.
The donor solution in 40 mM of potassium citrate resulted in a Dex-Phos flux not significantly different from those from the commercially available Faulding and American Regent injectable solutions, which contain sodium citrate for isotonicity. When Dex-Phos was formulated with 0.9% (154 mM) NaCl rather than 40 mM of sodium citrate, the drug flux was slightly increased (P<.05), but remained much smaller than that in the absence of background electrolyte.
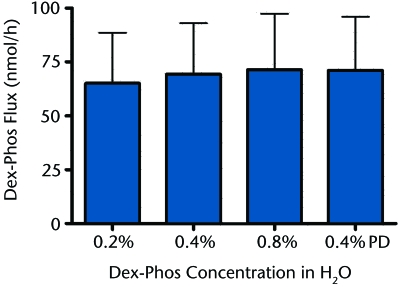
Next, improving Dex-Phos delivery by varying the drug concentration in the donor solution in the absence of background electrolyte was explored. In the case of cations, when no background electrolyte is present, the electromigration flux is rather independent of the drug concentration in the donor,10,11,31 For anions, however, when Ag-AgCl electrodes are used, there is Cl− release from the electrode and the potential for competition to evolve over time.32 Nevertheless, as shown in Figure 2, when the Dex-Phos concentration was varied by 4-fold (0.2% to 0.8% weight-to-volume ratio), there was no significant difference in the drug flux measured after 6 hours of iontophoresis, and these values did not differ from that obtained when the donor solution was continuously perfused at the high rate of 15 mL/h to ensure that the Cl− concentration never attained one tenth that of Dex-Phos.
Figure 2.
Dexamethasone phosphate (Dex-Phos) iontophoretic flux as a function of concentration in deionized water. In the 0.4% perfused Dex-Phos (PD) experiment, the donor solution was continuously perfused with 0.4% weight-to-volume ratio Dex-Phos.
Effect of Electroosmosis
To evaluate the effect of electroosmosis on the delivery of Dex-Phos, the flux of mannitol in the anode-to-cathode direction and its equivalent solvent flow were measured for the 4 delivery conditions summarized in Table 2. The mannitol fluxes also were measured in the cathode-to-anode direction and were not significantly different from the negligible passive diffusion of the neutral marker (1.0 [0.6] nmol/h). Electroosmosis occurred against Dex-Phos delivery from the cathode. Solvent flow was maximal when no background electrolyte was present in the donor (Dex-Phos 0.4% in H2O), the condition so far identified as “optimal” for Dex-Phos delivery. Electroosmotic flow was significantly higher when the background electrolyte was potassium citrate compared with sodium chloride (P<.05). The presence of Dex-Phos in the anode, as opposed to the cathode, when normal saline acted as background electrolyte, did not significantly affect the level of electroosmotic flow.
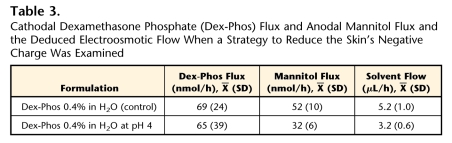
A strategy then was explored to reduce (or reverse) the skin's negative charge in order to decrease the electroosmotic flow and thereby enhance the delivery of the drug. The Dex-Phos 0.4% in H2O solution was prepared at pH 4 using a mixture of the diacid (Dex-H2-Phos) and the disodium salt (Dex-Na2-Phos). The idea was to reduce the negative charge on the skin, the isoelectric point of which has been shown to be between 4 and 5.33 As shown in Table 3, mannitol delivery was clearly reduced at pH 4, and convective solvent flow decreased, but this had no impact on the electrotransport of Dex-Phos.
Table 3.
Cathodal Dexamethasone Phosphate (Dex-Phos) Flux and Anodal Mannitol Flux and the Deduced Electroosmotic Flow When a Strategy to Reduce the Skin's Negative Charge Was Examined
Transport Numbers of Main Ionic Species
Finally, the transport numbers of the main ionic species present in the different experimental conditions studied were measured (Tab. 4). It was clear that sodium was the major charge carrier across the skin, contributing more than 70% in all cases. Only when the pH was reduced to 4 was the Na+ transport lowered (P<.05). In addition, although all donor solutions eliminated Cl−, the Ag-AgCl electrochemistry resulted in the progressive release of this anion into the donor solution such that a significant contribution to the movement of charge could be deduced for this anion.
Discussion and Conclusions
Electroosmosis
Dex-Phos delivery from the cathode proceeded against electroosmotic flow. The latter increased when the background electrolyte was removed from the drug solution in good agreement with previous reports.13,15
Lowering the pH of the Dex-Phos donor solution to 4 reduced the electroosmotic flow in the anode-to-cathode direction as previously observed.15,33 Consistent with the reduction of electroosmotic flow, the transport number of Na+ was significantly smaller as well, reflecting a change in the skin's permselectivity. However, the lower pH in the donor solution failed to improve drug delivery, suggesting that the altered electroosmotic flow has a negligible effect on the electrotransport of the drug.
Dex-Phos delivery from the anode was really inefficient and, therefore, should be avoided in the clinic. This conclusion contradicts an early study in which the drug was iontophoresed in vivo, in a monkey, from the anode and resulted in measurable levels in deep underlying structures.30 Although the exact reasons for this discrepancy are still unclear, 2 comments on the latter study can be made in that respect: (1) a relatively important drug concentration (>2 μg/g) in subdermal structures (depth >3 mm) was observed after a short application of the drug (20 minutes) in absence of current, which raises the question whether the skin barrier in this animal model was compromised; and (2) the current density used (0.94 mA/cm2) is nearly twice the maximum tolerable level in humans (0.5 mA/cm2)34 and could have altered the skin barrier.
Ionic Competition
Not all commercially available Dex-Phos solutions are equivalent for iontophoresis. The ideal donor solution contains no background electrolyte to compete with the drug to carry a charge across the skin. Trends similar to those observed in this study had been observed, but to a lesser extent due to a much higher passive diffusion contribution, when Dex-Phos was iontophoresed across an artificial membrane.35 The Dex-Phos/lidocaine formulation resulted in a Dex-Phos flux much less than those from all other formulations due to the high levels of chloride and citrate present. Although lidocaine hydrochloride increases the buffer capacity of the donor solution,9 this is not necessary when Ag-AgCl electrodes are used because problems associated with pH changes are avoided.32 In fact, in the experiments reported here, the pH of the donor solution never varied by more than 0.2 pH unit over the 6 hours of iontophoresis.
Even in optimal delivery conditions, the transport number of Dex-Phos was relatively small, with values just over 0.01. This was most likely due to the rather poor mobility of the drug inside the membrane resulting from its relatively large molecular weight. Similarly, the transport numbers of cations were found to fall off rapidly with molecular weight, with values approaching zero at 400 to 500 Da.36 The principal ionic species carrying electrical current during delivery of Dex-Phos from water was the subdermal counterion Na+. Even when citrate was present, the transport number of sodium was >80%. This value is in good agreement with the transport number of sodium previously observed when the competing counterion was gluconate, an anion with a molecular weight very similar to that of citrate.37 Interestingly, the release of Cl− from the Ag-AgCl cathode during iontophoresis of Dex-Phos from water did not significantly affect drug transport over the course of the experiment. Given the amount of Cl− produced (∼67 μmol, corresponding to a concentration of ∼19 mM), it is surprising (and, for the moment, not fully understood) that the Dex-Phos flux did not decrease somewhat after 6 hours of current passage. From a practical standpoint, however, this is a positive result, as an iontophoretic delivery device for Dex-Phos would not require complex characteristics, such as an ion-exchange membrane to sequester Cl−.32
Other Practical Issues
The steady-state flux of Dex-Phos from water (∼70 nmol/h at a current of 0.3 mA, or ∼2 μg/mA·min) translates to the delivery of about 0.16 mg from a typical current “dose” of 80 mA·min. Although this is rather less than that administered via injection (1 mL of a 0.4% weight-to-volume ratio solution contains 4 mg), it is significantly greater than that resulting from iontophoresis of the Dex-Phos formulations containing citrate (∼0.02 mg) and lidocaine (again, ∼0.02 mg), as reported previously.9
In vivo, the current “dose” typically is delivered over 20 minutes, and a steady-state flux is unlikely to be achieved in this time span (in the experiments reported here [data not shown], a lag time of about 30–60 minutes was observed). The kinetics of transport and uptake in vivo are typically more rapid than those seen in vitro due to the presence of a fully functional microcirculation, which can “clear” the drug to the underlying tissue and, ultimately, the systemic circulation. Furthermore, it is known that in vivo uptake and absorption can be influenced significantly by, for example, local vasoconstriction.38
Clinical Implications
The simple in vitro model used in this study cannot perfectly replicate the much more complex in vivo situation in humans. However, porcine skin, even when stored frozen before use, is a good model for human skin,33,39 and the trends observed here also may be expected in vivo. To optimize Dex-Phos delivery, the drug must be delivered from the cathode, and the formulation should exclude, as far as possible, the presence of competing co-ions. If commercially available Dex-Phos solutions are used, preference should be given to those that contain the least amounts of anions such as chloride and citrate. Ultimately, well-designed in vivo studies will be necessary to demonstrate the efficiency of iontophoresis to deliver clinically relevant corticosteroid doses in soft tissues, and the results presented here should be of particular importance for their design.
All authors provided concept/idea/research design and writing. Dr Sylvestre provided data collection and analysis. Dr Guy and Dr Delgado-Charro provided project management. Dr Guy provided fund procurement.
This research was presented at the Gordon Research Conference on Barrier Function of Mammalian Skin; August 7–12, 2005; South Hadley, Massachusetts.
This research was supported by the National Institutes of Health (EB–001420). Injectable Dex-Phos 0.4% (American Regent) was kindly provided by the Nicholas Institute of Sports Medicine and Athletic Trauma at Lenox Hill Hospital, New York, NY. Dr Sylvestre thanks Bourses Internationalistes J. Armand Bombardier, Natural Sciences and Engineering Research Council of Canada, Fonds Québéquois de la Recherche sur la Nature et les Technologies, and Universities UK for funding.
Sigma-Aldrich Company Ltd, The Old Brickyard, New Road, Gillingham, Dorset, SP8 4XT, United Kingdom.
American Regent Inc, One Luitpold Dr, PO Box 9001, Shirley, NY 11967.
Mayne Pharma PLC, Queensway, Royal Leamington Spa, Warwickshire, CV31 3RW, United Kingdom.
Organon Laboratories Ltd, Cambridge Science Park, Milton Road, Cambridge, CB4 0FL, United Kingdom.
AstraZeneca PLC, 15 Stanhope Gate, London, W1K 1LN, United Kingdom.
Acros Organics, Geel West Zone 2, Janssen Pharmaceuticalaan 3a, B-2440 Geel, Belgium.
Fisher Scientific UK Ltd, Bishop Meadow Rd, Loughborough, Leicestershire, LE11 5RG, United Kingdom.
Fluka, Industriestrasse 25, 9471 Buschs, Switzerland.
Barnstead International, 2555 Kerper Blvd, Dubuque, IA 52001-1478.
Zimmer Orthopaedic Surgical Products, PO Box 10, 200 West Ohio Ave, Dover, OH 44622.
Yokogawa Measurement Technologies Ltd, Solar House, Mercury Park, Wycomber Lane, Woodburn Green, Bucks, HP10 0HH, United Kingdom.
Dionex (UK) Ltd, 4 Albany Ct, Camberley, GU16 7QL, United Kingdom.
Hichrom, 1 The Markham Centre, Station Road, Theale, Barkshire, RG7 4PE, United Kingdom.
GraphPad Software, 11452 El Camino Real, #215, San Diego, CA 92130.
References
- 1.Harmon KG, Hawley C. Physician prescribing patterns of oral corticosteroids for musculoskeletal injuries. J Am Board Fam Pract. 2003;16:209–212. [DOI] [PubMed] [Google Scholar]
- 2.Cole BJ, Schumacher HR Jr. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13:37–46. [DOI] [PubMed] [Google Scholar]
- 3.Ines LP, da Silva JA. Soft tissue injections. Best Pract Res Clin Rheumatol. 2005;19:503–527. [DOI] [PubMed] [Google Scholar]
- 4.Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59:1178–1186. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher HR, Chen LX. Injectable corticosteroids in treatment of arthritis of the knee. Am J Med. 2005;118:1208–1214. [DOI] [PubMed] [Google Scholar]
- 6.Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Ann Fam Med. 2004;2:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann H, Hodges M, Evans B. Effectiveness of iontophoresis of anti-inflammatory medications in the treatment of common musculoskeletal inflammatory conditions: a systematic review. Phys Ther Rev. 2006;11:190–194. [Google Scholar]
- 8.Banga AK, Panus PC. Clinical applications of iontophoretic devices in rehabilitation medicine. Critical Reviews in Physical and Rehabilitation Medicine. 1998;10:147–179. [Google Scholar]
- 9.Petelenz TJ, Buttke JA, Bonds C, et al. Iontophoresis of dexamethasone: laboratory studies. J Control Release. 1992;20:55–66. [Google Scholar]
- 10.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619–658. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Charro MB, Guy RH. Transdermal iontophoresis for controlled drug delivery and non-invasive monitoring. S.T.P. Pharma Sciences. 2001;11:403–414. [Google Scholar]
- 12.Phipps JB, Gyory JR. Transdermal ion migration. Adv Drug Deliv Rev. 1992;9:137–176. [Google Scholar]
- 13.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 2001;46:281–305. [DOI] [PubMed] [Google Scholar]
- 14.Pikal MJ, Shah S. Transport mechanisms in iontophoresis, III: an experimental study of the contributions of electroosmotic flow and permeability change in transport of low and high molecular weight solutes. Pharm Res. 1990;7:222–229. [DOI] [PubMed] [Google Scholar]
- 15.Santi P, Guy RH. Reverse iontophoresis: parameters determining electroosmotic flow, I: pH and ionic strength. J Control Release. 1996;38:159–165. [Google Scholar]
- 16.Santi P, Guy RH. Reverse iontophoresis: parameters determining electro-osmotic flow, II: electrode chamber formulation. J Control Release. 1996;42:29–36. [Google Scholar]
- 17.Harris PR. Iontophoresis: clinical research in musculoskeletal inflammatory conditions. J Orthop Sports Phys Ther. 1982;4:109–112. [DOI] [PubMed] [Google Scholar]
- 18.Bertolucci LE. Introduction of anti-inflammatory drugs by iontophoresis: double blind study. J Orthop Sports Phys Ther. 1982;4:103–108. [DOI] [PubMed] [Google Scholar]
- 19.Pellecchia GL, Hamel H, Behnke P. Treatment of infrapatellar tendinitis: a combination of modalities and transverse friction massage versus iontophoresis. J Sport Rehabil. 1994;3:135–145. [Google Scholar]
- 20.Banta CA. A prospective, nonrandomized study of iontophoresis, wrist splinting, and anti-inflammatory medication in the treatment of early-mild carpal tunnel syndrome. J Occup Environ Med. 1994;36:166–173. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman EL, Braun BL, Lindgren BR. Temporomandibular joint iontophoresis: a double-blind randomized clinical trial. J Orofac Pain. 1996;10:157–165. [PubMed] [Google Scholar]
- 22.Hasson SM, Wible CL, Reich M, et al. Dexamethasone iontophoresis: effect on delayed muscle soreness and muscle function. Can J Sport Sci. 1992;17:8–13. [PubMed] [Google Scholar]
- 23.Neeter C, Thomee R, Silbernagel KG, et al. Iontophoresis with or without dexamethazone in the treatment of acute Achilles tendon pain. Scand J Med Sci Sports. 2003;13:376–382. [DOI] [PubMed] [Google Scholar]
- 24.Nirschl RP, Rodin DM, Ochiai DH, Maartmann-Moe C. Iontophoretic administration of dexamethasone sodium phosphate for acute epicondylitis: a randomized, double-blinded, placebo-controlled study. Am J Sports Med. 2003;31:189–195. [DOI] [PubMed] [Google Scholar]
- 25.Gokoglu F, Findikoglu G, Yorgancioglu ZR, et al. Evaluation of iontophoresis and local corticosteroid injection in the treatment of carpal tunnel syndrome. Am J Phys Med. 2005;84:92–96. [DOI] [PubMed] [Google Scholar]
- 26.Li LC, Scudds RA, Heck CS, Harth M. The efficacy of dexamethasone iontophoresis for the treatment of rheumatoid arthritic knees: a pilot study. Arthritis Care Res. 1996;9:126–132. [DOI] [PubMed] [Google Scholar]
- 27.Gudeman SD, Eisele SA, Heidt RS, et al. Treatment of plantar fasciitis by iontophoresis of 0.4% dexamethasone: a randomized, double-blind, placebo-controlled study. Am J Sports Med. 1997;25:312–316. [DOI] [PubMed] [Google Scholar]
- 28.Reid KI, Dionne RA, Sicard-Rosenbaum L, et al. Evaluation of iontophoretically applied dexamethasone for painful pathologic temporomandibular joints. Oral Surg Oral Med Oral Pathol. 1994;77:605–609. [DOI] [PubMed] [Google Scholar]
- 29.Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow): a double-blind study. Scand J Med Sci Sports. 2002;12:136–142. [DOI] [PubMed] [Google Scholar]
- 30.Glass JM, Stephen RL, Jacobson SC. The quantity and distribution of radiolabeled dexamethasone delivered to tissue by iontophoresis. Int J Dermatol. 1980;19:519–525. [DOI] [PubMed] [Google Scholar]
- 31.Marro D, Kalia YN, Delgado-Charro MB, Guy RH. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm Res. 2001;18:1701–1708. [DOI] [PubMed] [Google Scholar]
- 32.Scott ER, Phipps B, Gyory R, Padmanabhan RV. Electrotransport system for transdermal delivery: a practical implementation of iontophoresis. In: Wise DL, ed. Handbook of Pharmaceutical Controlled Release Technology. New York, NY: Marcel Dekker Inc; 2000:617–659.
- 33.Marro D, Guy RH, Delgado-Charro MB. Characterization of the iontophoretic permselectivity properties of human and pig skin. J Control Release. 2001;70:213–217. [DOI] [PubMed] [Google Scholar]
- 34.Ledger PW. Skin biological issues in electrically enhanced transdermal delivery. Adv Drug Deliv Rev. 1992;9:289–307. [Google Scholar]
- 35.Anderson CR, Boeh SD, Morris RL, et al. Quantification of total dexamethasone phosphate delivery by iontophoresis. International Journal of Pharmaceutical Compounding. 2003;7:155–159. [PubMed] [Google Scholar]
- 36.Mudry B, Carrupt PA, Guy RH, Delgado-Charro MB. Quantitative structure-permeation relationship for iontophoretic transport across the skin. J Control Release. 2007;122:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mudry B, Guy RH, Delgado-Charro MB. Electromigration of ions across the skin: determination and prediction of transport numbers. J Pharm Sci. 2006;95:561–569. [DOI] [PubMed] [Google Scholar]
- 38.Roberts MS, Lai PM, Cross SE, Yoshida NH. Solute structure as a determinant of iontophoresic transport. In: Potts RO, Guy RH, eds. Mechanisms of Transdermal Drug Delivery. New York, NY: Marcel Dekker Inc; 1997:291–349.
- 39.Mudry B, Guy RH, Delgado-Charro MB. Prediction of iontophoretic transport across the skin. J Control Release. 2006;111:362–367. [DOI] [PubMed] [Google Scholar]