Abstract
Background: Assessment of peak oxygen consumption (V̇o2peak) using traditional modes of testing such as treadmill or cycle ergometer can be difficult in individuals with stroke due to balance deficits, gait impairments, or decreased coordination.
Objective: The purpose of this study was to quantitatively assess the validity and feasibility of a modified exercise test using a total-body recumbent stepper (mTBRS-XT) in individuals after stroke.
Design: A within-subject design, with a sample of convenience, was used.
Participants. Eleven participants (7 male, 4 female) with a mean of 40.1 months (SD=32.7) after stroke, a mean age of 60.9 years (SD=12.0), and mild to severe lower-extremity Fugl-Myer test scores (range=13–34) completed the study.
Methods: Participants performed 2 maximal-effort graded exercise tests on separate days using the mTBRS-XT and a cycle ergometer exercise protocol to assess cardiorespiratory fitness. Measurements of V̇o2peak and peak heart rate (peak HR) were obtained during both tests.
Results: A strong relationship existed between the mTBRS-XT and the cycle ergometer exercise test for V̇o2peak and peak HR (r=.91 and .89, respectively). Mean V̇O2peak was significantly higher for the mTBRS-XT (16.6 mL×kg−1×min−1[SD=4.5]) compared with the cycle ergometer exercise protocol (15.4 mL×kg−1×min−1 [SD=4.5]). All participants performed the mTBRS-XT. One individual with severe stroke was unable to pedal the cycle ergometer. No significant adverse events occurred.
Conclusion: The mTBRS-XT may be a safe, feasible, and valid exercise test to obtain measurements of V̇o2peak in people with stroke. Health care professionals may use the mTBRS-XT to prescribe aerobic exercise based on V̇o2peak values for individuals with mild to severe deficits after stroke.
Maximal-effort exercise testing is performed to assess cardiorespiratory fitness. Information gathered from an exercise test, such as measurements of oxygen uptake (V̇o2), heart rate, and blood pressure, is used to guide exercise prescription. Although safe and effective exercise guidelines are available,1,2 fundamental questions regarding appropriate exercise test protocol selection and prescription for people after stroke remain unclear.3–5 Although numerous protocols are available for the general population,6 these protocols may not be feasible for people after stroke due to balance deficits, poor postural control, or incoordination of the hemiparetic limb during cycling or ambulation. Therefore, in an attempt to improve exercise test performance in people after stroke, modifications have been made to these protocols or modalities.
For treadmill testing, the use of 15% of body-weight support7 aids the individual with stroke in maintaining balance, whereas other treadmill protocols use lower incremental ambulating speeds without concomitant changes in incline.8,9 Several researchers10–14 have chosen the cycle ergometer as the preferred modality for exercise testing after stroke. However, to accommodate motor and postural deficits, modifications to the cycle ergometer such as using a seat with trunk support14 or securing the hemiparetic limb to the pedals3,11,14 have been used.
Despite modifications to the cycle ergometer, people with stroke may experience difficulty during exercise testing. Yates et al11 reported that 11 of the 14 subjects excluded from their study could not perform the cyclical motion of the cycle ergometer. In order to minimize the difficulties associated with exercise testing using a cycle ergometer, Tang and colleagues3 chose a semirecumbent cycle ergometer. This device provided posterior trunk support, and, if needed, the hemiparetic limb was secured to the pedal. Only 1 subject in their study was unable to complete the exercise test because the affected limb would not stay on the pedal.
One study incorporated both upper and lower extremities during exercise testing after stroke. Hill et al15 postulated that an all-extremity exercise protocol would be beneficial to decrease early onset of limb fatigue in the lower extremities. Use of this modality allowed the participants to remain in their wheelchair while their hands grasped the hand bars and feet were placed in the foot pedals of the ergometer. The authors stated that use of the combined upper- and lower-extremity cycle ergometer provided a better estimate of cardiorespiratory fitness secondary to incorporating more muscle mass, which may elicit a higher V̇o2peak in people after stroke.
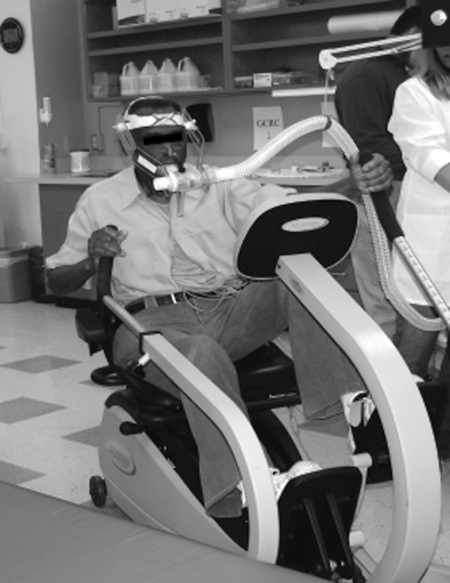
Individuals after stroke can experience decreased trunk control, impaired sitting or standing balance, and lower-extremity incoordination, making traditional exercise testing modalities inappropriate Supplemental Video.2 These deficits in motor function can affect exercise performance and may compromise maximal-effort testing, as evidenced by previous studies.5,11 Noting these challenges, the American College of Sports Medicine recommends the recumbent seated stepper (Fig. 1) for both exercise testing and training in individuals after stroke.2 This modality accommodates many common motor function deficits by providing trunk and distal limb support.2 In addition, the total-body recumbent stepper (TBRS) uses bilateral reciprocal movement of the arm coupled with the opposite leg, which allows for a push-and-pull motion.16
Figure 1.
Participant performing a maximal-effort exercise test using the Modified Total-Body Recumbent Stepper Exercise Test.
In consideration of these recommendations, we sought to establish a valid exercise testing protocol for individuals after stroke using a TBRS. In previous work, we developed an exercise test protocol using the TBRS for adults who are healthy.17 In order to assess the validity and reliability of data obtained using the TBRS exercise test (TBRS-XT), measurements of peak oxygen consumption (V̇o2peak) obtained using the TBRS-XT were compared with measurements obtained using the Bruce protocol. Our results suggested that the TBRS-XT is a valid and reliable exercise test for obtaining measurements of V̇o2peak in people who are healthy. Therefore, the purpose of the current study was to assess the validity and feasibility of a modified exercise test using a total-body recumbent stepper (mTBRS-XT) in individuals after stroke. We anticipated that: (1) a high correlation would be observed between V̇o2peak using the mTBRS-XT and V̇o2peak using the cycle ergometer and (2) V̇o2peak using the mTBRS-XT would be significantly higher than V̇o2peak using the cycle ergometer. A secondary goal was to assess whether the maximal-effort exercise tests would result in whole-body fatigue or only leg fatigue.
Method
Participants
Thirteen people were recruited to participate in this study. Data from 2 individuals were excluded from the study. One individual could not perform the cyclical movement of the cycle ergometer, and another individual demonstrated ST-segment elevation greater than 1 mm, which is an absolute indication for exercise test termination (n=1).6 Eleven participants (7 male, 4 female) with a mean age of 60.9 years (SD=12.0) completed this within-subject design study. Participant demographics are presented in Table 1.
Table 1.
Participant Demographics (N=11)
Inclusion criteria were: (1) ability to transfer from a sitting position to a standing position, (2) ability to walk 9.1 m (30 ft) independently with or without an orthotic or assistive device, and (3) a score of ≥24 on the Mini Mental Status Exam to screen for dementia and establish the ability to give informed consent for the study. Exclusion criteria consisted of the following: (1) hospitalization for myocardial infarction, heart surgery, or congestive heart failure during the preceding 3 months; (2) significant cardiac arrhythmia, hypertrophic cardiomyopathy, severe aortic stenosis, or pulmonary embolus; (3) recent symptoms of chest discomfort; (4) currently smoking or significant pulmonary pathology; and (5) musculoskeletal problems from conditions other than stroke that would limit the ability to exercise. Because the TBRS is a novel modality for assessing cardiorespiratory fitness in people after stroke, we had no preliminary data on which to base a power analysis. Therefore, a precision argument was used to justify the sample size for this study. Based on the early data (n=5), the standard error of the mean decreased drastically up to 10 participants, beyond which there is a diminishing of return. This project set out to recruit 15 participants to buffer against dropout.
Institutionally approved informed consent was obtained in writing prior to participation in the study. Stroke severity was assessed using the lower-extremity section of the Fugl-Meyer test (LEFM).18,19 Data collection for the exercise tests was performed at the general clinical research center of a medical center.
Exercise Testing
Participants performed 2 maximal-effort graded exercise tests in random-order assignment. The exercise testing sessions were separated by at least 48 hours, but no more than 7 days, and were controlled for time of day. Participants were instructed not to consume food, caffeine, or alcohol for 3 hours prior to exercise testing,6 but they were allowed to hydrate with water ad libitum. On the day of testing, participants were familiarized with each testing protocol prior to the exercise test. The testing administrator explained the Borg Rating of Perceived Exertion (RPE) Scale. Because the upper extremities were used during the mTBRS-XT, participants were instructed to nod “yes” or “no” when the test administrator asked, “Are you working harder than” the specific number on the Borg RPE Scale. The same procedure was used for the cycle ergometer exercise test. In addition, for individuals with type 2 diabetes, blood glucose levels were assessed. If blood glucose levels were less than 70 mg×dL−1 or higher than 300 mg×dL−1, the exercise test was rescheduled for another day.
The maximal-effort cycle tests were performed on a cycle ergometer* and the TBRS.† A ParvoMedics TrueOne 2400 metabolic cart‡ was used to continuously collect and analyze expired gases using a 2-way rebreather valve.§ The metabolic cart produced a 30-second average of the data collected for the sampling technique during all exercise tests. Prior to testing, all equipment was calibrated according to the manufacturers’ recommendations.
Baseline measurements for height, weight, heart rate, and blood pressure were obtained prior to exercise testing. An exercise physiologist and a physician continuously monitored heart rate and rhythm with a 12-lead electrocardiogram throughout each exercise testing session. Thirty seconds prior to the end of each stage, blood pressure was taken and recorded, and participants rated their perceived exertion using the Borg RPE Scale (6=no exertion at all, 20=maximal exertion).
Cycle Ergometer Exercise Test
Participants were given physical assistance if they were unable to independently transfer onto the cycle ergometer. Once the participant was seated on the cycle, seat height was adjusted to allow for a slight bend in the knee while avoiding full knee extension during pedaling. If the individual experienced difficulty keeping the hemiparetic limb on the pedal, the foot was secured on the pedal with an elastic wrap. Participants were instructed to pedal at 60 rpm for the duration of the test, and verbal encouragement was given if cadence dropped below 55 rpm. The resistance was set at 0 W for the first 3 minutes of the exercise test and then was increased by 10 W×min−1 until test termination.11 The exercise test was terminated using the following criteria: (1) the participant reached volitional fatigue and requested to end the test, (2) the participant's V̇o2peak plateaued or decreased despite continuation of exercise, (3) the participant was unable to maintain the cadence, or (4) an adverse cardiovascular event or response to the exercise test was observed.
Once the exercise test was completed, participants were asked to verbally report their reason for terminating the exercise test. They were asked to identify 1 of 2 choices: (1) generalized fatigue/whole-body fatigue or (2) limb fatigue.
mTBRS-XT
Once the participant was seated in the TBRS, necessary adjustments were made for leg and arm length according to methods used by Billinger and colleagues.17 The protocol stages lasted 2 minutes, with concomitant increases in resistance until the test was terminated (Tab. 2). Although the stepping cadence was modified from the original TBRS-XT protocol to 80 steps×min−1, exercise test progression was identical to that of our previous work.17 If participants were unable to consistently maintain a stepping cadence at 80 steps×min−1 with verbal cues and encouragement for participation, the test was terminated. Test termination criteria for the mTBRS-XT were the same as those for the cycle ergometer protocol. Once the mTBRS-XT was completed, participants were asked for their reason for terminating the exercise test, with the same choices as for the cycle ergometer test.
Table 2.
Modified Total-Body Recumbent Stepper Exercise Test (mTBRS-XT) Protocola
Loads 2 and 3 were omitted from the mTBRS-XT, which is identical to the original TBRS-XT17 protocol.
Data Analysis
The Pearson correlation coefficient was used to examine the relationship between the cycle ergometer exercise test and the mTBRS-XT for assessing V̇o2peak and peak heart rate. Because the mTBRS-XT uses both the upper and lower extremities during the exercise test and based on the article by Hill and colleagues,15 we hypothesized that V̇o2peak measured during the mTBRS-XT would be higher than V̇o2peak measured during the cycle ergometer exercise test. For physiological data (Tab. 3), a 1-tailed paired Student t test was conducted to determine whether a significant difference existed between the cycle ergometer exercise test and the mTBRS-XT. Statistical significance was set at alpha ≤.05. SPSS 15.0‖ was the software used for all data analyses.
Table 3.
Results From Maximal-Effort Graded Exercise Test Performance Using the Modified Total-Body Recumbent Stepper Exercise Test (mTBRS-XT) and Cycle Ergometer Exercise Testa
Data are means (SD). V̇o2=oxygen uptake, V̇o2peak=peak oxygen uptake, RPE=rating of perceived exertion, RER=respiratory exchange ratio, SBP=systolic blood pressure, DBP=diastolic blood pressure. Data analysis was performed using a one-tailed paired t test. Asterisks denote significant differences, where P<.05.
Results
Mean physiological values from the maximal-effort graded exercise tests are presented in Table 3. At test termination (maximal effort), mean physiological values were higher using the mTBRS-XT than the cycle ergometer for V̇o2peak (16.6 vs 15.4 mL×kg−1×min−1), peak heart rate (132.9 vs 130.7 bpm), and peak systolic blood pressure (144 vs 139 mm Hg). The correlation coefficient between the cycle ergometer exercise test and the mTBRS-XT for V̇o2peak (r=.91, P<.001; Fig. 2) and peak heart rate (r=.89, P<.001) indicated a strong relationship between the 2 tests for assessing cardiorespiratory fitness after stroke. A paired t test showed a statistically significant difference (P=.04) between the cycle ergometer exercise test and the mTBRS-XT for mean V̇o2peak (mL×kg−1×min−1) (Tab. 3). Seventy-three percent of the participants (8 of 11) reached 80% of maximal age-predicted heart rate (APHRmax) using the mTBRS-XT, and 7 of 11 participants reached 80% of APHRmax using the cycle ergometer exercise test. Forty-five percent of the participants (5 of 11) using the mTBRS-XT reached 90% of APHRmax, and 3 of 11 participants met the criteria for 90% of ARHRmax using the cycle ergometer exercise test. In this study, one individual had an ischemic ST-segment change on the electrocardiogram. The physician terminated the exercise test prior to peak effort. During cool-down, the ST-segment returned to normal. During rest, this individual reported that he did not want to mention to the staff that he had not taken his medication at his usual time before the exercise test. No other adverse events occurred during exercise testing for either test.
Figure 2.
Relationship between the Modified Total-Body Recumbent Stepper Exercise Test (mTBRS-XT) and the cycle ergometer exercise test for assessing peak oxygen uptake (V̇o2peak) (mL×kg−1×min−1), expressed as a solid line. Dashed line represents identity line.
Eight of 11 participants reported generalized/whole-body fatigue, whereas only 3 participants stated leg fatigue was the reason for test termination using the TBRS. Results for the cycle ergometer exercise test were reversed. Leg fatigue on the cycle ergometer was the reason for test termination for 8 of the 11 participants, whereas 3 participants reported generalized/whole-body fatigue. Baseline V̇o2 prior to starting the exercise test was higher for the cycle ergometer exercise test than the TBRS (Tab. 3).
Discussion
Individuals with stroke experience many challenges to maximal-effort exercise testing, including impaired sitting balance, inability to mount the cycle ergometer, and impaired coordination during the cyclical motion. We used a testing modality (the TBRS) that accommodated these physical challenges. All individuals enrolled in this study were able to perform the mTBRS-XT despite their vast motor impairment differences (mild to severe stroke, LEFM scores=13–34). This study provides important information to researchers and clinicians examining cardiorespiratory fitness in people with chronic stroke. Importantly, using this modality and exercise test allowed participants with stroke to engage in a maximal-effort exercise test and eliminated the need for numerous test protocols based on motor performance.10,20
The results from this study suggest that the mTBRS-XT is a valid, feasible, and safe exercise test protocol to obtain measurements of V̇o2peak in people with chronic stroke. We found the mTBRS-XT to have a strong relationship with the cycle ergometer exercise test for assessing V̇o2peak (r=.91). Although the cycle ergometer is a modality that is extensively used for exercise testing in people after stroke, the TBRS is a feasible alternative for assessing cardiorespiratory fitness. In addition, significantly higher (P=.04) group V̇o2peak values were found with both the mTBRS-XT and the cycle ergometer exercise test. Our results agree with those of Hill and colleagues,15 who concluded that the combined efforts of the upper and lower extremities may increase exercise capacity.
As evidenced in the current literature, numerous exercise test protocols for the cycle ergometer have been used for people after stroke. For example, in a recent study, Pang and colleagues10 used 2 different cycle protocols that varied in initial workload and intensity progression secondary to varying levels of motor performance. Potempa et al13 and Kelly et al20 chose a cycle protocol that started at 50 rpm at 0 W and then increased by 10 W×min−1 until volitional fatigue. Yates et al11 chose a cycle protocol that started at 60 rpm at 0 W for 3 minutes and then progressed at 10 W×min−1 until test termination. Chu et al21 chose a cycle protocol that began at a pedaling cadence between 50 and 70 rpm with a workload at 20 W×min−1 but then increased the resistance by 20 W×min−1 throughout the exercise test, whereas Eng and colleagues5 had their participants pedal at a rate of 50 to 70 rpm but began at 0 W and then progressed at 20 W×min−1 until test termination. As demonstrated by the variety of cycle protocols used for people after stroke, interpretation of exercise test results can be difficult for both researchers and clinicians. We addressed this issue by modifying an existing protocol (TBRS-XT) that demonstrated reliability and validity in subjects who were healthy17 while using the identical exercise test progression for people with stroke. In doing so, we eliminate uncertainty and maintain consistency when using the TBRS for exercise testing.
Eng and colleagues5 suggested the need for alternative exercise testing methods for people after stroke because of the challenges associated with cycle ergometry. In their study, 27 individuals met the initial screening criteria. Of the 15 individuals excluded from the study, 10 with severe stroke were unable to pedal the cycle ergometer due to decreased coordination, muscle weakness, or an inability to maintain appropriate leg position. The reasons for eliminating individuals from exercise testing participation were identical to those in the study by Yates and colleagues,11 where 21% of the individuals excluded from their study could not perform the cycle ergometer exercise test. Contrary to the studies by Eng et al5 and Yates et al,11 the results of our study demonstrate that people with varying levels of lower-extremity function (LEFM scores=13–34) after stroke can participate in the mTBRS-XT to assess cardiorespiratory fitness. In our study, all participants were able to perform the maximal-effort graded exercise test using the TBRS without difficulty because of balance deficits or motor impairments of the lower extremities. In addition, one participant with an LEFM score of less than 19, which indicates a severe stroke,19 was unable to pedal the cycle ergometer to perform the exercise test but was able to use the TBRS.
We found that 8 of 11 participants using the mTBRS-XT reached a peak heart rate expressed at 80% of their APHRmax and that 5 of 11 participants reached a peak heart rate at 90% of APHRmax. Our results for APHRmax are higher than those previously reported for a seated modality.11,15,20,22 Nine individuals were taking beta-blockers, which can lower maximal heart rate during an exercise test. Because we did not have access to information on these individuals prior to beta-blocker therapy, it is difficult to gauge the true effect of the beta-blocker on maximal heart rate for each individual. Interestingly, a previous study showed that individuals who were receiving beta-blocker therapy increased their maximal heart rate by 36% after an exercise intervention.23 Although not directly assessed in the study by Vanhees and colleagues,23 participants in their study reached 80% of APHRmax during a graded exercise test. Therefore, it is plausible that the participants in our study were able to reach this heart rate intensity despite the influence of beta-blockers. It is reasonable to suggest that a combined upper- and lower-extremity modality such as the TBRS may be a feasible and accurate method for assessing cardiorespiratory fitness and guiding exercise prescription in people with chronic stroke.
Another potential benefit incorporating bilateral upper- and lower-extremity involvement may be a reduction in leg fatigue, thus eliminating early test termination by participants. Anecdotally, individuals after stroke who use the combined efforts of the upper and lower extremities during a maximal-effort exercise test report less leg fatigue than when a cycle ergometer is used.15,20 Results from our study indicated that 8 of 11 participants reported generalized fatigue, whereas leg fatigue was the primary reason for test termination in 3 of 11 individuals using the TBRS. However, 8 of 11 participants reported leg fatigue on the cycle ergometer versus 3 of 11 participants who reported generalized fatigue, which may limit exercise test performance. One interesting and unexpected finding from this study was that mean baseline V̇o2 values were higher for the cycle ergometer than for the TBRS. One potential explanation may be related to the difficulty some individuals experienced mounting the cycle ergometer. Increased energy expenditure (V̇o2) was observed prior to the start of the exercise test for the cycle ergometer, which could have affected exercise test performance.
Although we believe the present study included a wide variety of functional performance levels, the sample size (N=11) was small and may limit the ability to generalize the results to people with stroke. One disadvantage of the TBRS is that blood pressure measurements are difficult to obtain because all extremities are simultaneously moving during the exercise test. We had the participants release their grasp on one of the arm poles and relax the arm at their side during blood pressure measurements. Maintaining a constant stepping cadence of 80 steps×min−1 during the mTBRS-XT was difficult for some individuals and required verbal cues to maintain the appropriate stepping rate. Finally, future research studies comparing cardiorespiratory fitness values between the mTBRS-XT and a protocol using a combined arm and leg ergometer15 may be advantageous because both modalities use all extremities.
Conclusion
The results of this study suggest that the mTBRS-XT is a valid, feasible, and safe exercise test protocol to assess cardiorespiratory fitness for most individuals after stroke. In addition, lower LEFM scores did not exclude individuals after stroke from participating in the mTBRS-XT. This is important because individuals after stroke with various levels of lower-extremity performance should be able to participate in exercise training programs and gain the cardiorespiratory benefits associated with aerobic exercise training.
Although the cycle ergometer is a frequently used modality for exercise testing after stroke, the mTBRS-XT demonstrated higher V̇o2peak values than the cycle ergometer. The physiological values from the mTBRS-XT may be more representative of cardiorespiratory fitness because all extremities are engaged during the exercise test. The results from exercise tests using this protocol may be used to prescribe intensity of exercise training in individuals after stroke.
Supplementary Material
Ms Billinger provided concept/idea/research design and writing. Ms Billinger and Mr Tseng provided data collection and analysis. Ms Billinger and Dr Kluding provided project management. Mr Tseng and Dr Kluding provided subjects. Dr Kluding provided fund procurement, facilities/equipment, and consultation (including review of manuscript before submission). The authors thank Kayla Schippers for her assistance with database management.
This study was approved by the Institutional Review Board at the University of Kansas Medical Center.
This research was presented at the regional conference of the Central States Chapter of the American College of Sports Medicine; October 20–21, 2006; Kansas City, Missouri; and the Combined Sections Meeting of the American Physical Therapy Association; February 14–18, 2007; Boston, Massachusetts.
This research was funded by National Institute of Disability and Rehabilitation Research grant H133F050006 and supported, in part, by University of Kansas Medical Center General Clinical Research Center grant M01 RR 023940, National Center for Research Resources/National Institutes of Health.
Lode BV, Zernikepark 16, 9747 AN Groningen, the Netherlands.
NuStep Inc, 51111 Venture Dr, Ann Arbor, MI 48108.
Parvomedics, 8152 South 1715 East, Sandy, UT 84093.
Hans Rudolph Inc, 7200 Wyandotte St, Kansas City, MO 64114.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109:2031–2041. [DOI] [PubMed] [Google Scholar]
- 2.Durstine JL, Moore GE, eds. ACSM's Exercise Management for Persons With Chronic Diseases and Disabilities. 2nd ed. Champaign, IL: Human Kinetics Inc; 2003.
- 3.Tang A, Sibley KM, Thomas SG, et al. Maximal exercise test results in subacute stroke. Arch Phys Med Rehabil. 2006;87:1100–1105. [DOI] [PubMed] [Google Scholar]
- 4.Arsura E. Evaluating cardiorespiratory fitness after stroke: does the best provide less? Chest. 2005;127:1473–1474. [DOI] [PubMed] [Google Scholar]
- 5.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- 7.Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697–1702. [DOI] [PubMed] [Google Scholar]
- 8.Macko RF, Katzel LI, Yataco A, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke. 1997;28:988–992. [DOI] [PubMed] [Google Scholar]
- 9.Macko RF, Smith GV, Dobrovolny CL, et al. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. [DOI] [PubMed] [Google Scholar]
- 10.Pang MY, Eng JJ, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke: influence of stroke-specific impairments. Chest. 2005;127:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates JS, Studenski S, Gollub S, et al. Bicycle ergometry in subacute-stroke survivors: feasibility, safety, and exercise performance. J Aging Phys Act. 2004;12:64–74. [DOI] [PubMed] [Google Scholar]
- 12.Rimmer JH, Braunschweig C, Silverman K, et al. Effects of a short-term health promotion intervention for a predominantly African-American group of stroke survivors. Am J Prev Med. 2000;18:332–338. [DOI] [PubMed] [Google Scholar]
- 13.Potempa K, Lopez M, Braun LT, et al. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–105. [DOI] [PubMed] [Google Scholar]
- 14.Chen HY, Chen SC, Chen JJ, et al. Kinesiological and kinematical analysis for stroke subjects with asymmetrical cycling movement patterns. J Electromyogr Kinesiol. 2005;15:587–595. [DOI] [PubMed] [Google Scholar]
- 15.Hill DC, Ethans KD, MacLeod DA, et al. Exercise stress testing in subacute stroke patients using a combined upper- and lower-limb ergometer. Arch Phys Med Rehabil. 2005;86:1860–1866. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn ME, Connelly DM, Overend TJ, Petrella RJ. Validity of values for metabolic equivalents of task during submaximal all-extremity exercise and reliability of exercise responses in frail older adults. Phys Ther. 2008;88:747–756. [DOI] [PubMed] [Google Scholar]
- 17.Billinger SA, Loudon JK, Gajewski BJ. Validity of a Total body recumbent stepper exercise test (TBRS-XT) to assess cardiorespiratory fitness. J Strength Cond Res. In press. [DOI] [PubMed]
- 18.Duncan PW, Goldstein LB, Matchar D, et al. Measurement of motor recovery after stroke: outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. [DOI] [PubMed] [Google Scholar]
- 19.Daly JJ, Roenigk K, Holcomb J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–178. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JO, Kilbreath SL, Davis GM, et al. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil. 2003;84:1780–1785. [DOI] [PubMed] [Google Scholar]
- 21.Chu KS, Eng JJ, Dawson AS, et al. Water-based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moldover JR, Daum MC, Downey JA. Cardiac stress testing of hemiparetic patients with a supine bicycle ergometer: preliminary study. Arch Phys Med Rehabil. 1984;65:470–473. [PubMed] [Google Scholar]
- 23.Vanhees L, Fagard R, Amery A. Influence of beta adrenergic blockade on effects of physical training in patients with ischaemic heart disease. Br Heart J. 1982;48:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.