Abstract
GLUT8 is a class 3 sugar transport facilitator which is predominantly expressed in testis and also detected in brain, heart, skeletal muscle, adipose tissue, adrenal gland, and liver. Since its physiological function in these tissues is unknown, we generated a Slc2a8 null mouse and characterized its phenotype. Slc2a8 knockout mice appeared healthy and exhibited normal growth, body weight development and glycemic control, indicating that GLUT8 does not play a significant role for maintenance of whole body glucose homeostasis. However, analysis of the offspring distribution of heterozygous mating indicated a lower number of Slc2a8 knockout offspring (30.5:47.3:22.1%, Slc2a8+/+, Slc2a8+/−, and Slc2a8−/− mice, respectively) resulting in a deviation (p = 0.0024) from the expected Mendelian distribution. This difference was associated with lower ATP levels, a reduced mitochondrial membrane potential and a significant reduction of sperm motility of the Slc2a8 knockout in comparison to wild-type spermatozoa. In contrast, number and survival rate of spermatozoa were not altered. These data indicate that GLUT8 plays an important role in the energy metabolism of sperm cells.
Keywords: GLUT8, glucose transport, sperm motility, mitochondrial membrane potential
Introduction
Facilitated diffusion of glucose into mammalian cells along a concentration gradient is catalyzed by specific carriers (GLUT proteins). The GLUT family comprises 14 members and, according to sequence similarities, can be divided into three classes (Joost & Thorens 2001). GLUT8 belongs to the class 3 transporters and is an intrinsic membrane protein that catalyzes the facilitative transport of glucose and probably also fructose and galactose (Doege et al. 2000, Ibberson et al. 2000, Joost & Thorens 2001). A unique feature of class 3 transporters is the presence of endosomal targeting motifs that dictate their intracellular retention (Lisinski et al. 2001, Augustin et al. 2005, Schmidt et al. 2006). So far, translocation of class 3 transporters to the plasma membrane in response to a stimulus has only been demonstrated for GLUT8 in blasto-cysts (Carayannopoulos et al. 2000) and for the H+/myo-inositol transporter HMIT which belongs to the class 3 transporters due to its sequence similarities (Uldry et al. 2004).
GLUT8 is expressed predominantly in testis; smaller amounts of GLUT8 mRNA were detected in most other tissues including brain (cerebellum, brain stem, hippocampus, and hypothalamus), spleen, liver, and insulin-sensitive tissues such as heart, skeletal muscle, and adipose tissue. GLUT8 was detected in preimplantation embryos, in which it was reported to be essential for the development and survival of the embryo (Pinto et al. 2002). In testis, the GLUT8 protein was found to be associated with the acrosomal region of mature spermatozoa (Schürmann et al. 2002a), whereas others detected GLUT8 in differentiating spermatocytes of type 1 stage but not in mature spermatozoa (Ibberson et al. 2002). GLUT8 may also play a role in adipocyte metabolism because its expression increases markedly during fat cell differentiation, and is sensitive to prolonged hypoxia and glucose deprivation (Scheepers et al. 2001). However, in adipose cells, GLUT8 does not undergo insulin-stimulated translocation to the plasma membrane as described for the GLUT4 isoform (Lisinski et al. 2001). In liver, GLUT8 was detected in perivenous hepatocytes, indicating that GLUT8 might have a function in the regulation of glycolytic flux (Gorovits et al. 2003). Recombinant GLUT8 expressed in COS-7 cells exhibits glucose transport activity when reconstituted into lecithin liposomes (Doege et al. 2000). In addition, high-affinity glucose transport activity (Km = 2 mM) was observed in Xenopus oocytes after injection of GLUT8 mRNA carrying a mutation of the amino-terminal dileucine motif (Ibberson et al. 2000). This activity was specifically inhibited by Dfructose and D-galactose, indicating that GLUT8 might be a multifunctional sugar transporter (Ibberson et al. 2000).
In order to analyze the specific role of the intracellular GLUT8 in glucose metabolism and function of sperm cells we generated a null mutant lacking Slc2a8 and examined its phenotype with the focus on number and motility. While our study was in progress, it was described that targeted disruption of the Slc2a8 gene in mice caused mild alterations in brain and heart, such as an increased proliferation of hippocampal cells and a slightly impaired transmission of the electrical wave through the atrium leading to a reduction in P-wave (Membrez et al. 2006). Here we report data demonstrating that GLUT8 is required for maintaining mitochondrial membrane potential and motility of spermatozoa. This study indicates that the intracellular compartment in which GLUT8 is expressed is needed for transport of metabolites required for energy production.
Materials and Methods
Antibody
A polyclonal antibody against two GLUT8-specific peptides corresponding to a sequence of the big intracellular loop (WGSEEGWEEPPVGAEG) and of the C-terminus (KGRTLEQVTAHFEGR) of mouse GLUT8 was raised in rabbit.
Inactivation of the Slc2a8 gene
To generate a floxed Slc2a8 allele we constructed a targeting vector (see Figure 1A). Exons 5 and 7 of Slc2a8 were flanked with two loxP sites, and a PGKneo/HSVtk cassette (Neo/tk) with a third loxP site which was introduced downstream of the flanked exon 7. To verify a single introduction of the targeting construct in the homologously recombined ES cell clone, we blotted and hybridized the Xba III-digested ES cell DNA with the indicated probe giving a single band of 12.5 kb band for the wild type. Homologously recombined ES cell clones containing the targeted allele were transiently transfected with pCre to generate ES clones carrying a deleted allele and clones carrying the floxed Slc2a8 allele. The two types of ES cell clones were identified by PCR with specific primers matching to a 5′-flanking region of exon 5 and the 3′-flanking region of exon 7. ES cells carrying the deleted allele were used for a morula aggregation. Blastocysts were then transferred into a pseudopregnant (day 2.5) female mouse. Male chimeric mice were mated with C57BL/6 females. Offspring carrying the transgene were backcrossed on to C57BL/6 3 times and subsequently intercrossed. The littermates of this intercross were used for the phenotypic characterization. Genotyping was performed by PCR (forward primer: 5′-CATCTTCTGTGCAGTCCATC-3′, reverse primer: 5′-GGTACCAAAGGCACTCATACTG-3′).
Figure 1.
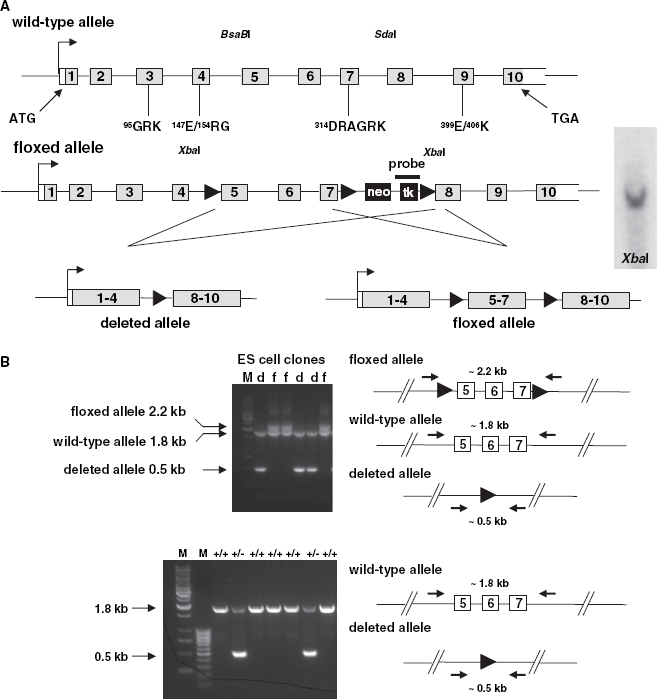
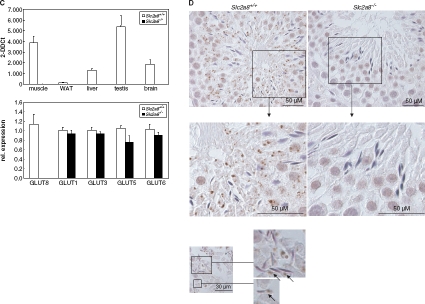
Generation of Slc2a8flox allele and targeting of Slc2a8. (A) Targeting strategy which resulted in the introduction of loxP sites upstream of exons 5, downstream of exon 7 of Slc2a8 and of a PGKneo/HSVtk cassette (Neo/tk) with a third loxP site. Verification of a single introduction of the targeting construct in the homologously recombined ES cell clone by Southern blot analysis as described in Materials and Methods (right panel). (B) After transient expression of Cre recombinase, the two types of ES cell clones carrying a deletion of exons 5–7 (d) or the floxed Slc2a8 allele (f) were identified by PCR with specific primers matching to a 5′-flanking region of exon 5 and the 3′-flanking region of exon 7 (upper panel). Identification of Slc2a8+/+, Slc2a8+/−, and Slc2a8−/− littermates by PCR with specific primers as described under Materials and Methods (lower panel). (C) Slc2a8 mRNA levels of 10 weeks old Slc2a8+/+ and Slc2a8−/− littermates in muscle, white adipose tissue (WAT), liver, testis and brain were assayed by quantitative RT-PCR as described in Materials and Methods (upper panel). mRNA levels of GLUT8, GLUT1, GLUT3, GLUT5 and GLUT6 in testis of Slc2a8+/+ and Slc2a8−/− males were assayed by qRT-PCR (lower panel). (D) Immunohistochemical detection of GLUT8 in seminiferous tubules. Sections of testis from Slc2a8+/+ and Slc2a8−/− mice were fixed with paraformaldehyde and incubated with the anti-GLUT8 antiserum. The immunostaining was performed with peroxidase-conjugated secondary antibody as described in Materials and Methods. Sections were counterstained with Mayer’s hematoxylin (M, DNA ladders).
Animals
The animals were housed in air conditioned rooms (temperature 20±2°C, relative moisture 50–60%) under a 12 h/12 h light/dark cycle. They were kept in accordance with the NIH guidelines for the care and use of laboratory animals, and all experiments were approved by the ethics committee of the Ministry of Agriculture, Nutrition and Forestry (State of Brandenburg, Germany).
Southern blot analysis
DNA of ES cell clones was digested with XbaI, separated on a 0.7% agarose gel, and blotted onto a Hybond-N+ -nylon membrane (Amersham Pharmacia Biotech). To verify a homologous recombination of the targeting construct in the ES cell clones, a 522 bp fragment of the Slc2a8-gene, external to the targeting construct, was used for hybridization after labelling with [α32P]dCTP by using a random priming kit (Amersham Pharmacia Biotech). An 8.8 kb band of the wild-type allele and a 12.5 kb band of the floxed allele indicated a homologous recombination of the targeting construct (data not shown). For identification of a single introduction of the targeting construct in the homologous recombined ES cell clone, a 500 bp sequence that corresponds to the HSVtk-cassette was labelled and a single integration site of the targeting construct was indicated by a single band of 12.5 kb (Figure 1A).
RNA preparation and first strand cDNA synthesis
RNA was extracted from different tissues and cDNA synthesis was performed as previously described (Buchmann et al. 2007). Quality of cDNA was controlled performing a PCR with murine GAPDH primers (forward: 5′-ACC ACA GTC CAT GCC ATC AC-3′; reverse, 5′-TCC CAC CAC CCT GTT GCT GTA-3′).
Quantitative real-time PCR
Quantitative real-time PCR analysis (qRT-PCR) was performed with the Applied Biosystems 7300 Real-Time PCR System as described previously (Buchmann et al. 2007). The TaqMan gene expression assay (Mm00444635_g1) was used to detect the GLUT8-mRNA expression. The assay amplifies the region between exons 5 and 6, which is deleted in Slc2a8−/− mice. For the determination of other GLUT encoding genes, the following TaqMan gene expression assays were used: GLUT1 (Mm0044 1473_m1), GLUT3 (Mm00441483_m1), GLUT5 (Mm00600311_m1), GLUT6 (Mm00554217_m1). Data were normalized referring to Livak & Schmittgen (2001), whereas a b-actin expression assay (Mm00607939_si; Applied Biosystems) was used as endogenous control.
Immunohistochemical detection of GLUT8
Paraffin sections of testis from Slc2a8+/+ and Slc2a8−/− males were dewaxed in toluene and rehydrated, antigen demasking was performed by heat treatment (microwave, 2.5 min, 850 W) in Target Retrieval Solution (ChemMateTM, Dako Cytomation, Hamburg, Germany). Endogenous peroxidase was deactivated with 3% hydrogen peroxide for 10 min. Unspecific binding sites were blocked with DAKO antibody diluent for 30 min. The affinity purified polyclonal anti-GLUT8 antibody in a concentration of 5 μg/ml was applied overnight at 4°C in a humid chamber. Specific antibody binding was visualized by biotin-conjugated donkey anti-rabbit IgG (1:800; Dianova, Hamburg, Germany), for 30 min at 37°C, followed by incubation with a streptavidin-biotin-horseradish peroxidase complex (StreptAB-complex/POD) for 30 min, and diaminobenzidine as substrate according to the manufacturer’s specifications. The sections were dehydrated in graded ethanol and toluene and mounted in Histomount. Microscopic investigation and photo documentation were done with the combined light and fluorescence microscope ECLIPSE E-100 (Nikon, Düsseldorf, Germany) in combination with the video camera CCD-1300CB (Vosskühler, Osnabrück, Germany) and the Analysis System LUCIA G (Nikon, Düsseldorf, Germany).
Preparation and characterization of Slc2a8+/+ and Slc2a8−/− spermatozoa
For preparation of spermatozoa caudae epididymis and vasa deferentia were excised and rinsed with buffer as described (Schürmann et al. 2002b). Briefly rinsed spermatozoa were transferred to 1 ml medium supplemented with 5 mg of bovine serum albumin per ml and 15 mM NaHCO3, semen was allowed to exclude (15 min at 37°C, 5% CO2 from 3 to 5 small incisions). Cells were diluted to 4 ml and collected twice by sedimentation (400 g, 5 min). Sperm count and motility of the sperm cells were analyzed with a CASA (computer-assisted sperm analyzer, MTG, medeaLAB, Altdorf, Germany) as described by Krause (1995). Parameter settings were used as described by Wennemuth et al. (2003). For all assays sperm cells were kept in the indicated media not longer than 15 min in order to suppress capicitation (Wennemuth et al. 2003).
Cytoplasmic calcium levels of spermatozoa were assayed by loading the cells with 1 mM of the fluorescent calcium indicator Fluo3/acetoxymethyl (AM) ester (Gentaur; excitation: 506 nm, emission: 525nm recorded in FL-1, 530±30 nm band pass filter, FACSCalibur, BD) for 20 min at 37°C/5% CO2. After transport into the cells, AM hydrolysis occurs and, thereafter, the dye is trapped within the cell. Upon binding to calcium Fluo-3 exhibits a marked increase in fluorescence intensity (Perl et al. 2006). The mitochondrial calcium levels were estimated with 4 mM Rhod2/AM ester, which is compartmentalized into the mitochondria (Perl et al. 2006). Sperm cells were incubated with the dye for 20 min at 37°C/5% CO2 and emission was recorded in FL-2 (585±42 nm band pass filter).
The survival rate of spermatozoa was determined by staining with membrane-permanent nucleic acid dye 50 nM SYBR 14 according to manufacturer’s instructions (Molecular Probes) for 10 min at 37°C/ 5% CO2. Stained and unstained sperm were assayed by flow cytometry (excitation 488 nm, emission 520 nm allocated in the FL1 channel; FACSCalibur, BD).
Mitochondrial transmembrane potential (ΔΨm)of spermatozoa was likewise analysed by flow cytometry. 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimid-azolocarbocyanine iodide (JC-1) is a potential dependent J-aggregate forming lipophilic cation, which accumulates in mitochondria and indicates the membrane potential by an emission shift from green (527 nm) to red (590 nm, high transmembrane potential). Sperm where incubated with 2 μM JC-1 for 20 min at 37°C. JC1 fluorescence excited at 488 nm was collected with a 530±30 nm filter in the FL1 channel (green fluorescence) and a 585±42 nm filter in the FL2 channel (orange fluorescence). A preliminary staining with 50 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) for 10 min was used as a positive control to disrupt the ΔΨm.
The ATP values of spermatozoa where determined by using the ATP Bioluminescense Assay Kit CLS II (Roche, Mannheim, Germany). The results were referred to the protein concentration of the samples.
Serum parameters
Blood glucose levels, plasma insulin, triglycerides and free fatty acids were analyzed as previously described (Buchmann et al. 2007).
Staining of acrosome
Sections of epididymal tubules of 12 weeks old Slc2a8+/+ and Slc2a8−/− mice were incubated with 50 μg/ml FITC-conjugated Pisum sativum agglutinin (Sigma, St. Louis, MO, USA) for 30 min at 37°C in order to stain the acrosome as described earlier (Schürmann et al. 2002a).
Glucose uptake
Uptake of [14C]-2-deoxyglucose by sperm of 12 weeks old Slc2a8+/+ and Slc2a8−/− mice was determined as described (McLean et al. 1997). Briefly, isolated sperm were diluted to 0.5×106sperm/ml with 50 mM TES, pH7.4, containing 130 mM NaCl and were centrifuged (700 g). Washed sperm were diluted in the above buffer containing 1 mM deoxy-D-glucose, and 2 μM CaCl2 and 1 μCi/μ1 [14C]-2-deoxyglucose and incubated for 15 min at 37°C. Glucose uptake was stopped by addition of a cold 0.1 M glucose solution and centrifugation at 12000 g for 5 min. Radioactivity of pellets was determined in a Beckman LS 6000LL scintillation counter.
Statistical analysis
Statistical significance was determined by two-tailed Student’s t-test or by Pearsons’s chi-square test with 2 degrees of freedom for fertility mating. All values are the mean ±SEM. Statistical significance was set at p <0.05.
Results
Inactivation of GLUT8 by targeting the Slc2a8 gene in mice
For generation of GLUT8 knockout mice (Slc2a8−/− mice) we used the Cre/loxP recombination system which allows both the conventional deletion of the gene and the generation of tissue-specific null mutants. ES cells were transfected with the targeting construct (Figure 1A), and homologous recombinants were re-transfected with crerecombinase to generate deletion of the neo and tk cassettes (floxed allele) or of the full segments (deleted allele). For the generation of Slc2a8−/− mice, we performed a morula fusion with ES cells carrying the deleted allele as shown in Figure 1A (lower left panel). Male chimeric mice were mated with C57BL/6 females, and F1 progeny carrying the transgene were backcrossed 3 times on to the C57BL/6 background. Quantitative real-time PCR failed to detect full-length mRNA of Slc2a8 in all investigated tissues of Slc2a8−/− mice (Figure 1C, upper panel). Furthermore, no upregulation of GLUT1, GLUT3, GLUT5, or GLUT6 was observed in testis (Figure 1C, lower panel). Immunohistochemistry of testis sections performed with a specific anti-GLUT8 antibody raised against sequences corresponding to the intracellular loop 6 and the C-terminus of GLUT8 confirmed the absence of GLUT8 protein in testis of Slc2a8−/− mice while a positive staining of GLUT8 was detected in spermatocytes, spermatides, and mature spermatozoa of Slc2a8+/+ littermates (Figure 1D). As shown in the lower panel of Figure 1D also in spermatozoa GLUT8 is located in an intracellular compartment rather than in the area of the acrosome as we have previously described (Schürmann et al. 2002a).
Slc2a8−/− mice were viable with normal growth and no apparent abnormality. In sections from several tissues (liver, skeletal muscle, and adipose tissue) from Slc2a8−/− mice, no morphological or pathological abnormalities were observed (data not shown). Body weight development (Figure 2A) and body composition of Slc2a8+/+ and Slc2a8−/− mice were identical (Figure 2B). In addition, no differences in serum glucose, triglyceride, free fatty acid or insulin levels were observed between fed wild-type and knockout mice (Table I). Due to these results we conclude that GLUT8 does not play an important role for the regulation of the energy balance and glucose homeostasis.
Figure 2.
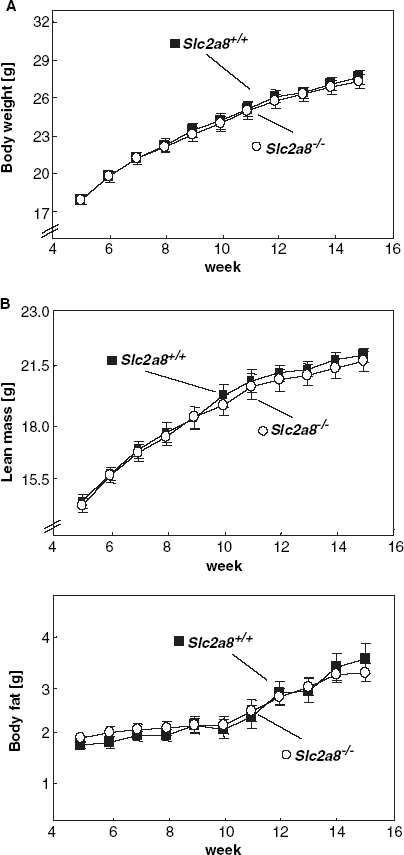
Characterization of body weight and body composition of wild-type and Slc2a8−/− mice. (A) Body weight, (B) lean mass (upper panel) fat mass (lower panel) development of male Slc2a8+/+ and Slc2a8−/− mice (means±SEM from 36 Slc2a8+/+ and 26 Slc2a8−/− mice).
Table I.
Comparison of plasma parameter in Slc2a8+/+ and Slc2a8−/− males. Glucose, triacylglycerol (TG), free fatty acids (FFA) and insulin were measured in fed 12 to 15-week-old mice kept on standard diet. Data are shown as mean±SEM from 19–28 mice (as indicated in brackets).
| Glucose (mg/dl) | TG (mg/dl) | FFA (mg/dl) | Insulin (μg/I) | |
|---|---|---|---|---|
| Slc2a8+/+ | 138.5±5.5 (n = 22) | 72.1±3.6 (n = 23) | 0.80±0.04 (n = 23) | 3.3±0.4 (n = 28) |
| Slc2a8−/− | 142.3±11.3 (n = 19) | 70.0±6.4 (n = 19) | 0.76±0.04 (n = 19) | 3.9±0.6 (n = 25) |
In the F2 progeny which was used for characterization of the phenotype, we obtained Slc2a8+/+, Slc2a8+/−, and Slc2a8−/− mice in a ratio of 30.5:47.3:22.1 (Table II). Since the number of homozygous knockout mice was significantly lower (p = 0.0024) than the expected 25%, breeding pairs of wild-type males and females, Slc2a8−/− males and females as well as Slc2a8−/− males and Slc2a8+/+ females were formed, and mean litter size and number of pups born over a time period of 6 months were compared. No significant differences in the average litter size or the number of live pups were obtained between the indicated crossings (Table III), indicating that loss of GLUT8 does not cause a reduction in fecundity.
Table II.
Numbers of offspring of Slc2a8+/+, Slc2a8+/−, and Slc2a8−/− genotypes obtained by crossing heterozygous mice.
| Genotype | No. of offspring | % | Expected (%) |
|---|---|---|---|
| Slc2a8+/+ | 218 (113 males) | 30.5 | 25 |
| Slc2a8+/− | 338 (186 males) | 47.3 | 50 |
| Slc2a8−/− | 158 (89 males) | 22.1 | 25 |
Table III.
Test of fertility of Slc2a8−/− mice. Slc2a8+/+ males and females (n = 7), Slc2a8−/− males and Slc2a8+/+ females (n = 8), and Slc2a8−/− males and females (n = 9), were paired and mean number of litters and number of pups were recorded over a period of 6 months.
| Slc2a8 genotype | |||
|---|---|---|---|
| Male | Female | Mean litter size | No. of pups |
| +/+ | +/+ | 5.43±0.37 | 31.14±1.47 |
| −/− | +/+ | 4.33±0.6 | 24.78±4.79 |
| −/− | −/− | 4.75±0.6 | 29.38±3.98 |
Intracellular and mitochondrial calcium levels are not altered in Slc2a8−/− spermatozoa
The high expression levels of GLUT8 in testis and the slightly altered genotype distribution observed with heterozygous breeding pairs suggested an impaired function of mature spermatozoa in the absence of GLUT8. We therefore analyzed different parameters which have been associated with defective fertility, such as structural abnormalities (Escalier & David 1984, Lalouette et al. 1996), alterations of intracellular (Perl et al. 2006) or mitochondrial calcium concentrations (Okunade et al. 2004) or reduced mitochondrial membrane potential (Perl et al. 2006). Analysis of testis sections revealed no histopathology or indications that spermatogenesis was abnormal (Figure 1D). In addition, no differences in intracellular (Figure 3A) or mitochondrial Ca2+ concentration (Figure 3B) were detected with specific fluorescent calcium indicators in spermatozoa isolated from Slc2a8+/+ and Slc2a8−/− mice.
Figure 3.
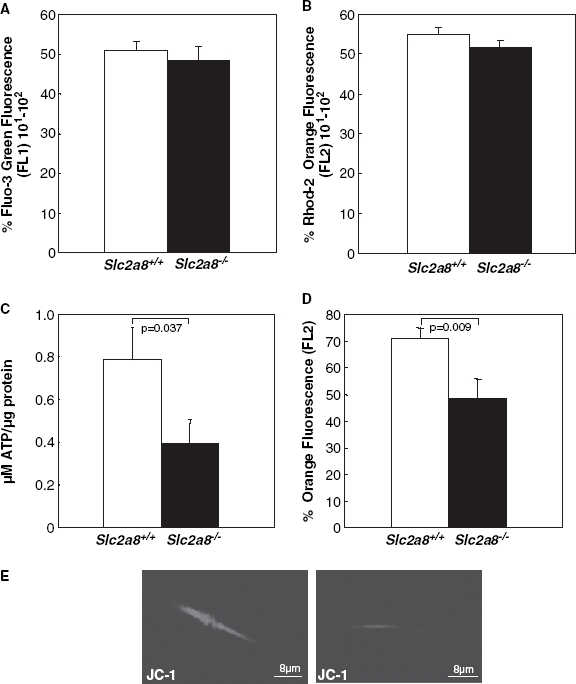
Detection of intracellular (A) and mitochondrial (B) calcium concentrations and assessment of (C, E) mitochondrial transmembrane potential and (D) ATP levels of spermatozoa from Slc2a8+/+ and Slc2a8−/− littermates. Spermatozoa were isolated of Slc2a8+/+ and Slc2a8−/− males at the age of 10–12 weeks. (A) Intracellular calcium levels were detected after incubation of spermatozoa with Fluo2/AM ester, (B) mitochondrial calcium levels with Rhod2/AM ester and analysis by flow cytometry. (C) ATP levels were measured as described in Materials and Methods. (D, E) Mitochondrial membrane potential was observed after staining of spermatozoa with JC1 (E) and subsequent flow cytometry analysis (D). Data present mean±SEM of 10 wild-type and 12 knockout mice. This Figure is reproduced in colour in Molecular Membrane Biology online.
ATP levels and mitochondrial membrane potential are reduced in Slc2a8−/− spermatozoa
We next measured the ATP levels of isolated control and Slc2a8−/− spermatozoa and detected significantly (p = 0.037) reduced ATP levels of knockout sperm cells (0.39±0.11 μM/μg protein) in comparison to that of wild-type litter mates (0.78±0.15 μM/μg protein; Figure 3C). Beside glycolysis the mitochondrial phosphorylation plays crucial roles for the energy supply required for sperm motility (Turner 2006, Marchetti et al. 2002, 2004). Therefore, we analyzed the mitochondrial membrane potential (ΔΨm) of Slc2a8+/+ and Slc2a8−/− spermatozoa by using the potentiometric fluorescent dye JC-1. As shown in Figure 3D ΔΨm was reduced by more than 20% in Slc2a8−/− spermatozoa. JC-1 staining as shown in Figure 3E detects mitochondria within the mid-piece of wild-type sperm. This staining was much lower in Slc2a8−/− spermatozoa. In addition, sperm viability was monitored by staining with Sybr 14 (Garner & Johnson 1995) but viability of Slc2a8+/+ and Slc2a8−/− spermatozoa was not different (33.5±3.5 vs 31.9±2).
Glucose uptake into Slc2a8−/− sperm is not altered
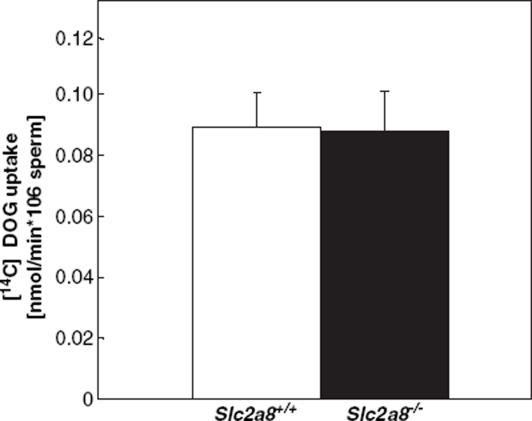
In order to evaluate the effects of Slc2a8 disruption on glucose transport into spermatozoa we measured uptake of labeled deoxy-glucose of isolated sperm cells from wild-type and Slc2a8−/− sperm. As expected from the intracellular localization of GLUT8 (Figure 1D) we did not detect differences in glucose transport between the two genotypes indicating that a reduced energy supply is not responsible for altered ATP levels of Slc2a8−/− sperm.
Diminished motility of spermatozoa lacking GLUT8
In order to characterize the number and motility of Slc2a8+/+ and Slc2a8−/− spermatozoa we excised epididymal tissue of Slc2a8+/+ and Slc2a8−/− males at the age of 10–12 weeks, and analyzed them as described in Material and Methods. The sperm count of Slc2a8−/− males was not altered in comparison to that of Slc2a8+/+ males (Figure 5B, left panel). In contrast, motility of Slc2a8−/− spermatozoa meaning the number of motile sperm cells as assessed with a computer-assisted sperm analyzer (CASA) was significantly (p = 3.3×10−9) reduced by about 50% compared to that of wild-type spermatozoa (Figure 5B, right panel). Within motile sperm no differences concerning the fast (≥25 μm/sec; 56.75±1.6 vs. 56.83±2.1%), slow (<25 μm/sec; 32.75±1.1 vs. 33.17±1.6%), and non-progressive local motility (between <5 μm/sec and > μm/ sec; 10.5±1.3 vs. 10.0±2.3%) was detected between Slc2a8+/+ and Slc2a8−/− spermatozoa, respectively.
Figure 5.
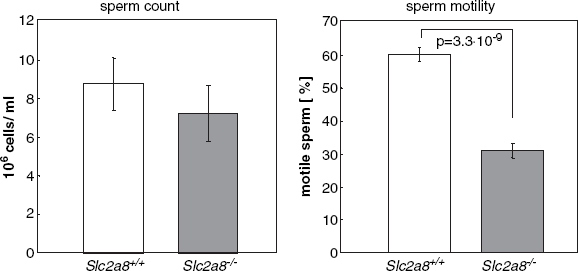
GLUT8 is required for normal sperm motility. Sperm count and sperm motility from Slc2a8+/+ and Slc2a8−/− mice. Spermatozoa were isolated from epididymal tissues of 10–12 weeks old Slc2a8+/+ and Slc2a8−/− mice and counted (left panel), and their mobility was analyzed as described in Materials and Methods (means±SEM from 6 mice of each genotype).
Electron microscopic analysis of Slc2a8+/+ and Slc2a8−/− testis
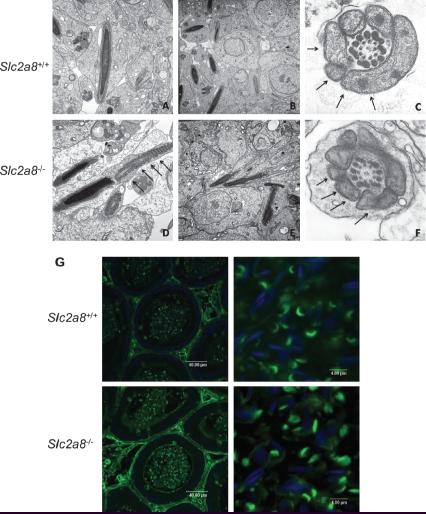
To determine whether structural abnormalities are responsible for impaired motility of Slc2a8−/− sperm cells we examined testis of Slc2a8+/+ and Slc2a8−/− mice by electron microscopy. As shown in Figure 6, in early and late spermatides of wild-type mice, normal development of acrosome and surrounding membranes was visible (Figure 6A, B) whereas development of mitochondria was not consistent within the epithelium of Slc2a8−/− testis. We observed a lack of condensation of mitochondria in some late spermatides (Figure 6E, F) but also late spermatides with normally developed mitochondria (Figure 6D).
Figure 6.
Electron microscopy of testis of Slc2a8+/+ (A–C) and Slc2a8−/− mice (D–F) and analysis of cauda epididymal sperm with Pisum sativum agglutinine. (A) Early spermatides, (B, D, E) late spermatides, (C, F) cross section of a late spermatide, cut in the mid-piece region of the tail. Arrows depict the mitochondria (magnification approx. A and D × 18,000; B and E × 12,000; C and F × 40,000). (G) Sections of the cauda epididymis of Slc2a8+/+ and Slc2a8−/− males at the age of 10–12 weeks were stained with Pisum sativum agglutinine and analyzed by confocal laser scanning microscopy. Nuclei were co-stained with DAPI.
In addition, we stained sections of cauda epididymus with Pisum sativum agglutinine which detects the acrosome. As shown in Figure 6G no differences between wild-type and knockout sperm could be observed indicating that the epididymal maturation of Slc2a8−/− sperm was not affected.
Discussion
The present data show that the facilitative glucose transporter GLUT8 is required for maintenance of the mitochondrial transmembrane potential (ΔΨm) of spermatozoa. Ablation of GLUT8 reduces ATP levels of sperm and sperm motility which results in a slight but significant deviation of offspring from the Mendelian distribution of genotypes. However, sperm number and survival rate as well as glucose transport of sperm cells were unaffected by deletion of GLUT8. Our data give new evidence for an intracellular function of GLUT8, presumably the transfer of sugars or metabolites through membranes in order to maintain energy flow.
Motility and fertility of spermatozoa are regulated in a complex way. The role of glycolysis for ATP production is controversially discussed in the literature. An important study by Narisawa et al. (2002) has shown that under conditions of defective mitochondrial phosphorylation the sperm is still able to generate ATP and to fertilize. In addition, targeted deletion of sperm-specific GAPDH, an enzyme located in the fibrous sheath resulted in male sterility which was associated with severe aberrations in sperm motility (Miki et al. 2004). On the other hand, Ford (2006) demonstrated that glycolysis is not required to support normal sperm function. Inhibition of glycolysis by α-chlorohydrin did not alter mouse sperm motility. Therefore, beside energy provided by glycolysis sperm depend on production of reactive oxygen intermediates (Fisher & Aitken 1997) and maintenance of ΔΨm (Marchetti et al. 2002, 2004). Male mice lacking the enzyme transaldolase (TAL) which indirectly contributes to the generation of NADPH are sterile because spermatozoa lost ΔΨm and mitochondrial integrity (Perl et al. 2006). The effects on ΔΨm and ATP levels in Slc2a8−/− males did not lead to infertility; Slc2a8 knockout sperm were able to fertilize Slc2a8 wild-type oocytes. In addition, no differences in the number of litters or in the number of pups born after crossing Slc2a8−/− males with Slc2a8+/+ females were observed in comparison to an intercross of Slc2a8+/+ mice (Table III). However, the reduced number of motile Slc2a8 knockout sperm resulted in a moderate but significant reduction of Slc2a8+/− (47.3 instead of 50%) and Slc2a8−/− (22.1 instead of 25%) offspring in the intercross of heterozygous mice when Slc2a8 wild-type and Slc2a8 knockout sperm competed for the oocytes (Table II). Altered mitochondria condensation as detected by ultrastructural analysis (Figure 6) might participate in the reduction of motility. We hypothesize that knockout sperm cells which exhibit defective mitochondrial condensation during development are those that are immobile after maturation. For the detection of mitochondrial membrane potiential and ATP levels all sperm cells were used including immotile sperm cells. Thereby reduced ΔΨm and ATP levels of the Slc2a8−/− spermatozoa were detected, indicating that the energy supply required for sperm motility was impaired. However, we did not detect differences in glucose uptake between Slc2a8+/+ and Slc2a8−/− sperm (Figure 4). In addition, spermatozoa express the high-affinity glucose transporter GLUT3 at the cell surface (Haber et al. 1993, Burant & Davidson 1994). Therefore, it seems unlikely that deletion of GLUT8 which is primarily localized to intracellular membranes of all cell types (spermatocytes, spermatides, mature spermatozoa) impairs glucose uptake in Slc2a8 knockout cells. Rather, we propose that GLUT8 functions in a different, intracellular transport process which is required for mitochondrial function and generation of ATP.
Figure 4.
Uptake of [14C]-2deoxyglucose by sperm of 12 weeks old Slc2a8+/+ and Slc2a8−/− mice. Each bar represents the mean and SE of observations from 6 mice.
The role of GLUT8 in the regulation of the energy homeostasis and the metabolism is so far poorly understood. Recently, Membrez et al. (2006) described a phenotypical characterization of Slc2a8−/− mice and observed normal embryonic and postnatal development, glucose homeostasis and response to mild stress, respectively. Here we confirmed the results of Membrez et al. (2006) who observed no differences in body weight between Slc2a8+/+ and Slc2a8−/− mice kept on a standard chow diet. We therefore conclude that GLUT8 does not play a major role in the regulation of energy balance.
GLUT8 can be expressed in blastocysts and translocated to their surface in response to insulin (Carayannopoulos et al. 2000). In addition, suppression of GLUT8 expression by antisense mRNA caused increased apoptosis of blastocysts, suggesting that GLUT8 plays an essential role in early embryogenesis (Pinto et al. 2002). However, our data do not confirm this conclusion. Since mating of knockout mice produced viable, normally developing off-spring in numbers comparable to those of a wild-type intercross (Table III).
In summary, our data indicate that deletion of GLUT8 results in reduced sperm motility due to impaired mitochondrial function in spermatozoa, suggesting that GLUT8 is involved in the regulation of energy metabolism of sperm cells.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (GK1208, FOR441, AU178/ 3-1). The skilful technical assistance of Brigitte Rischke and Elisabeth Meyer is gratefully acknowledged.
References
- Augustin R, Riley J, Moley KH. GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment. Traffic. 2005;6:1196–1212. doi: 10.1111/j.1600-0854.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Buchmann J, Meyer C, Neschen S, Augustin R, Schmolz K, Kluge R, Al-Hasani H, Jurgens H, Eulenberg K, Wehr R, Dohrmann C, Joost Hg, Schürmann A. Ablation of the cholesterol transporter adenosine triphosphate-binding cassette transporter G1 reduces adipose cell size and protects against diet-induced obesity. Endocrinology. 2007;148:1561–1573. doi: 10.1210/en.2006-1244. [DOI] [PubMed] [Google Scholar]
- Burant CF, Davidson NO. GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol. 1994;267((6 Pt 2)):R1488–1495. doi: 10.1152/ajpregu.1994.267.6.R1488. [DOI] [PubMed] [Google Scholar]
- Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulinstimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA. 2000;97:7313–7318. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem. 2000;275:16275–16280. doi: 10.1074/jbc.275.21.16275. [DOI] [PubMed] [Google Scholar]
- Escalier D, David G. Pathology of the cytoskeleton of the human sperm flagellum: axonemal and peri-axonemal anomalies. Biol Cell. 1984;50:37–52. doi: 10.1111/j.1768-322x.1984.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ford WCL. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- Garner DL, Johnson LA. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol Reprod. 1995;53:276–284. doi: 10.1095/biolreprod53.2.276. [DOI] [PubMed] [Google Scholar]
- Gorovits N, Cui L, Busik JV, Ranalletta M, Hauguel de-Mouzon S, Charron MJ. Regulation of hepatic GLUT8 expression in normal and diabetic models. Endocrinology. 2003;144:1703–1711. doi: 10.1210/en.2002-220968. [DOI] [PubMed] [Google Scholar]
- Haber RS, Weinstein SP, O’Boyle E, Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993;132:2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem. 2000;275:4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology. 2002;143:276–284. doi: 10.1210/endo.143.1.8587. [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- Krause W. Computer-assisted semen analysis systems: comparison with routine evaluation and prognostic value in male fertility and assisted reproduction. Hum Reprod. 1995;10(Suppl. 1):66. doi: 10.1093/humrep/10.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- Lalouette A, Lablack A, Guenet JL, Montagutelli X, Segretain D. Male sterility caused by sperm cell-specific structural abnormalities in ebouriffé, a new mutation of the house mouse. Biol Reprod. 1996;55:355–363. doi: 10.1095/biolreprod55.2.355. [DOI] [PubMed] [Google Scholar]
- Lisinski I, Schürmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001;358:517–522. doi: 10.1042/0264-6021:3580517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods. 2001;25:402–440. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Comparison of four fluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum Reprod. 2004;19:2267–2276. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Jones LG, Jr, Froman DP. Reduced glucose transport in sperm from roosters (Gallus domesticus) with heritable subfertility. Biol Reprod. 1997;57:791–795. doi: 10.1095/biolreprod57.4.791. [DOI] [PubMed] [Google Scholar]
- Membrez M, Hummler E, Beermann F, Haefliger JA, Savioz R, Pedrazzini T, Thorens B. GLUT8 is dispensable for embryonic development but influences hippocampal neurogenesis and heart function. Mol Cell Biol. 2006;26:4268–4276. doi: 10.1128/MCB.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch Do, Strader LF, Perreault SD, Eddy EM, O’Brien Da. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millán Jl. Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol. 2002;22:5554–5562. doi: 10.1128/MCB.22.15.5554-5562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunade GW, Miller Ml, Pyne GJ, Sutliff RL, O’connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- Perl A, Qian Y, Chohan KR, Shirley CR, Amidon W, Banerjee S, Middleton FA, Conkrite KL, Barcza M, Gonchoroff N, Suarez SS, Banki K. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc Natl Acad Sci USA. 2006;103:14813–14818. doi: 10.1073/pnas.0602678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AB, Carayannopoulos MO, Hoehn A, Dowd L, Moley KH. Glucose transporter 8 expression and translocation are critical for murine blastocyst survival. Biol Reprod. 2002;66:1729–1733. doi: 10.1095/biolreprod66.6.1729. [DOI] [PubMed] [Google Scholar]
- Scheepers A, Doege H, Joost HG, Schürmann A. Mouse 3T3-L1 adipocytes by glucose. Biochem Biophys Res Commun. 2001;288:969–974. doi: 10.1006/bbrc.2001.5866. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Briese S, Leicht K, Schürmann A, Joost HG, Al-Hasani H. Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex. J Cell Sci. 2006;119:2321–2331. doi: 10.1242/jcs.02943. [DOI] [PubMed] [Google Scholar]
- Schürmann A, Axer H, Scheepers A, Doege H, Joost HG. The glucose transport facilitator GLUT8 is predominantly associated with the acrosomal region of mature spermatozoa. Cell Tissue Res. 2002a;307:237–242. doi: 10.1007/s00441-001-0499-2. [DOI] [PubMed] [Google Scholar]
- Schürmann A, Koling S, Jacobs S, Saftig P, Krauss S, Wennemuth G, Kluge R, Joost HG. Reduced sperm count and normal fertility in male mice with targeted disruption of the ADP-ribosylation factor-like 4 (Arl4) gene. Mol Cell Biol. 2002b;22:2761–2768. doi: 10.1128/MCB.22.8.2761-2768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RM. Moving to the beat: a review of mammalian sperm mobility. Reprod Fertil Dev. 2006;18:25–28. doi: 10.1071/rd05120. [DOI] [PubMed] [Google Scholar]
- Uldry M, Steiner P, Zurich MG, Beguin P, Hirling H, Dolci W, Thorens BR. egulated exocytosis of an H+/myo-inositol symporter at synapses and growth cones. EMBO J. 2004;23:531–540. doi: 10.1038/sj.emboj.7600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennemuth G, Carlson AE, Harper AJ, Babcock DF. Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development. 2003;130:1317–1326. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]