Abstract
The traditional subcutaneous tumor model is less than ideal for studying colorectal cancer. Orthotopic mouse models of colorectal cancer, which feature cancer cells growing in their natural location, replicate human disease with high fidelity. Two techniques can be used to establish this model. Both techniques are similar and require mouse anesthesia and laparotomy for exposure of the cecum. One technique involves injection of a colorectal cancer cell suspension into the cecal wall. Cancer cells are first grown in culture, harvested when subconfluent and prepared as a single cell suspension. A small volume of cells is injected slowly to avoid leakage. The other technique involves transplantation of a piece of subcutaneous tumor onto the cecum. A mouse with a previously established subcutaneous colorectal tumor is euthanized and the tumor is removed using sterile technique. The tumor piece is divided into small pieces for transplantation to another mouse. Prior to transplantation, the cecal wall is lightly damaged to facilitate tumor cell infiltration. The time to developing primary tumors and liver metastases will vary depending on the technique, cell line, and mouse species used. This orthotopic mouse model is useful for studying the natural progression of colorectal cancer and testing new therapeutic agents against colorectal cancer.
Protocol
I. Cell Preparation
Colorectal cancer cells are grown in culture and harvested when subconfluent.
A single cell suspension is prepared in phosphate buffered saline and kept on ice.
II. Tumor Preparation
A mouse with a previously established subcutaneous colorectal tumor is euthanized.
The subcutaneous tumor is removed using sterile technique and divided into 2-3 mm pieces
The tumor pieces are kept in phosphate buffered saline on ice.
III. Mouse Preparation
Note: In our laboratory we use inhaled isoflurane to anesthetize the mouse; alternatively, one can use injectable anesthetics to achieve the same effect
The depth of anesthesia is assessed using toe pinch. There should be no withdraw reflex with toe pinch.
Antibiotics may be given at this point.
The anesthetized mouse, which was previously shaved, is properly positioned.
The abdomen is prepped with a betadine solution.
The abdomen and surgical site are draped in a sterile fashion.
IV. Laparotomy
A small nick is made in the skin
The abdominal wall musculature is grasped and lifted up
The abdominal cavity is entered and a single blade of the scissors is used to push the intra-abdominal contents away
The incision is extended to 2-3 cm
V. Exposure of the Cecum
The cecum with its blind ending pouch is identified and exteriorized
The cecum is isolated from the rest of the mouse using a pre-cut, sterile gauze
Warm saline is used to keep the cecum moist
VI. Injection of Cells into the Cecal Wall
A 27 G or finer needle is used to inject a 50 µL volume of cells into the cecal wall
The needle is removed
The injection site is inspected to ensure no leakage
The cecum is returned to the abdominal cavity
VII. Transplantation of Tumor onto the Cecum
Note: In addition to injecting cells into the cecal wall, an alternative approach is to transplant tumor onto the cecum
A figure of 8 stitch is placed onto the cecum using 6-0 or 7-0 sized suture
The cecal wall is lightly damaged
Then, the tumor piece is positioned
The stitch is tied down
The cecum is returned to the abdominal cavity
VIII. Mouse Abdominal Wall Closure and Recovery
The mouse abdominal wall is closed using three interrupted stitches using 3-0 or 4-0 sized suture
Alternatively, one can use a simple running stitch
Post-operative analgesics and a fluid bolus may be given at this point
The mouse is allowed to recover from anesthesia.
Note: With inhaled anesthetics this typically takes 30 seconds
IX. Results - Primary Tumor and Liver Metastasis
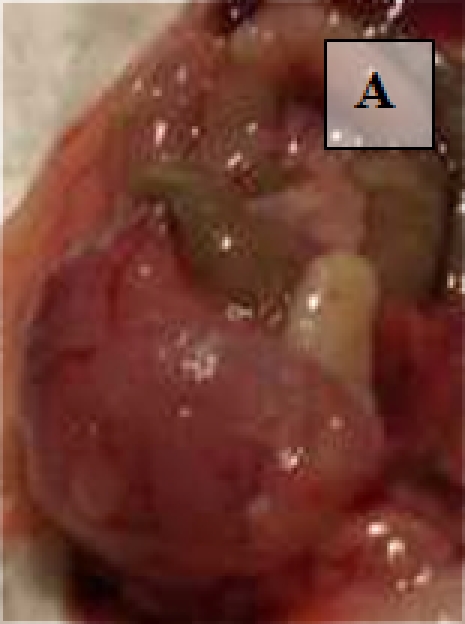
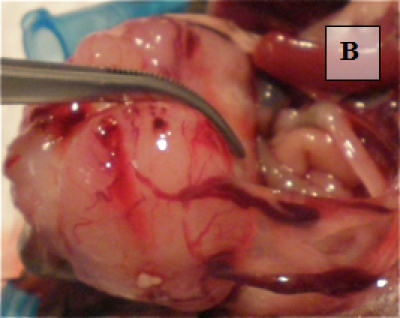
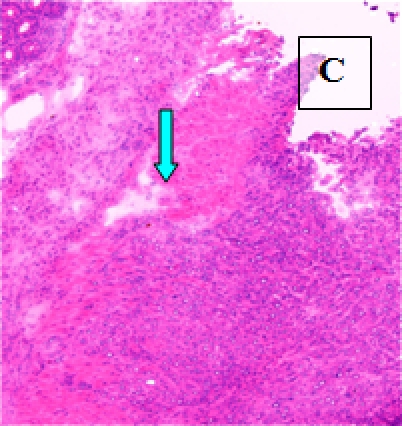 Primary Tumor - shown in situ (A) and with evidence of neovascularization (B); on H&E staining, tumors are locally invasive (C)
Primary Tumor - shown in situ (A) and with evidence of neovascularization (B); on H&E staining, tumors are locally invasive (C)
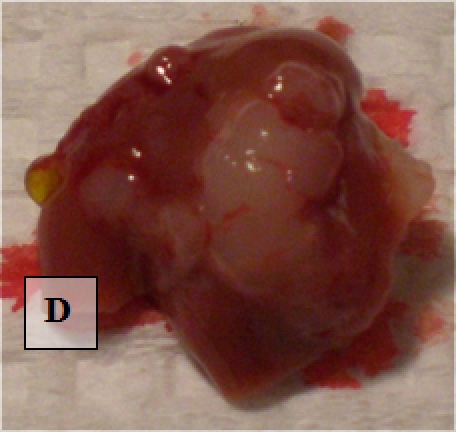 Liver Metastases - shown ex vivo (D)
Liver Metastases - shown ex vivo (D)
Discussion
Although mouse subcutaneous tumor models are easy to establish and monitor, it is clear that this model cannot replicate the original anatomic site of colorectal cancer. Due to the difference in microenvironment, colorectal cancer cells growing under the skin have been shown to change their phenotype and almost always fail to progress and metastasize 1,2. In fact, tumor response to therapy can vary dramatically depending on whether cancer cells are implanted in an ectopic (subcutaneous) versus orthotopic location 3. Orthotopic mouse models of colorectal cancer, which feature cancer cells growing in their natural location, replicate human disease with high fidelity. The two techniques to establish this model have unique advantages and disadvantages. Injection of a cell suspension into the cecal wall introduces colorectal cancer cells which have been previously growing in an in vitro environment and are arguably homogeneous. Cells may have reduced invasive or metastatic potential after several passages in culture. Transplantation of a piece of subcutaneous tumor introduces a more heterogeneous population of cancer cells that have been established in vivo. However, tumors contain stromal cells and frequently have necrotic portions which may affect the consistency of this model (personal communication, I. Fidler, MD Anderson). From a technical standpoint, our laboratory has found that injection of a cell suspension into the cecal wall is more difficult and carries the risks of intraluminal injection and leakage post-injection. Interestingly, an orthotopic mouse model of rectal cancer has been described that does not require mouse laparotomy 4. Mice are anesthetized and the rectal mucosa is prolapsed with digital pressure. A small volume of colorectal cancer cells in single cell suspension is injected submucosally. Primary, invasive rectal cancers develop in mice as early as one week after injection; however, none of the mice develop liver metastases and the authors did not comment on the frequency of lung metasases.
Acknowledgments
Department of Surgery, University of California, San Francisco Raymond Shaheen, M.D.
References
- Heijstek MW, Kraneburg O, Rinkes Borel IH. Mouse models of colorectal cancer and liver metastases. Dig Surg. 2005;22:16–25. doi: 10.1159/000085342. [DOI] [PubMed] [Google Scholar]
- Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16–26. [PubMed] [Google Scholar]
- Wilmanns C, Fan D, O'Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer. 1992;52:98–104. doi: 10.1002/ijc.2910520118. [DOI] [PubMed] [Google Scholar]
- Kashtan H, Rabau M, Mullen JB, Wong AHC, Roder JC, Shiptz B, Stern HS, Gallinger S. Intra-rectal injection of tumor cells: a novel animal model of rectal cancer. Surg Oncol. 1992;1:251–256. doi: 10.1016/0960-7404(92)90072-s. [DOI] [PubMed] [Google Scholar]


