Abstract
Carnitine plays an essential role in the transfer of long-chain fatty acids across the inner mitochondrial membrane. This transfer requires enzymes and transporters that accumulate carnitine within the cell (OCTN2 carnitine transporter), conjugate it with long chain fatty acids (carnitine palmitoyl transferase 1, CPT1), transfer the acylcarnitine across the inner plasma membrane (carnitine-acylcarnitine translocase, CACT), and conjugate the fatty acid back to Coenzyme A for subsequent beta oxidation (carnitine palmitoyl transferase 2, CPT2). Deficiency of the OCTN2 carnitine transporter causes primary carnitine deficiency, characterized by increased losses of carnitine in the urine and decreased carnitine accumulation in tissues. Patients can present with hypoketotic hypoglycemia and hepatic encephalopathy, or with skeletal and cardiac myopathy. This disease responds to carnitine supplementation. Defects in the liver isoform of CPT1 present with recurrent attacks of fasting hypoketotic hypoglycemia. The heart and the muscle, which express a genetically distinct form of CPT1, are usually unaffected. These patients can have elevated levels of plasma carnitine. CACT deficiency presents in most cases in the neonatal period with hypoglycemia, hyperammonemia, and cardiomyopathy with arrhythmia leading to cardiac arrest. Plasma carnitine levels are extremely low. Deficiency of CPT2 present more frequently in adults with rhabdomyolysis triggered by prolonged exercise. More severe variants of CPT2 deficiency present in the neonatal period similarly to CACT deficiency associated or not with multiple congenital anomalies. Treatment for deficiency of CPT1, CPT2 and CACT consists in a low-fat diet supplemented with medium chain triglycerides that can be metabolized by mitochondria independently from carnitine, carnitine supplements, and avoidance of fasting and sustained exercise.
Keywords: Carnitine, Primary carnitine deficiency, Carnitine palmitoyl transferase deficiency, carnitine acylcarnitine translocase deficiency, hypoglycemia, arrhythmia, cardiomyopathy, SLC22A5, OCTN2
INTRODUCTION
Carnitine (β-hydroxy-γ-trimethylammonium butyrate) is a hydrophilic molecule that plays an essential role in the transfer of long-chain fatty acids inside mitochondria for β oxidation. Carnitine binds acyl residues and help in their elimination. This mechanism is essential in binding/removing abnormal organic acids in several organic acidemias and explains the secondary carnitine deficiency that can result from them. Carnitine conjugation decreases the number of acyl residues attached to CoA and increases the ratio between free and acylated CoA [Bieber, 1988]. Less defined functions of carnitine include the shuttling of fatty acids between different intracellular organelles (peroxisomes, microsomes, mitochondria) involved in fatty acid metabolism. The conjugation of different acyl residues with carnitine produces acylcarnitine species that can be used as a diagnostic tool to screen for or diagnose inborn errors of metabolism. The formation of these acylcarnitine conjugates is the basis of expanded newborn screening by tandem mass spectrometry (MS/MS).
The average adult diet provides about 75% of daily carnitine requirements, mostly from meat and dairy products [Borum, 1995; Rebouche and Engel, 1984; Stanley, 2004]. The remainder of the carnitine needs are satisfied by endogenous synthesis [Scaglia and Longo, 1999]]. Strict vegetarians maintain normal carnitine levels, indicating that humans not only synthesize carnitine, but also effectively conserve it through renal tubular reabsorption [Rebouche, 2004].
CARNITINE AND FATTY ACID OXIDATION
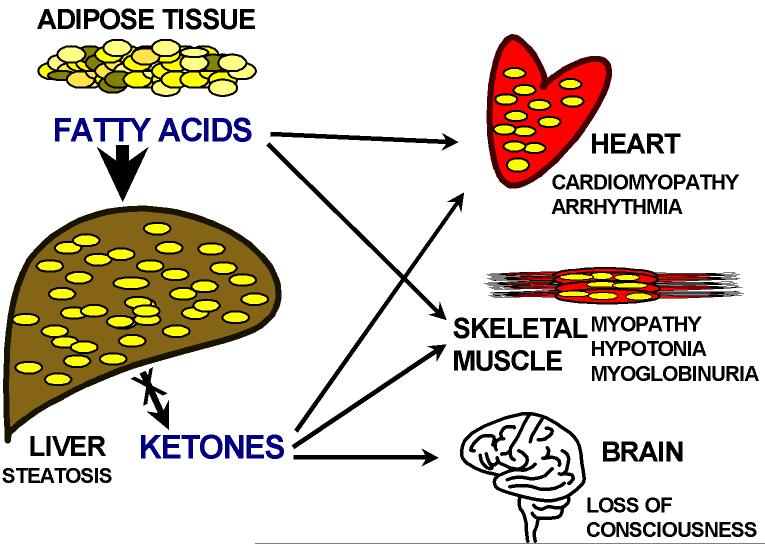
Carnitine is required for the transfer of long-chain fatty acids from the cytoplasm to the mitochondrial matrix for their oxidation [Roe and Ding, 2001]. During periods of fasting, fatty acids turn into the predominant substrate for energy production via oxidation in the liver, cardiac muscle, and skeletal muscle (Fig. 1). The brain does not directly utilize fatty acids for oxidative metabolism, but oxidizes ketone bodies derived from acetyl CoA and acetoacetyl CoA produced by β-oxidation of fatty acids in the liver. When the oxidation of fatty acids is defective, fats are still released from the adipose tissue with fasting and will reach the liver, skeletal muscle and heart where they can accumulate (Fig. 1). The inability of the liver to metabolize them will results in steatosis and decreased production of ketones. Ketones can be used an alternate energy source by the heart, skeletal muscle, and brain, sparing glucose. In the liver, acetyl-CoA activates pyruvate carboxylase to favor gluconeogenesis. The net result of both actions is glucose sparing and production (although this latter not from fat itself). If fatty acid oxidation is defective, fat cannot be utilized, glucose is consumed without regeneration via gluconeogenesis and there is a drop in glucose levels (hypoglycemia). The lack of usable supplies of energy will impair brain function with loss of consciousness. Fats can go directly to the heart and skeletal muscle where they can accumulate and impair organ/tissue function (cardiomyopathy/myopathy). Free fatty acids and long-chain acylcarnitines can alter the electrical activity of cardiac cells resulting in arrhythmia. In certain diseases, the muscle fibers can also break down during sustained exercise resulting in myoglobinuria.
Fig. 1. Fatty acid oxidation during fasting.
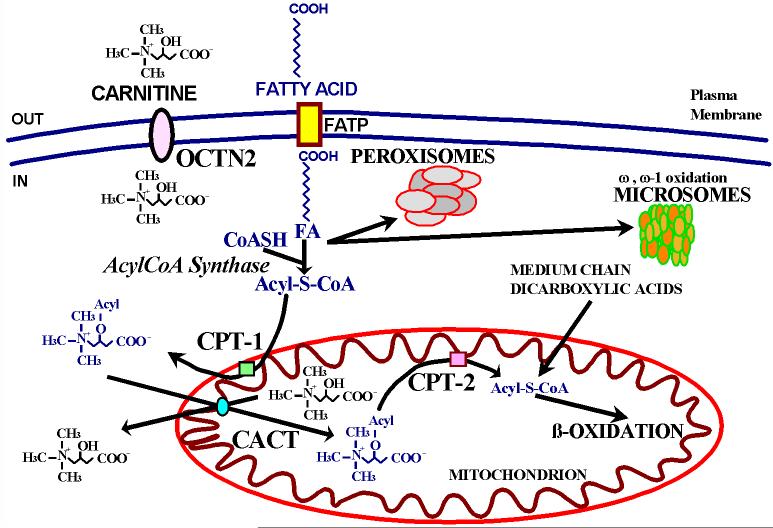
Fatty acids are mobilized from adipose tissue stores and transported in the circulation primarily bound to albumin. After their entry into the cells by a specific membrane transporter, fatty acids are conjugated to Coenzyme A by acyl CoA synthase (Fig. 2). Fatty acids must then be conjugated to carnitine to enter mitochondria. Carnitine is accumulated inside the cell by the high-affinity OCTN2 carnitine transporter in the heart, muscle, and kidney. Hepatocytes in the liver have a different low-affinity, high-capacity transporter [Scaglia et al., 1999]. Carnitine forms a high-energy ester bond with long chain carboxylic acids by the action of carnitine palmitoyl transferase 1 (CPT-1), located in the inner aspect of the outer mitochondrial membrane. Acylcarnitine is then translocated across the inner mitochondrial membrane by the carnitine acylcarnitine translocase (CACT) and cleaved by CPT-2 in the inner aspect of the inner mitochondrial membrane. Carnitine is released in the mitochondrial matrix and can then return to the cytoplasm for another cycle (using CACT), while the fatty acid is conjugated back to Coenzyme A in the mitochondrial matrix and can enter (in aerobic conditions and in the presence of low levels of ATP) β-oxidation with production of acetyl-CoA for oxidative phosphorylation or production of ketone bodies in the liver. Inherited defects of all these steps are transmitted as autosomal recessive traits in humans.
Fig. 2. The carnitine cycle in fatty acid oxidation.
FATP: Fatty Acid Transporter Protein; FA: Fatty Acid; CPT-1: Carnitine Palmitoyl Tansferase-1; CPT-2: Carnitine Palmitoyl Tansferase-2; CACT: Carnitine Acyl Carnitine Translocase. Modified from [Scaglia and Longo, 1999].
PRIMARY CARNITINE DEFICIENCY
Primary carnitine deficiency (OMIM 212140) is an autosomal recessive disorder of fatty acid oxidation due to the lack of functional OCTN2 carnitine transporters. Primary carnitine deficiency has a frequency of about 1:40,000 newborns in Japan [Koizumi et al., 1999] and 1:37,000-1:100,000 newborns in Australia [Wilcken et al., 2001]. In the USA and Europe, the frequency of primary carnitine deficiency has not been defined, but from the reported cases, it seems similar to that in Japan.
The lack of the plasma membrane carnitine transporter results in urinary carnitine wasting, low serum carnitine levels (0-5 μM, normal 25-50 μM), and decreased intracellular carnitine accumulation. Patients with primary carnitine deficiency lose most (90-95%) of the filtered carnitine in urine and their heterozygous parents lose 2 to 3 times the normal amount, explaining their mildly reduced plasma carnitine levels [Scaglia et al., 1998].
Affected patients can have a predominant metabolic or cardiac presentation. The metabolic presentation is more frequent before two years of age. Typically, these children start refusing feedings and become irritable for an upper respiratory tract infection or an acute gastroenteritis. Subsequently, they become lethargic and minimally responsive. In most cases, they have hepatomegaly in addition to signs and symptoms of the triggering condition. Laboratory evaluation usually reveals hypoglycemia with minimal or no ketones in urine and hyperammonemia with variably elevated liver function tests. Creatine kinase (CK) can also be mildly elevated. If children are not treated promptly with intravenous glucose, they progress to coma and death. Cardiomyopathy is more frequent in older patients associated sometimes with hypotonia. Chest radiograms may show an enlarged heart and decreased ventricular ejection fraction can be measured by echocardiography. Cardiomyopathy can also be seen in older patients with a metabolic presentation, even if asymptomatic from a cardiac standpoint. A few patients, have been completely asymptomatic for all of their life and have been diagnosed following the birth of an affected child [Spiekerkoetter et al., 2003]. Other children, diagnosed because of an affected sibling, had only mild developmental delays [Wang et al., 2001].
Key to the diagnosis is the measurement of plasma carnitine levels. Free and acylated carnitine are extremely reduced (free carnitine < 5 μM, normal 25-50 μM) and urine organic acids do not show any consistent anomaly, although a non-specific dicarboxylic aciduria has been reported [Scaglia et al., 1998]. Diagnosis is confirmed by demonstrating reduced carnitine transport in skin fibroblasts from the patient. This is usually reduced below 10% of the value of matched controls. There is a correlation between residual carnitine transport activity in fibroblasts and severity of the mutations, with nonsense mutations associated with absent carnitine transport activity. However, there is no correlation between genotype and clinical presentation [Amat Di San Filippo and Longo 2004; Dobrowolski et al., 2005; Scaglia et al., 1998; Wang et al., 2000a; Wang et al., 2001; Wang et al., 2000b; Wang et al., 1999]. Heterozygous parents of affected children have half-normal carnitine transport in their fibroblasts and might have borderline low levels of plasma carnitine [Scaglia et al., 1998]. Cardiac hypertrophy has been reported in heterozygotes approaching middle age [Koizumi et al., 1999]. It is unclear whether this is associated with any health problem.
Several patients with primary carnitine deficiency have been identified by newborn screening programs in the past few years. The only anomaly on the acylcarnitine profile is a low level of free carnitine and all acylcarnitine species. Carnitine is transferred by the placenta to the growing fetus and plasma levels decrease rapidly after birth [Wilcken et al., 2001]. However, plasma carnitine levels can be in the normal range if obtained too early in life. For this reason, several cases referred to us have been from states performing a repeated newborn screening after one week of age. These patients are usually completely asymptomatic at time of diagnosis and confirmation should be obtained by measuring plasma carnitine levels (free and total) and with appropriate transport studies in fibroblasts. Recently, a few infants were found with extremely low carnitine levels on newborn screening. However, their carnitine levels increased briskly on carnitine supplementation. These patients did not have primary carnitine deficiency, but their mothers did and remained asymptomatic all of their lives. Therefore, low carnitine levels in infants might unmask primary carnitine deficiency in the mother.
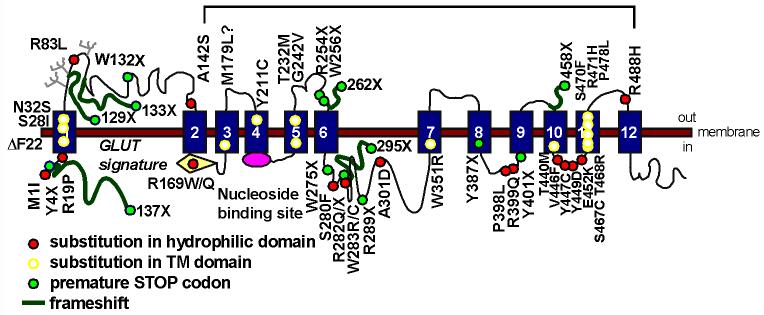
DNA studies have identified heterogeneous mutations in the SLC22A5 gene encoding the OCTN2 carnitine transporter in patients with primary carnitine deficiency. Our database includes 49 different mutations, summarized in Table I and Fig. 3. Most families have private mutations and a few mutations, occurring at mutation-prone DNA sequences, have been reported more than once.
TABLE I. Mutations in the carnitine transporter OCTN2 in patients with primary carnitine deficiency.
Residual carnitine transport of missense mutations was measured in mammalian cells after transfection with the mutant cDNA. - = No residual transport activity expected (STOP codons); ? = no expression studies reported; # = mutation identified in more than one family; @ = mutation found with another mutation on the same allele.
| Codon | Exon | Nucleotide change (cDNA) |
Reference | Transport (% normal) |
|---|---|---|---|---|
| 1 | c.-91_ 22del113 | [Nezu et al., 1999] | - | |
| M1I | 1 | c.3 G>T | [Dobrowolski et al., 2005] | 0 |
| R2fsX137 | 1 | c.4_5insC | [Nezu et al., 1999] | - |
| Y4X | 1# | c.12 C>G | [Wang et al., 2001] | - |
| R19P | 1 | c.56 G>C | [Wang et al., 2001] | 4 |
| ΔF22 | 1# | c.64_66delTTC | [Lamhonwah et al., 2002], submitted |
0 |
| S28I | 1 | c.83 G >T | [Rahbeeni et al., 2002] | ? |
| N32S | 1# | c.95 A>G | [Christensen E 2000; Lamhonwah et al., 2002] |
? |
| P78fsX129 | 1 | c.232delC | submitted | - |
| R83L | 1 | c.248 G>T | [Makhseed et al., 2004] | <1 |
| I89fsX133 | 1# | c.254_264dup11 GGCTCGCCACC |
[Lamhonwah et al., 2002; Wang et al., 2001] |
- |
| W132X | 2# | c.396G>A | [Koizumi et al., 1999; Nezu et al., 1999; Tang et al., 1999] | - |
| A142S@ | 2 | c.424 G>T | submitted | 5@ |
| V153fsX193 | 2 | c.457_458delTG | [Dobrowolski et al., 2005] | - |
| R169W | 3# | c.505 C>T | [Lamhonwah et al., 2002; Wang et al., 2000b] |
<1 |
| R169Q | 3 | c.506 G>A | [Burwinkel et al., 1999] | ? |
| M179L | 3 | c.535 A>T | [Koizumi et al., 1999] | 80 |
| Y211C | 3 | c.632 A>G | [Vaz et al., 1999] | ? |
| IVS3 | c.652+1 G>A | [Lamhonwah et al., 2002] | - | |
| T232M | 4 | c.695 C>T | [Dobrowolski et al., 2005] | 2 |
| G242V | 4 | c.725 G>T | [Wang et al., 2000b] | 1 |
| R254X | 4# | c.759 C>T | [Tang et al., 2002] | - |
| W256X | 4 | c.768 G>A | submitted | - |
| L269fsX295 | 4 | c.806delT | [Cederbaum et al., 2002] | - |
| W275X | 5 | c.825 G>A | [Dobrowolski et al., 2005] | - |
| S280F | 5 | c.839 C>T | submitted | <1 |
| R282fsX295 | 5 | c.839delC | [Lamhonwah et al., 2002] | - |
| R282X | 5# | c.844 C>T | [Burwinkel et al., 1999; Vaz et al., 1999; Wang et al., 1999] | - |
| R282Q | 5 | c.845 G>A | submitted | 10 |
| W283R | 5 | c.847 T>C, c.847 T>A | [Mayatepek et al., 2000], | 1 |
| W283C | 5 | c.849 G>T | [Koizumi et al., 1999] | 2 |
| R289X | 5 | c.865 C>T | [Dobrowolski et al., 2005] | - |
| A301D | 5 | c.902 C>A | [Wang et al., 2000b] | 3 |
| T337fsX348 | 6 | c.1008delA | [Lamhonwah et al., 2002] | - |
| W351R | 6 | c.1051 T>C | [Wang et al., 2000b] | <1 |
| Y387X | 7 | c.1161 T>G | [Tang et al., 2002] | - |
| P398L | 7 | c.1193 C>T | submitted | <1 |
| R399Q | 7 | c.1196 G>A | [Wang et al., 2001] | 4 |
| Y401X | 7# | c.1202_1203insA | [Lamhonwah et al., 2002; Wang et al., 1999] |
- |
| IVS7 | c.1267del +3_+23 | [Dobrowolski et al., 2005] | - | |
| G435fsX458 | 8 | c.1302delG | [Wang et al., 1999] | - |
| T440M | 8# | c.1319 C>T | [Lamhonwah et al., 2002] | <1 |
| V446F | 8 | c.1336 G>T | [Mayatepek et al., 2000] | 0.5 |
| Y447C | 8# | c.1340 A>G | [Amat Di San Filippo and Longo 2004; Rahbeeni et al., 2002] | 0 |
| Y449D | 8 | c.1345 T>G | [Amat Di San Filippo and Longo 2004] | 11 |
| E452K | 8 | c.1354 G>A | [Wang et al., 2000a] | 4 |
| S467C | 8 | c.1400 C>G | [Koizumi et al., 1999] | 11 |
| T468R | 8# | c.1403 C>G | [Lamhonwah et al., 2002], submitted |
<1 |
| S470F | 8 | c.1409 C>T | [Lamhonwah et al., 2002] | ? |
| R471H | 8 | c.1412 G>A | [Spiekerkoetter et al., 2003], submitted |
1.5 |
| P478L | 8 | c.1433 C>T | [Tang et al., 1999] | 0 |
| IVS8 | c.1451–1 G>A | [Nezu et al., 1999] | - | |
| R488H@ | 9 | c.1463 G>A | submitted | 5@ |
Fig. 3. Mutations in the OCTN2 carnitine transporter in primary carnitine deficiency.
Patients with primary carnitine deficiency respond to dietary carnitine supplementation (100-400 mg/kg/day), if started before irreversible organ damage occurs. The dose of carnitine should be adapted to each individual patient by serial measurements of plasma carnitine levels. Carnitine has few side effects. It can cause diarrhea and intestinal discomfort with high doses. This is usually self limiting, resolving by reducing carnitine dosage. Sometimes, bacterial metabolism in the intestine can result in carnitine degradation, with production of trimethylamine, a non-toxic chemical with a very unpleasant odor. This responds to oral therapy with metronidazole, an antibiotic active against anaerobic bacteria. The long-term prognosis is favorable as long as children remain on carnitine supplements. Repeated attacks of hypoglycemia or sudden death from arrhythmia even without cardiomyopathy have been reported in patients discontinuing carnitine against medical advice.
Primary carnitine deficiency should be differentiated from other causes of carnitine deficiency. These include a number of organic acidemias, defects of fatty acid oxidation and of the carnitine cycle (28). In all these disorders, analysis of urine organic acids, plasma amino acids and acylcarnitine profile, in conjunction with the clinical presentation, allows a definitive diagnosis. Low carnitine levels can also be seen in patients with generalized renal tubular dysfunction, such as renal Fanconi syndrome. In this case, the urinary wasting of other compounds, such as bicarbonate, phosphorus and amino acids, allows a net differentiation, since patients with primary carnitine deficiency have selective carnitine losses.
CARNITINE PALMITOYL TRANSFERASE 1 (CPT-1) DEFICIENCY
Carnitine palmitoyl transferase 1 (CPT-1) conjugates fatty acids to carnitine allowing their subsequent mitochondrial import (Fig. 2). There are three different isoforms of CPT-1 with tissue specific expression encoded by different genes: liver-type (CPT-1A) encoded by a gene on 11q13, muscle-type (CPT-1B) encoded by a gene on 22qter, and brain-type (CPT-1C) whose gene maps to 19q13. Only deficiency of the liver type, CPT-1A, has been demonstrated in humans [Bonnefont et al., 2004]. CPT-1 deficiency (OMIM 255120) is usually triggered by fasting or viral illnesses. Affected children present, usually between birth and 18 months of age, with altered mental status and hepatomegaly. Laboratory evaluation indicates nonketotic hypoglycemia, mild hyperammonemia, elevated liver function tests, and elevated free fatty acids. In this disease, plasma carnitine levels are not decreased, but usually increased. Urine organic acids might show low levels of ketones, dicarboxylic aciduria with prominent elevation of the C12 dicarboxylic (dodecanedioic) acid, and presence of 3-hydroxyglutaric acid [Korman et al., 2005]. Some patients had elevated levels of CK and metabolic acidosis attributable to distal renal tubular acidosis during acute attacks. Diagnosis is suspected from the elevation of free and short chain acylcarnitine, with low levels of long-chain acylcarnitine. Diagnosis is confirmed by assay of CPT-1 in fibroblasts, whose activity is usually reduced to 5-20% of normal. Children with severe episodes may have delays secondary to the initial brain insult. Therapy consists in avoidance of fasting benefiting from nighttime feeds with uncooked cornstarch and a low fat diet rich in medium chain triglycerides, which do not need the carnitine cycle to enter β oxidation in liver mitochondria. Several different mutations have been identified in patients with CPT-1 deficiency and there is some correlation between the severity of the enzymatic impairment caused by the mutation and the clinical presentation [Bennett et al., 2004; Bonnefont et al., 2004; Stoler et al., 2004]. CPT1 deficiency can be identified by newborn screening using MS/MS. Although the value of free carnitine is usually elevated in patients with CPT1 deficiency, an elevated ratio between free carnitine (C0) and the sum of palmitoylcarnitine and stearoylcarnitine (C16 + C18) allows distinction with cases of exogenous carnitine supplementation [Fingerhut et al., 2001]. The ratio C0/(C16+C18) can become more elevated in second screening samples due to the physiological decline in C16 and C18 past the immediate neonatal period.
CARNITINE-ACYLCARNITINE TRANSLOCASE DEFICIENCY
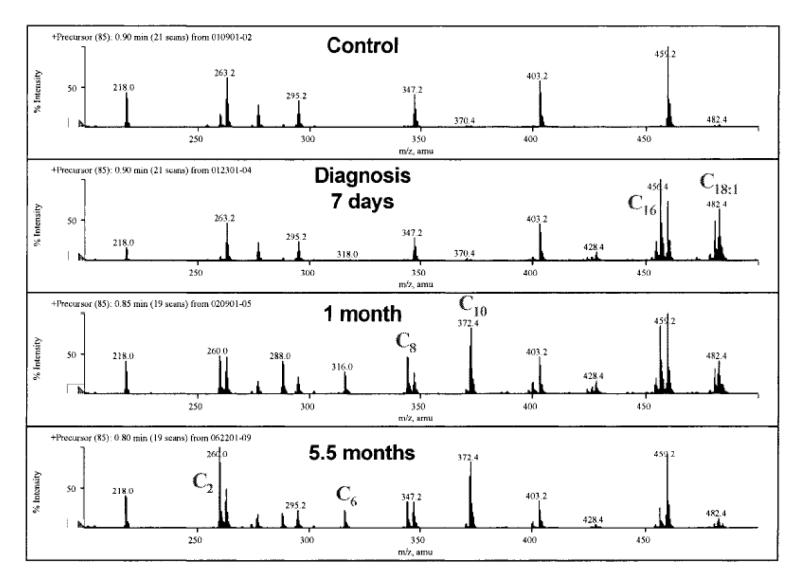
Carnitine-acylcarnitine translocase (CACT) is located in the inner mitochondrial membrane and operates a carnitine/acylcarnitine exchange across this membrane (Fig. 2) [Rubio-Gozalbo et al., 2004]. CACT deficiency (OMIM 212138) presents most often in the neonatal period with seizures, irregular heart beat, and apnea. Many times these episodes are triggered by fasting or by the physiologic birth stress. Patients with presentation later in life (up to 15 months of age) have been reported. In these milder cases, attacks are triggered by fever, infections and fasting as other fatty acid oxidation defects. Fasting hypoglycemia and seizures have been reported in these patients. In neonatal cases and during acute attacks, laboratory examination reveals nonketotic hypoglycemia and hyperammonemia with elevation of CK and liver function tests. Carnitine levels are usually extremely reduced (<5 μM). Plasma acylcarnitine profile shows a marked increase in long-chain acylcarnitines and decreased levels of free carnitine (Fig. 4). Urine organic acids can show severe dicarboxylic aciduria, with excess unsaturated species. The episodes repeat over time with progressive neurological, cardiac, and hepatic deterioration. Diagnosis is suspected from the abnormal plasma acylcarnitine profile with low free carnitine and elevated C16-18. This abnormal profile, however, is not distinguishable from that of neonatal CPT-2 deficiency and direct assay of carnitine-acylcarnitine translocase in fibroblasts is needed for diagnostic confirmation. The gene for this condition maps to 3p21. DNA studies have found heterogeneous mutations in different patients and can also be used for diagnostic confirmation [Iacobazzi et al., 2004a; Rubio-Gozalbo et al., 2004]. Complete deficiency of this transporter is associated with rapidly progressive disease. Residual activity has been associated with a milder phenotype and near normal development with appropriate therapy. Pre-symptomatic identification of affected infants has lead to better outcome with appropriate therapy. Unfortunately, most cases have presented very early in life and it is unclear whether the results of newborn screening would return early enough to completely prevent neurological sequelae. Therapy consists in frequent feedings with a diet rich in carbohydrates, low in fat most of which should be medium chain triglycerides, and supplemented with carnitine. This therapy improves the acylcarnitine profile and prevents further attacks of hypoglycemia and arrhythmia [Iacobazzi et al., 2004b].
Fig. 4. Plasma acylcarnitine profiles from a normal control (top) and a patient with CACT deficiency.
The acylcarnitine profiles were obtained at time of diagnosis (7 days of age) and after therapy with medium chain triglycerides and carnitine supplements (1 and 5.5 months of age). Note the progressive decline in C16 and C18:1 and the increase in medium-chain acylcarnitines (C6—C10) reflecting treatment with medium-chain triglycerides (from [Iacobazzi et al., 2004b].
CARNITINE PALMITOYL TRANSFERASE 2 DEFICIENCY
Carnitine palmitoyl transferase 2 (CPT-2) deficiency presents most frequently in adolescents or young adults (OMIM # 255110) with predominant muscular involvement, but can also present in infancy (OMIM # 600649) and in the neonatal period (OMIM # 608836) [Bonnefont et al., 2004]. The neonatal form presents shortly after birth (few hours-4 days) with respiratory distress, seizures, altered mental status, hepatomegaly, cardiomegaly, cardiac arrhythmia, and, in many cases, dysmorphic features, renal dysgenesis, and neuronal migration defects. These malformations are similar to those seen in severe forms of other inborn errors of metabolism, such as glutaric acidemia type 2/Zellweger/pyruvate dehydrogenase deficiency, indicating that fatty acid oxidation plays an important role in fetal development. The neonatal form of CPT-2 deficiency is rapidly fatal. The infantile variety usually presents between 6 and 24 months of age with recurrent attacks of hypoketotic hypoglycemia causing loss of consciousness and seizures, liver failure and transient hepatomegaly. Several children also have heart involvement with cardiomyopathy and arrhythmia. Episodes are triggered by infections/fever/fasting. Laboratory studies usually indicate hyperammonemia, metabolic acidosis, hypoketotic hypoglycemia with elevated levels of creatine kinase. Carnitine levels are reduced, with an increase in the long-chain acylcarnitine fraction very similar to that observed in CACT deficiency. Diagnosis is confirmed by enzyme assay in fibroblasts or DNA analysis. The neonatal form of CPT2 deficiency responds poorly to therapy. The same therapy used for CACT deficiency (see above) is somehow effective in the infantile form of CPT2 deficiency. The myopathic form of CPT-2 deficiency presents in young adults with muscle pain with or (in most cases) without myoglobinuria with elevation of serum creatine kinase precipitated by strenuous exercise, cold, fever or prolonged fasting. Myoglobinuria can cause kidney failure and death. Unlike patients with phosphorylase and phosphofructokinase deficiency, these patients have a normal rise in lactic acid during muscle exercise. Diagnosis is suggested even in asymptomatic patients by an abnormal acylcarnitine profile obtained from blood spotted on filter paper with increased (C16+C18:1)/C2 ratio [Gempel et al., 2002]. Diagnosis can be confirmed by DNA studies or enzyme assay in cultured fibroblasts. The late onset CPT-2 deficiency responds to limitation of exercise, restriction of fat and long-chain fatty acids with increased dietary carbohydrates [Orngreen et al., 2003], and fasting avoidance. The gene for CPT-2 maps to 1p32 and heterogenous mutations have been identified in patients with CPT2 deficiency [Bonnefont et al., 2004]. There is some genotype-phenotype correlation, with mutations in the neonatal/infantile types that reduce enzyme activity below a critical threshold preventing long chain fatty acid oxidation in all tissues. By contrast, most patients with the myopathic form of CPT2 deficiency have at least one copy of a mild mutation (such as S113L or P50H) allowing residual fatty acid oxidation at least in fibroblasts [Bonnefont et al., 2004]. The neonatal form of CPT2 deficiency diagnosed through newborn screening was useful to establish the cause of death in some patients with this fatal disease [Albers et al., 2001].
CONCLUSIONS
Expansion of newborn screening programs to identify disorders of fatty acid oxidation and the carnitine cycle poses new challenges for the medical practitioner and for the clinical geneticist. Among disorders of the carnitine cycle, primary carnitine deficiency responds extremely well to therapy with carnitine supplements. Low C0 can be identified on newborn screening collected 1-2 days after birth. CPT-1A deficiency can be identified by elevated C0/(C16+C18) and responds to low fat diet supplemented with medium chain triglycerides and uncooked cornstarch. Both primary carnitine deficiency and CPT-1A deficiency can be better identified on the second newborn screening sample, collected after the birth stress and the transplacental transfer of carnitine become lesser factors. CACT deficiency and neonatal CPT-2 deficiency have the same abnormal acylcarnitine profile at birth consisting of low C0 with increased C16-C18 species. In many cases, children will be symptomatic before the results of newborn screening become available. CACT deficiency responds to therapy with fasting avoidance and low-fat diet supplemented with medium chain triglycerides. The neonatal form of CPT-2 deficiency is very severe and responds poorly to therapy. Milder forms of CPT-2 deficiency benefit from the same treatment used for CACT deficiency.
Acknowledgments
Grant support: National Institutes of Health; Contract grant number: R01 DK 53824
Author biographies
Nicola Longo is Professor of Pediatrics and Director of the Metabolic Service at the University of Utah. His research covers inherited disorders of fatty acid oxidation and the development of new therapies for metabolic disorders.
Cristina Amat di San Filippo is a Research Associate working on the molecular bases of primary carnitine deficiency.
Marzia Pasquali is Associate Professor of Pathology at the University of Utah in Salt Lake City and Medical Director of the Biochemical Genetics and Newborn Screening at ARUP Laboratories. She has a strong interest in the development of new testing for the diagnosis of inborn errors of metabolism.
REFERENCES
- Albers S, Marsden D, Quackenbush E, Stark AR, Levy HL, Irons M. Detection of neonatal carnitine palmitoyltransferase II deficiency by expanded newborn screening with tandem mass spectrometry. Pediatrics. 2001;107:E103. doi: 10.1542/peds.107.6.e103. [DOI] [PubMed] [Google Scholar]
- Di San Filippo C Amat, Longo N. Tyrosine Residues Affecting Sodium Stimulation of Carnitine Transport in the OCTN2 Carnitine/Organic Cation Transporter. J Biol Chem. 2004;279:7247–7253. doi: 10.1074/jbc.M309171200. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Boriack RL, Narayan S, Rutledge SL, Raff ML. Novel mutations in CPT 1A define molecular heterogeneity of hepatic carnitine palmitoyltransferase I deficiency. Mol Genet Metab. 2004;82:59–63. doi: 10.1016/j.ymgme.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Borum PR. Carnitine in neonatal nutrition. J Child Neurol. 1995;10(Suppl 2):S25–31. [PubMed] [Google Scholar]
- Burwinkel B, Kreuder J, Schweitzer S, Vorgerd M, Gempel K, Gerbitz KD, Kilimann MW. Carnitine transporter OCTN2 mutations in systemic primary carnitine deficiency: a novel Arg169Gln mutation and a recurrent Arg282ter mutation associated with an unconventional splicing abnormality. Biochem Biophys Res Commun. 1999;261:484–487. doi: 10.1006/bbrc.1999.1060. [DOI] [PubMed] [Google Scholar]
- Cederbaum SD, Koo-McCoy S, Tein I, Hsu BY, Ganguly A, Vilain E, Dipple K, Cvitanovic-Sojat L, Stanley C. Carnitine membrane transporter deficiency: a long-term follow up and OCTN2 mutation in the first documented case of primary carnitine deficiency. Mol Genet Metab. 2002;77:195–201. doi: 10.1016/s1096-7192(02)00169-5. [DOI] [PubMed] [Google Scholar]
- Christensen EHJ, Hansen SH, Sorensen N, Nezu J, Tsuji A, Skovby F. Sudden infant death following pivampicillin treatment in a patient with carnitine transporter deficiency. J Inherit Metab Dis. 2000;23(Suppl 1):117. (Abs 234-P).
- Dobrowolski SF, McKinney JT, di San Filippo C Amat, Sim K Giak, Wilcken B, Longo N. Validation of dye-binding/high-resolution thermal denaturation for the identification of mutations in the SLC22A5 gene. Hum Mutat. 2005;25:306–313. doi: 10.1002/humu.20137. [DOI] [PubMed] [Google Scholar]
- Fingerhut R, Roschinger W, Muntau AC, Dame T, Kreischer J, Arnecke R, Superti-Furga A, Troxler H, Liebl B, Olgemoller B, Roscher AA. Hepatic carnitine palmitoyltransferase I deficiency: acylcarnitine profiles in blood spots are highly specific. Clin Chem. 2001;47:1763–1768. [PubMed] [Google Scholar]
- Gempel K, Kiechl S, Hofmann S, Lochmuller H, Kiechl-Kohlendorfer U, Willeit J, Sperl W, Rettinger A, Bieger I, Pongratz D, Gerbitz KD, Bauer MF. Screening for carnitine palmitoyltransferase II deficiency by tandem mass spectrometry. J Inherit Metab Dis. 2002;25:17–27. doi: 10.1023/a:1015109127986. [DOI] [PubMed] [Google Scholar]
- Iacobazzi V, Invernizzi F, Baratta S, Pons R, Chung W, Garavaglia B, Dionisi-Vici C, Ribes A, Parini R, Huertas MD, Lauria G, Palmieri F, Taroni F. Molecular and functional analysis of SLC25A20 mutations causing carnitine-acylcarnitine translocase deficiency. Hum Mutat. 2004a;24:312–320. doi: 10.1002/humu.20085. [DOI] [PubMed] [Google Scholar]
- Iacobazzi V, Pasquali M, Singh R, Matern D, Rinaldo P, di San Filippo C Amat, Palmieri F, Longo N. Response to therapy in carnitine/acylcarnitine translocase (CACT) deficiency due to a novel missense mutation. Am J Med Genet A. 2004b;126:150–155. doi: 10.1002/ajmg.a.20573. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Nozaki J, Ohura T, Kayo T, Wada Y, Nezu J, Ohashi R, Tamai I, Shoji Y, Takada G, Kibira S, Matsuishi T, Tsuji A. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum Mol Genet. 1999;8:2247–2254. doi: 10.1093/hmg/8.12.2247. [DOI] [PubMed] [Google Scholar]
- Korman SH, Waterham HR, Gutman A, Jakobs C, Wanders RJ. Novel metabolic and molecular findings in hepatic carnitine palmitoyltransferase I deficiency. Mol Genet Metab. 2005;86:337–343. doi: 10.1016/j.ymgme.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Olpin SE, Pollitt RJ, Vianey-Saban C, Divry P, Guffon N, Besley GT, Onizuka R, De Meirleir LJ, Cvitanovic-Sojat L, Baric I, Dionisi-Vici C, Fumic K, Maradin M, Tein I. Novel OCTN2 mutations: no genotype-phenotype correlations: early carnitine therapy prevents cardiomyopathy. Am J Med Genet. 2002;111:271–284. doi: 10.1002/ajmg.10585. [DOI] [PubMed] [Google Scholar]
- Makhseed N, Vallance HD, Potter M, Waters PJ, Wong LT, Lillquist Y, Pasquali M, di San Filippo C Amat, Longo N. Carnitine transporter defect due to a novel mutation in the SLC22A5 gene presenting with peripheral neuropathy. J Inherit Metab Dis. 2004;27:778–780. doi: 10.1023/b:boli.0000045837.23328.f4. [DOI] [PubMed] [Google Scholar]
- Mayatepek E, Nezu J, Tamai I, Oku A, Katsura M, Shimane M, Tsuji A. Two novel missense mutations of the OCTN2 gene (W283R and V446F) in a patient with primary systemic carnitine deficiency. Hum Mutat. 2000;15:118. doi: 10.1002/(SICI)1098-1004(200001)15:1<118::AID-HUMU28>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, Takada G, Matsuishi T, Yoshino M, Kato H, Ohura T, Tsujimoto G, Hayakawa J, Shimane M, Tsuji A. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- Orngreen MC, Ejstrup R, Vissing J. Effect of diet on exercise tolerance in carnitine palmitoyltransferase II deficiency. Neurology. 2003;61:559–561. doi: 10.1212/01.wnl.0000078195.05396.20. [DOI] [PubMed] [Google Scholar]
- Rahbeeni Z, Vaz FM, Al-Hussein K, Bucknall MP, Ruiter J, Wanders RJ, Rashed MS. Identification of two novel mutations in OCTN2 from two Saudi patients with systemic carnitine deficiency. J Inherit Metab Dis. 2002;25:363–369. doi: 10.1023/a:1020143632011. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Engel AG. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes. Evidence for alterations in tissue carnitine transport. J Clin Invest. 1984;73:857–867. doi: 10.1172/JCI111281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C, Ding J. Mitochondrial fatty acid oxidation disorders. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8 McGraw-Hill; New York: 2001. pp. 2297–2326. [Google Scholar]
- Rubio-Gozalbo ME, Bakker JA, Waterham HR, Wanders RJ. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Mol Aspects Med. 2004;25:521–32. doi: 10.1016/j.mam.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Scaglia F, Longo N. Primary and secondary alterations of neonatal carnitine metabolism. Semin Perinatol. 1999;23:152–161. doi: 10.1016/s0146-0005(99)80047-0. [DOI] [PubMed] [Google Scholar]
- Scaglia F, Wang Y, Longo N. Functional characterization of the carnitine transporter defective in primary carnitine deficiency. Arch Biochem Biophys. 1999;364:99–106. doi: 10.1006/abbi.1999.1118. [DOI] [PubMed] [Google Scholar]
- Scaglia F, Wang Y, Singh RH, Dembure PP, Pasquali M, Fernhoff PM, Longo N. Defective urinary carnitine transport in heterozygotes for primary carnitine deficiency. Genet Med. 1998;1:34–39. doi: 10.1097/00125817-199811000-00008. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Huener G, Baykal T, Demirkol M, Duran M, Wanders R, Nezu J, Mayatepek E. Silent and symptomatic primary carnitine deficiency within the same family due to identical mutations in the organic cation/carnitine transporter OCTN2. J Inherit Metab Dis. 2003;26:613–615. doi: 10.1023/a:1025968502527. [DOI] [PubMed] [Google Scholar]
- Stanley CA. Carnitine deficiency disorders in children. Ann N Y Acad Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- Stoler JM, Sabry MA, Hanley C, Hoppel CL, Shih VE. Successful long-term treatment of hepatic carnitine palmitoyltransferase I deficiency and a novel mutation. J Inherit Metab Dis. 2004;27:679–684. doi: 10.1023/b:boli.0000042979.42120.55. [DOI] [PubMed] [Google Scholar]
- Tang NL, Ganapathy V, Wu X, Hui J, Seth P, Yuen PM, Wanders RJ, Fok TF, Hjelm NM. Mutations of OCTN2, an organic cation/carnitine transporter, lead to deficient cellular carnitine uptake in primary carnitine deficiency. Hum Mol Genet. 1999;8:655–660. doi: 10.1093/hmg/8.4.655. [DOI] [PubMed] [Google Scholar]
- Tang NL, Hwu WL, Chan RT, Law LK, Fung LM, Zhang WM. A founder mutation (R254X) of SLC22A5 (OCTN2) in Chinese primary carnitine deficiency patients. Hum Mutat. 2002;20:232. doi: 10.1002/humu.9053. [DOI] [PubMed] [Google Scholar]
- Vaz FM, Scholte HR, Ruiter J, Hussaarts-Odijk LM, Pereira RR, Schweitzer S, de Klerk JB, Waterham HR, Wanders RJ. Identification of two novel mutations in OCTN2 of three patients with systemic carnitine deficiency. Hum Genet. 1999;105:157–161. doi: 10.1007/s004399900105. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kelly MA, Cowan TM, Longo N. A missense mutation in the OCTN2 gene associated with residual carnitine transport activity. Hum Mutat. 2000a;15:238–245. doi: 10.1002/(SICI)1098-1004(200003)15:3<238::AID-HUMU4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Korman SH, Ye J, Gargus JJ, Gutman A, Taroni F, Garavaglia B, Longo N. Phenotype and genotype variation in primary carnitine deficiency. Genet Med. 2001;3:387–392. doi: 10.1097/00125817-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Taroni F, Garavaglia B, Longo N. Functional analysis of mutations in the OCTN2 transporter causing primary carnitine deficiency: lack of genotype-phenotype correlation. Hum Mutat. 2000b;16:401–407. doi: 10.1002/1098-1004(200011)16:5<401::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ye J, Ganapathy V, Longo N. Mutations in the organic cation/carnitine transporter OCTN2 in primary carnitine deficiency. Proc Natl Acad Sci U S A. 1999;96:2356–2360. doi: 10.1073/pnas.96.5.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcken B, Wiley V, Sim KG, Carpenter K. Carnitine transporter defect diagnosed by newborn screening with electrospray tandem mass spectrometry. J Pediatr. 2001;138:581–584. doi: 10.1067/mpd.2001.111813. [DOI] [PubMed] [Google Scholar]