Abstract
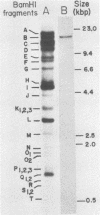
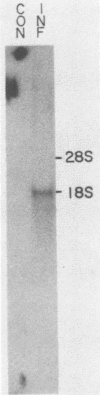
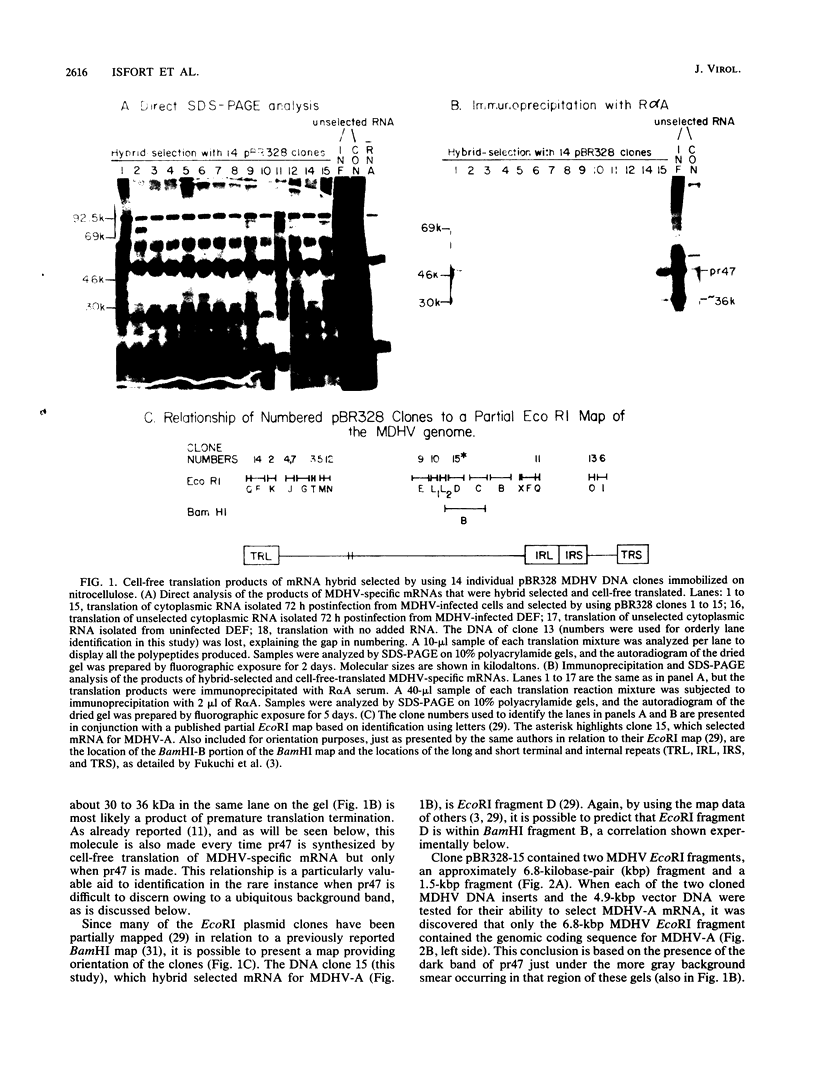
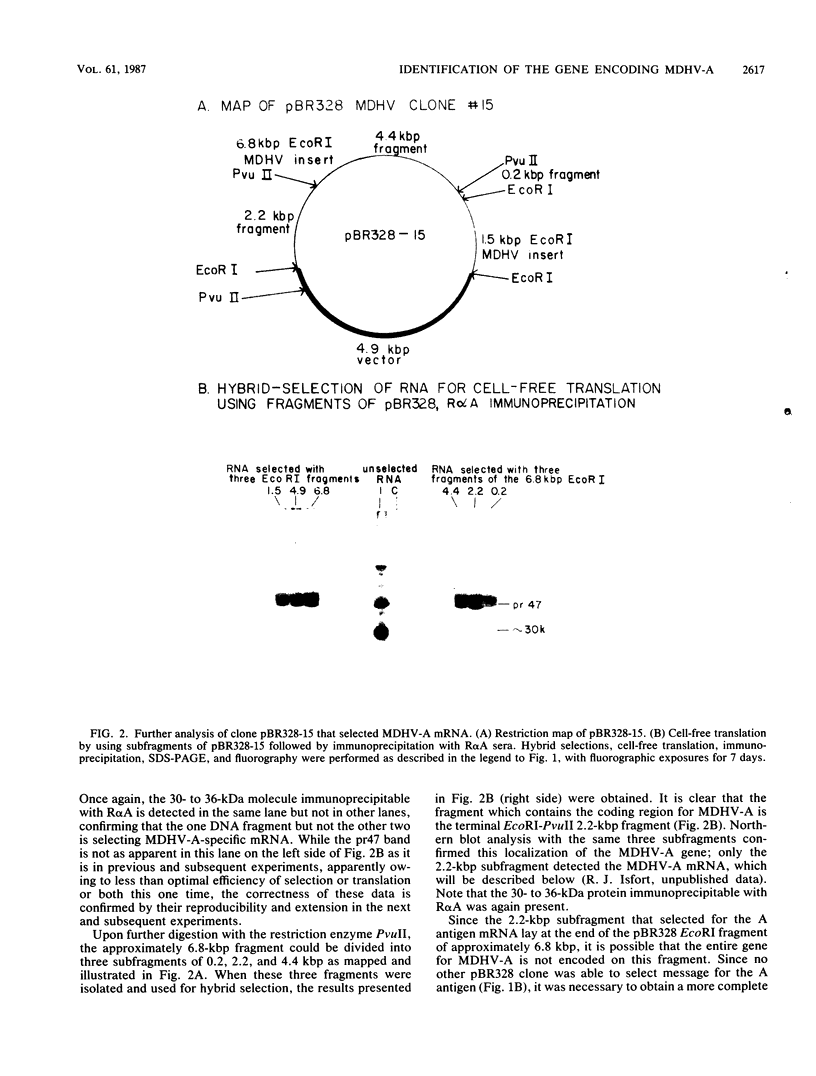
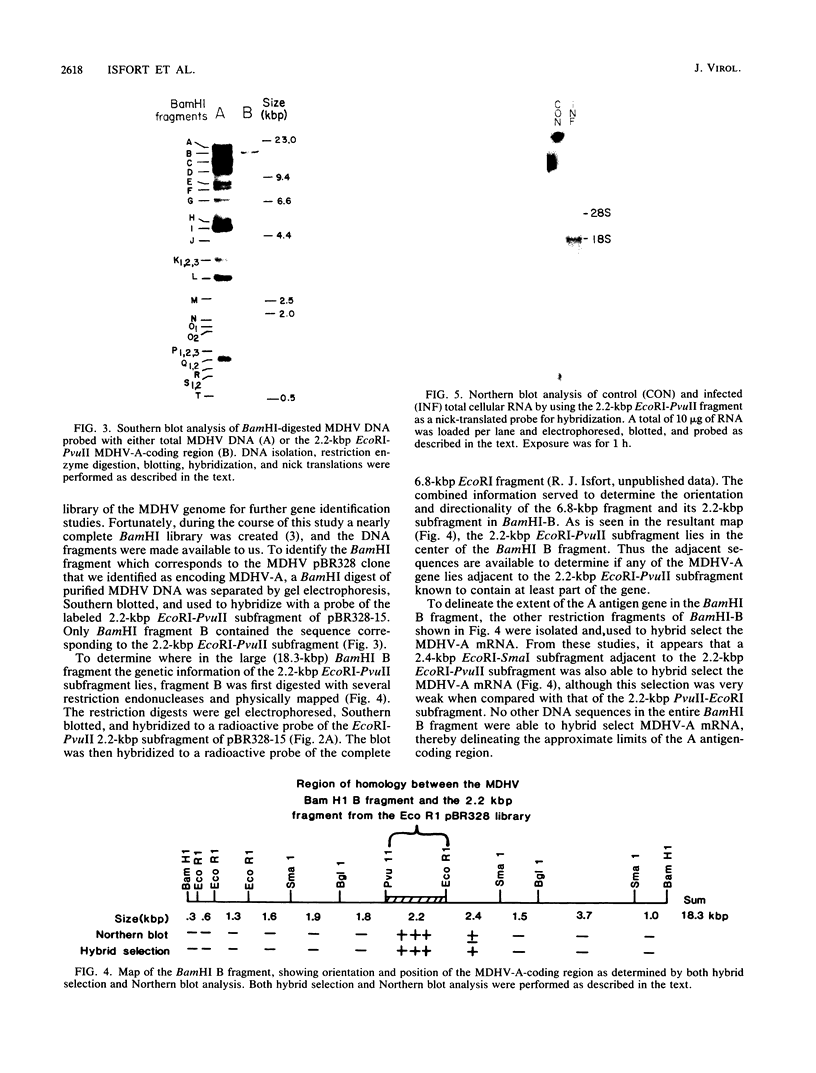
The gene encoding the glycoprotein Marek's disease herpesvirus A antigen (MDHV-A) precursor polypeptide pr47 was delineated by using Northern blot (RNA blot) analysis and hybrid selection of its mRNA with cloned MDHV DNA, cell-free translation of the mRNA, and immunoprecipitation of the polypeptide. The resulting piece of DNA with strongly positive hybrid selection results was a 2.2-kilobase-pair (kbp) PvuII-EcoRI restriction fragment localized to the center of the 18.3-kbp MDHV BamHI B fragment of the total virus genome. The localization was specific since no other small restriction subfragment of the larger BamHI B fragment was able to hybrid select significant MDHV-A mRNA and the gene mapped only in the BamHI B fragment of the total virus genome. Northern blot analysis confirmed the localization of the MDHV-A gene on the 2.2-kbp fragment and detected its mRNA as a 1.8-kilobase species, a size consistent with encoding a 47-kilodalton polypeptide. This is the first report of an MDHV gene being mapped to the MDHV viral genome. This opens the way for the use of recombinant DNA technology to study the nature of the gene encoding a secreted virus-specific glycoprotein that could possibly be involved in immunoprevention, immunosuppression, or immunoevasion, immune phenomena known or speculated to be involved in this oncogenic herpesvirus system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. P., Nazerian K., Velicer L. F., Kung H. J. Extensive homology exists between Marek disease herpesvirus and its vaccine virus, herpesvirus of turkeys. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3365–3369. doi: 10.1073/pnas.81.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Stubblefield E., Nazarian K., Varmus H. E. DNA of a chicken herpesvirus is associated with at least two chromosomes in a chicken lymphoblastoid cell line. Virology. 1980 Aug;105(1):234–240. doi: 10.1016/0042-6822(80)90170-1. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Monoclonal antibodies reactive with the surface and secreted glycoproteins of Marek's disease virus and herpesvirus of turkeys. J Gen Virol. 1983 Dec;64(Pt 12):2597–2610. doi: 10.1099/0022-1317-64-12-2597. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Most virus-specific polypeptides in cells productively infected with Marek's disease virus or herpesvirus of turkeys possess cross-reactive determinants. J Gen Virol. 1983 Apr;64(Pt 4):961–965. doi: 10.1099/0022-1317-64-4-961. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Sithole I., Kung H. J., Velicer L. F. Molecular characterization of Marek's disease herpesvirus B antigen. J Virol. 1986 Aug;59(2):411–419. doi: 10.1128/jvi.59.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Stringer R. A., Kung H. J., Velicer L. F. Synthesis, processing, and secretion of the Marek's disease herpesvirus A antigen glycoprotein. J Virol. 1986 Feb;57(2):464–474. doi: 10.1128/jvi.57.2.464-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Long P. A., Clark J. L., Velicer L. F. Marek's Disease Herpesviruses II. Purification and Further Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1192–1201. doi: 10.1128/jvi.15.5.1192-1201.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P. A., Kaveh-Yamini P., Velicer L. F. Marek's Disease Herpesviruses I. Production and Preliminary Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1182–1191. doi: 10.1128/jvi.15.5.1182-1191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazerian K. Studies on intracellular and membrane antigens induced by Marek's disease virus. J Gen Virol. 1973 Oct;21:193–195. doi: 10.1099/0022-1317-21-1-193. [DOI] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Witter R. L. Genomic expansion of Marek's disease virus DNA is associated with serial in vitro passage. J Virol. 1985 Jun;54(3):690–696. doi: 10.1128/jvi.54.3.690-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Beiss B., Israel E. Virus-mediated abrogation of chicken lymphocyte responsiveness to mitogenic stimulus. Avian Dis. 1980 Jul-Sep;24(3):580–590. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]