Abstract
The solid-state x-ray image intensifier (SSXII) is an EMCCD-based x-ray detector designed to satisfy an increasing need for high-resolution real-time images, while offering significant improvements over current flat panel detectors (FPDs) and x-ray image intensifiers (XIIs). FPDs are replacing XIIs because they reduce/eliminate veiling glare, pincushion or s-shaped distortions and are physically flat. However, FPDs suffer from excessive lag and ghosting and their performance has been disappointing for low-exposure-per-frame procedures due to excessive instrumentation-noise. XIIs and FPDs both have limited resolution capabilities of ~3 cycles/mm. To overcome these limitations a prototype SSXII module has been developed, consisting of a 1k × 1k, 8 μm pixel EMCCD with a fiber-optic input window, which views a 350 μm thick CsI(Tl) phosphor via a 4:1 magnifying fiber-optic-taper (FOT). Arrays of such modules will provide a larger field-of-view. Detector MTF, DQE, and instrumentation-noise equivalent exposure (INEE) were measured to evaluate the SSXIIs performance using a standard x-ray spectrum (IEC RQA5), allowing for comparison with current state-of-the-art detectors. The MTF was 0.20 at 3 cycles/mm, comparable to standard detectors, and better than 0.05 up to 7 cycles/mm, well beyond current capabilities. DQE curves indicate no degradation from high-angiographic to low-fluoroscopic exposures (< 2% deviation in overall DQE from 1.3 mR to 2.7 μR), demonstrating negligible instrumentation-noise, even with low input signal intensities. An INEE of < 0.2 μR was measured for the highest-resolution mode (32 μm effective pixel size). Comparison images between detector technologies qualitatively demonstrate these improved imaging capabilities provided by the SSXII.
Keywords: solid-state x-ray image intensifier, SSXII, EMCCD, detectors, high-resolution, MTF, DQE, INEE
1. INTRODUCTION
With the onset of improved, patient-specific treatments for vascular disease and advancing diagnostic techniques, there is an increasing need for high-quality, high-resolution images obtainable in real time1. Current state-of-the-art medical x-ray image intensifiers (XII) have inherent limitations2. They are physically cumbersome and suffer from various distortions as a result of the signal amplification process, including susceptibility to the Earth’s magnetic field. As a result, XIIs are being replaced with flat panel detectors (FPDs). FPDs are used for dynamic x-ray imaging in fluoroscopy and angiography because they do not suffer from veiling glare or pincushion and s-shaped distortion. However, their performance has been disappointing for low exposure per frame procedures because these detectors suffer from excessive instrumentation noise, lag and ghosting3,4,5,6. Additionally, both XIIs and FPDs only have resolution capabilities of roughly 3 line pairs per millimeter (lp/mm). To overcome these limitations, our group has been developing a new digital dynamic x-ray image receptor designated the solid state x-ray image intensifier (SSXII), based on electron-multiplying CCDs (EMCCDs), which has the advantages of FPDs but with no lag or ghosting, negligible instrumentation noise and higher-resolution capability due to the smaller pixel sizes7,8,9,10,11.
Linear-system analytical quantities such as the modulation transfer function (MTF) and the detective quantum efficiency (DQE) are used to provide an objective evaluation of the inherent performance of the SSXII system in terms of resolution and signal-to-noise response12. These figures-of-merit also enable a direct comparison with current detector technologies, assuming care is taken to ensure standardized x-ray imaging conditions are utilized, as per the IEC guidelines (report 62220-1)13, as these metrics are sensitive to the x-ray energies used. The minimum operating exposure of the detector can be assessed by measuring the instrumentation-noise equivalent exposure (INEE)7,14,15,16. The INEE metric also facilitates direct comparison between detector technologies by providing the precise exposure at which the detector noise is equivalent to the quantum-noise, below which the instrumentation-noise dominates and degrades image quality and the resulting signal-to-noise ratio (SNR).
Previous theoretical analysis of these metrics has indicated that electron-multiplying CCD (EMCCD) technology, adapted for x-ray imaging, has the potential for providing a nearly ideal imaging platform8. Variable signal amplification occurs directly on the EMCCD sensor, in solid-state, prior to the analog-to-digital converter. This allows for the effective elimination of read-noise degradations17, even at the low signal intensities experienced in fluoroscopy. Small pixel sizes, on the order of 10 μm, allow for extremely high-resolution imaging, limited only by the phosphor blur and demands of the field-of-view (FOV) requirements dictated by the imaging procedure. Depending on the imaging task, various component configurations may be utilized to provide extremely high-resolution in a small region-of-interest (ROI) or coarser resolution with a broader FOV. Measurements with the first SSXII prototype demonstrated bar patter resolution of 20 lp/mm in a small FOV9. The work presented here involves a system modification to increase the FOV with the installation of a 4:1 magnifying fiber-optic taper (FOT).
In this study, we present a detailed performance assessment of the SSXII using the above metrics and will discuss how these measurements compare with theoretical results published previously, in which a parallel cascade model was developed from which the detector MTF, DQE and INEE was calculated8. The detector performance is then compared with FPDs in order to quantitatively establish improvements provided by the SSXII. Qualitative comparisons are then done by showing images acquired with each of the different detector technologies (a FPD, XII, and SSXII) under nearly identical imaging conditions.
2. MATERIALS AND METHODS
2.1 The Solid-State X-Ray Image Intensifier
The prototype SSXII module7,8, shown in figure 1, is composed of an electron-multiplying CCD (EMCCD) camera system (Photonic Science LTD, East Sussex, UK) in which a custom fiber optic input window has been installed. The camera utilizes an EMCCD sensor (Texas Instruments, Dallas, TX), featuring 1000 × 1000 8 μm pixels and has a standard 12-bit camera-link data output. It views a 350 μm thick CsI(Tl) scintillating phosphor via a 4:1 fiber-optic taper (FOT) (Incom, Inc., Charlton, MA) providing an effective pixel size of 32 μm. Larger pixel sizes can be achieved with variable binning. The EMCCD sensor operates like a standard frame-transfer CCD, with the addition of a unique multiplication register prior to the readout of the signal. This multiplication register provides a built-in adjustable gain which amplifies the signal, in solid-state, prior to the addition of read-noise - allowing for the effective elimination of read-noise degradations at lower x-ray exposures. A Peltier cooler is used to cool the sensor (Δ45 °C), reducing thermal electron noise to negligible levels. This is a crucial element as dark-noise is propagated, along with the signal, through the multiplication register potentially decreasing image SNR in the higher gain modes. Easily interchangeable front-end components allow for task-based optimization of the detector system for both diagnostic and interventional medical imaging procedures.
Figure 1.
Figure 1(a). Schematic of the solid-state x-ray image intensifier (SSXII) module which includes a CsI(Tl) scintillating plate, a fiber-optic taper (FOT) and an EMCCD camera with fiber-optic input window. Note: the FOT holder has been omitted so that the inner components can be seen.
Figure 1(b). A photograph of the assembled SSXII detector module with the power supply unit attached.
A single module may be appropriate for high-resolution ROI imaging18 and high-resolution ROI computed tomography19,20,21,22,23,24 at a targeted site of interest (i.e. the tip of a catheter or interventional sight) located using a lower-resolution, large FOV detector27. However, the modular design is extensible to an array configuration for general radiographic imaging, with the ability to select various high-resolution ROIs using adaptive binning and modular selection to provide a complete all-in-one detector capable of both gross and acute imaging7.
2.2 Image Quality Metrics: MTF, DQE and INEE
Measurements of the MTF and DQE were taken according to the IEC 62220-1 guidelines, using the RQA 5 spectrum, allowing for a fair comparison among various detectors which also have been measured using this spectrum. This spectrum was obtained using a 74 kVp x-ray beam with 21mm Al (alloy 1100) added filtration25, providing a HVL of ~7.1 mm Al, determined using spectrum generating software26. The SSXII was placed in front of the XII/FPD on a c-arm system (Toshiba Medical Systems Corp., Tustin CA), with a source-to-image distance of 95 cm (the current maximum). The raw images were corrected for offset and gain prior to being analyzed. The MTF was measured using the standard edge spread technique28, imaging a tungsten plate with a precision edge angulated at 1.5 degrees to the detector axis. Measurements were done both before and after the installation of the 4:1 FOT in order to assess the effects of using larger taper ratios on the detector resolution. NPS measurements were obtained using a minimum of 60 flat images, acquired at various exposures ranging from 1.3 mR to 2.7 μR, as measured with an ionization chamber (Model 35050A dosimeter with model 96035B ionization chamber, Keithley Instruments Inc., Cleveland, OH). To compensate for potential degradation due to read-noise inclusion, the variable EMCCD gain was adjusted so as to maintain an output signal level of at least 25% of saturation (1000 digital units). The DQE was then calculated from these results and compared with a theoretical analysis which has been described previously.
MTF measurements were available for the Paxscan 2520 FPD in which the authors utilized the RQA 5 standard29. Additionally, MTF and DQE measurements were done on a Paxscan 2020 FPD, with the image processing disabled, under the same imaging conditions used for the SSXII. These FPD results were used for comparison purposes.
The INEE was measured using a non-standardized spectrum, which was required to get the low entrance exposures necessary (< 1 μR) to allow use of higher EMCCD gains (> 100x). Like the MTF and DQE, the INEE is also dependent on the incident x-ray energy, however, this analysis was simply meant to quantify the operating range of exposures and to demonstrate quantum-noise limited operation, even at extremely low detector entrance exposures. A 70kVp x-ray beam was used with added filtration resulting in a half value layer of 8.75 mm Al. The signal variance of 60 flat images was measured over a range of exposures (1 – 0.3 μR) and a linear curve was fit to a plot of this variance versus measured exposure, with the intercept representing the instrumentation-noise, i.e the variance at zero exposure, in squared digital units and the slope representing the conversion factor relating digital units to equivalent exposure values. The INEE was then determined using the slope and intercept of this fit.
2.3 Phantom Comparison: SSXII Versus Current State-of-the-art
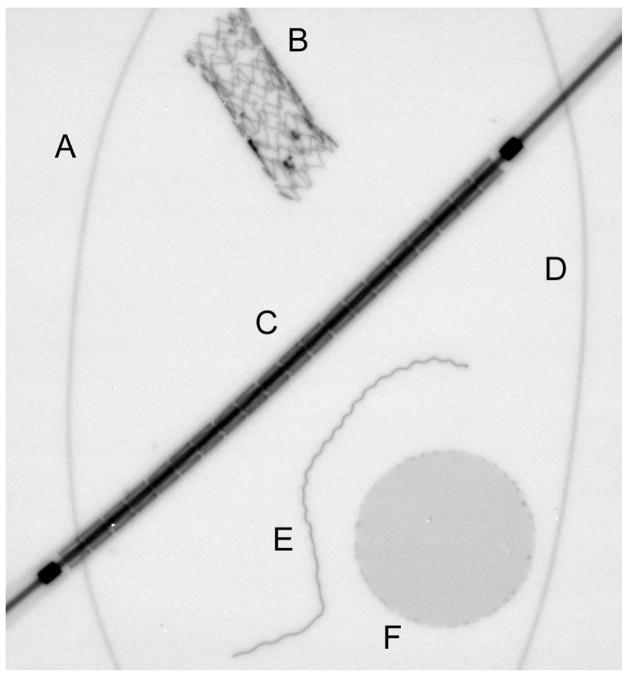
To qualitatively show how these performance improvements translate to actual images, an image comparison was done. A phantom was constructed containing objects relevant to neurovascular image-guided interventional procedures. Figure 2 shows the phantom, consisting of two contrast filled polyethylene tubes with inner diameters of 150 and 200 μm, a 50 μm diameter platinum coil which has been stretched, a stent crimped on a 1mm diameter balloon tipped catheter (Boston Scientific, Natick, MA), an expanded asymmetric stent with platinum markers1 and a mesh patch used for flow diversion on the first generation of asymmetric stents30. Images were acquired of the phantom for the SSXII with 32 μm pixels, a FPD (Paxscan 2020, Varian Medical Systems, Inc. Palo Alto, CA) with a pixel size of 194 μm and an XII (Toshiba Medical Systems Corp.) with 120 μm pixels. In order to gauge inherent detector performance, images were acquired in the absence of scattering material, with the phantom placed as close to the detector input as possible so as to minimize any effects of geometric unsharpness. As there was no scattering material present in this analysis, the x-ray scatter grid was removed from all detectors. Imaging was done using the RQA 5 x-ray spectrum.
Figure 2.
Radiograph taken with the SSXII of the neurovascular device phantom consisting of : A) 150 μm diameter contrast filled polyethylene tubing, B) asymmetric stent with platinum markers, C) stent crimped on a 1 mm diameter balloon tipped catheter, D) 200 μm diameter contrast filled polyethylene tubing, E) stretched platinum coil and F) stainless-steel mesh patch.
3. RESULTS AND DISCUSSION
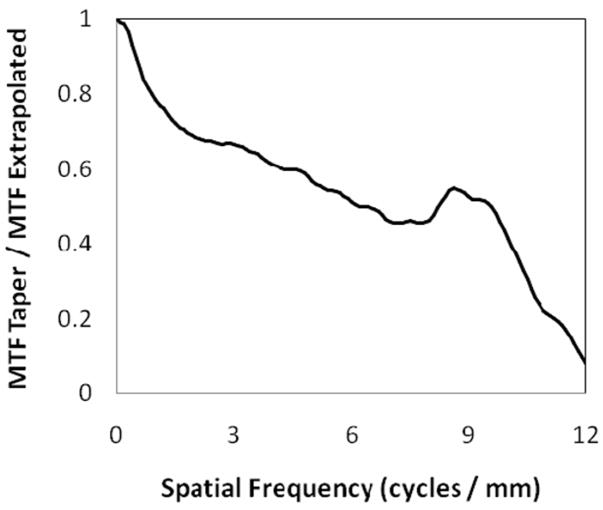
Figure 3 shows the MTF up to 12 cycles/mm, measured for the SSXII with a 4:1 FOT (MTFTaper). An average of three separate measurements was used in order to help reduce uncertainty and to provide a smoother curve. The MTF was also measured without the FOT installed (MTFNo Taper) in order to determine the resolution modifying effects of the taper. In varying the FOT magnification ratio from 1:1 to 4:1, the output frequency modulation was expected to differ only by the difference in the multiplication of the sinc function of the respective pixel sizes, which in this case was 8 μm versus 32 μm, assuming similar fiber size. This correction factor, sinc(32)/sinc(8), was then multiplied with MTFNo Taper to get an extrapolated value, MTFExtrapolated, which could be used for direct comparison with MTFTaper. Given that phosphor blur is the dominant degrading factor across all frequencies8, it was expected that these two results would converge. However, there is clearly a discrepancy between the two measurements. This difference is quantified in figure 4 where MTFTaper/MTFExtrapolated was plot as a function of spatial frequency. This additional reduction of modulation transfer may be attributable to several different physical phenomena. There may be x-rays passing through the CsI(Tl) or k-fluorescent x-rays escaping the phosphor which in turn interact with the FOT, potentially resulting in Compton scattering back into the phosphor. These scattered photons may then be converted into light, eventually reaching the sensor and resulting in a loss of image contrast. There also may be some amount of veiling glare associated with the FOT or light spread across the individual fibers in the taper. Further investigation will be required to fully diagnose the cause and potential solution of this additional modulation. Despite this, the MTF indicates good resolution performance both with and without the 4:1 FOT. Both configurations have demonstrated 10 lp/mm bar-pattern resolution capabilities with no geometric magnification.
Figure 3.
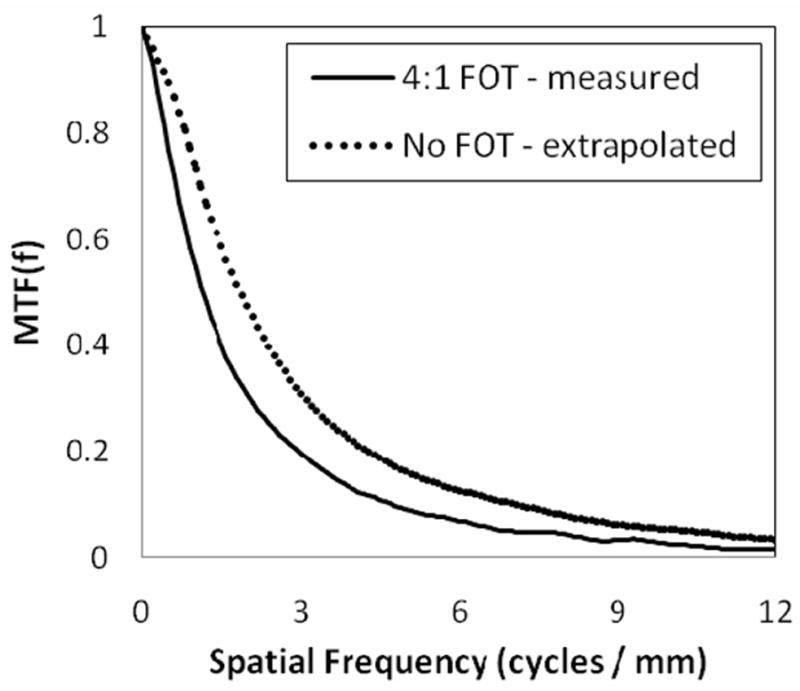
MTF of the SSXII with (solid line) and without (dotted line) a 4:1 FOT. For comparison, the MTFNo Taper was corrected for the difference in effective pixel size to allow comparison between the two curves. Resolution well beyond 6 cycles/mm is demonstrated in both curves.
Figure 4.
The difference between the two MTF curves in fig. 3 is determined as a function of spatial frequency by dividing the two, showing additional modulation resulting from the fiber-optic taper.
The measured DQE of the SSXII is shown in figure 5. The theoretical DQE is also shown, demonstrating reasonably good agreement given the large number of parameters involved in the calculated result, and thus indicating good understanding of the detector system as described in more detail previously8. Figure 6 shows the frequency dependent DQE for a wide range of exposures, from angiographic (1.3 mR) to fluoroscopic levels (2.7 μR). The EMCCD gain was varied from 10x to 340x to provide appropriate signal amplification. A semi logarithmic plot was used to better illustrate that there is no noticeable degradation in the DQE across all frequencies, thus demonstrating the SSXII’s ability to maintain quantum noise-limited performance, even at low fluoroscopic exposures.
Figure 5.
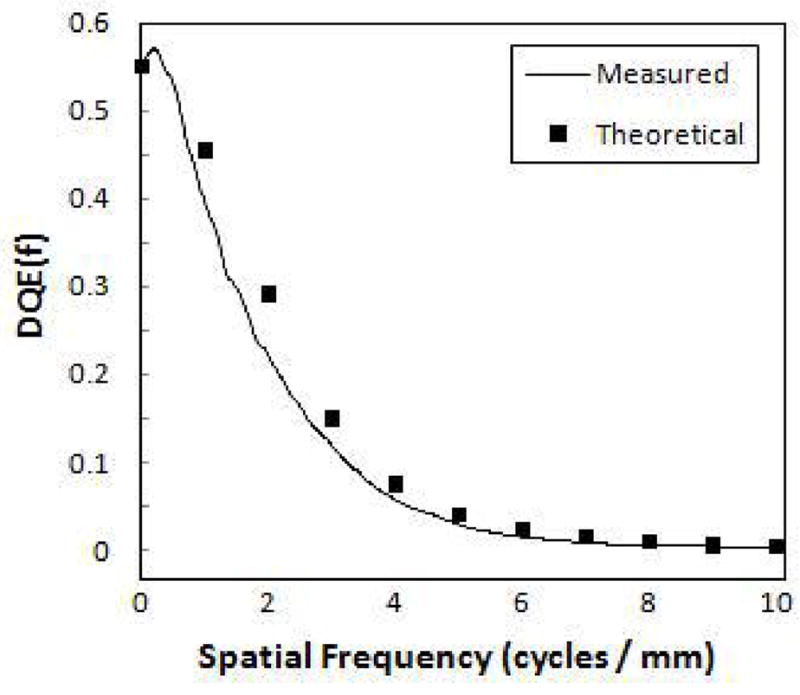
The measured DQE of the SSXII. Calculated results from a parallel cascade model demonstrate good agreement.
Figure 6.
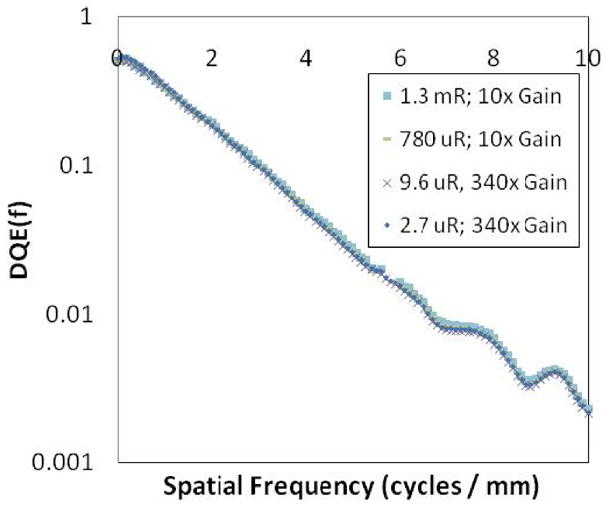
DQE of the SSXII on a semi-log plot for various exposures, indicating there is no drop as a result of instrumentation noise inclusion, even at low exposures.
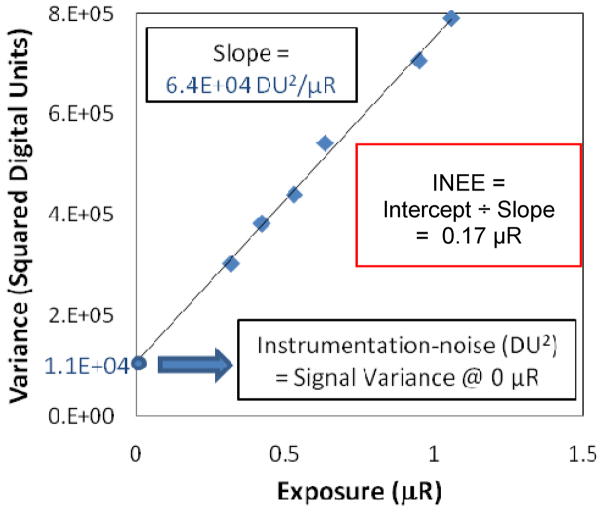
The INEE was measured using the variance versus exposure plot shown in figure 7. The INEE was determined from the linear regression coefficients, by dividing the intercept by the slope, resulting in a value of 0.17 μR in the highest resolution mode (32 μm pixels). It is at this exposure that the quantum noise and instrumentation noise are equivalent, demonstrating a capability to effectively operate at even the lowest fluoroscopic exposure levels. This value is dependent on the incident x-ray statistics, and thus the pixel size, as the number of incident x-rays per pixel depends on the pixel area. With the implementation of 2 × 2 binning, the INEE would be further lowered by a factor of 4. For comparison, the INEE for indirect FPDs has been measured indirectly by Roos et al. to be 2.75 μR, with a pixel size of 194 μm31. This would translate to an INEE of 99 μR for a detector of the same design with 32 μm pixels, or a factor of ~36x increase, demonstrating superior noise performance of the SSXII over FPDs at low exposures. A measure of the INEE is a simple but effective method for determining the precise exposure value at which the quantum noise equals the instrumentation noise, thus decreasing the DQE by 50%. This allows quantification of the inherent noise limitations of the detector, while also incorporating the detector’s sensitivity.
Figure 7.
Measured variance versus exposure data and the corresponding linear regression are used to measure an instrumentation-noise equivalent exposure (INEE) of 0.17 μR
In order to put these results in perspective, two different indirect FPDs were used for comparison. Samei et al. have published MTF measurements from the Varian Paxscan 2520 (127 μm pixels)29. Additionally, MTF and DQE measurements have been performed in our laboratory on a Varian Paxscan 2020 (194 μm pixels). To minimize the averaging affects of lag, images were acquired at 1 frame per second. In both cases, the RQA 5 spectrum was utilized, allowing for a direct comparison. Highly non-linear processing, recursive filtering and lag are generally implemented on clinical machines in an attempt to improve perceived image quality, all of which may reduce or alter noise measurements. Thus, for an accurate account of inherent detector noise and performance, it is imperative that any imaging processing be disabled prior to the measurement of these fundamental parameters. Figure 8 shows the measured MTF of the SSXII and the two FPDs, demonstrating similar resolving capabilities at low frequencies (< 3 cycles/mm). This plot also demonstrates that the SSXII MTF extends well beyond the limits of current state-of-the-art FPDs. The SSXII MTF was measured to be 0.2 at 3 cycles/mm and 0.05 at 7 cycles/mm. Depending on the imaging task, a thinner phosphor could be implemented to further improve resolution performance as this is the main limitation.
Figure 8.
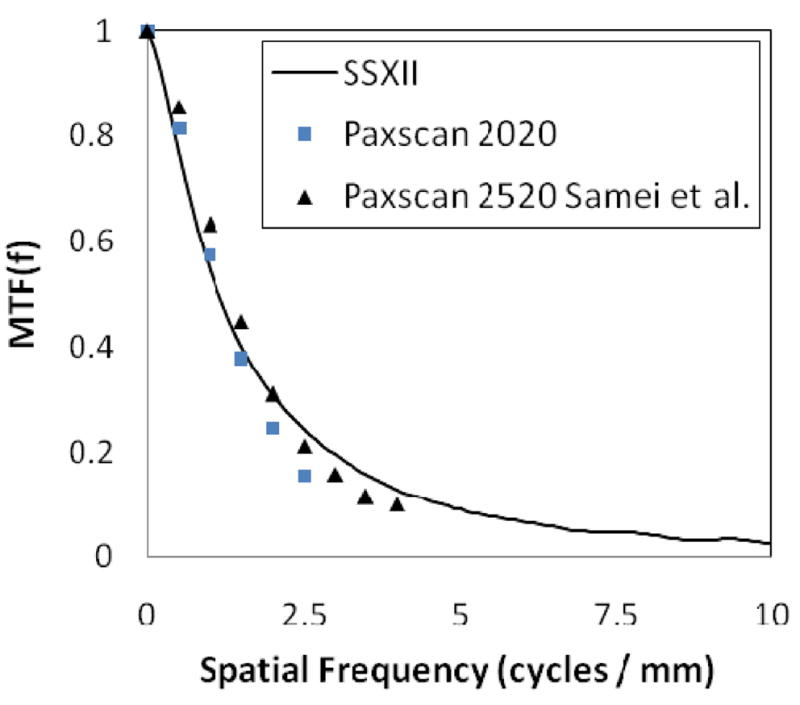
MTF comparison between the SSXII and two FPDs.
Figure 9 shows a comparative plot of the DQE for the SSXII and the Paxscan 2020 obtained using the same imaging conditions. The FPD DQE is shown to be substantially less (50% less) than that of the SSXII at 2.7 μR, as a result of instrumentation-noise degradation (INEE ~ 2.75 μR), whereas the SSXII DQE was shown to remain constant (see figure 6) down to these lower exposures as a result of having negligible instrumentation-noise (INEE < 0.2 μR). Furthermore, the SSXII DQE extends well beyond the FPD Nyquist frequency of 2.5 cycles/mm.
Figure 9.
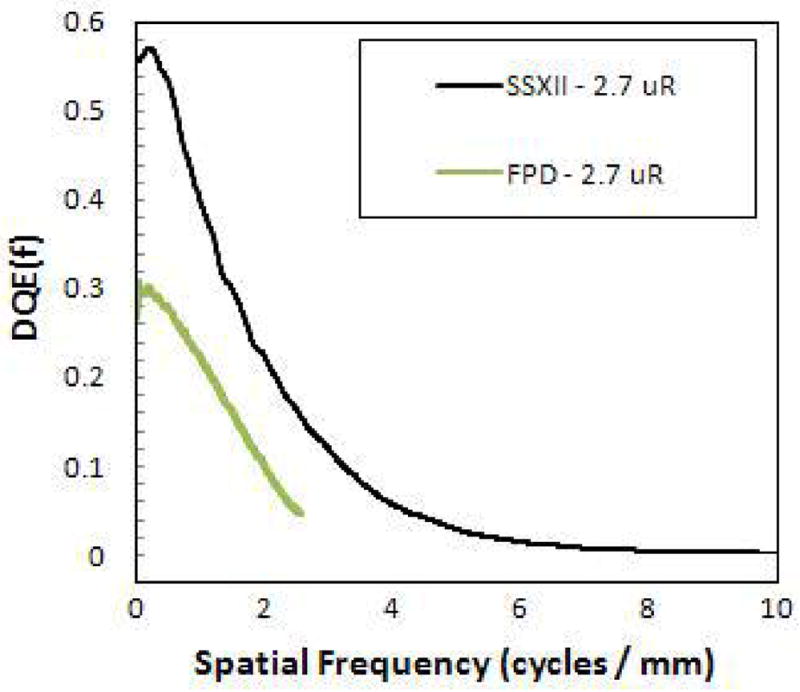
DQE comparison between the SSXII and a FPD demonstrating substantial improvements of the SSXII at low exposures.
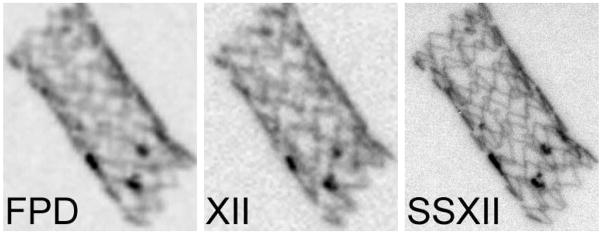
Figure 10 and figure 11 show comparison images acquired of the neurovascular device phantom (figure 2) using a FPD, an XII and the new SSXII using RQA 5 and an exposure of 2.5 mR. The pixel sizes of each detector are 194, 120 and 32 μm respectively. Figure 10 shows the expanded asymmetric stent in which platinum markers have been placed at the periphery of the flow diverting polyurethane patch. It is difficult to discern the individual stent struts in the FPD image and the XII does slightly better as a result of having a smaller pixel size. However, the SSXII outperforms both, clearly resolving the struts. Figure 11 shows a different region of the neurovascular device phantom. The SSXII image is sharper and individual stent struts can be distinguished on the crimped stent. Also, individual turns of the platinum coil and the puddling of molten steel on the edges of the mesh patch as a result of the cut-out process can be seen.
Figure 10.
Images of an expanded stent with platinum markers taken with a FPD, an XII and the SSXII with 194, 120, and 32 μm pixel sizes respectively, demonstrating the improved resolution of the SSXII where individual stent struts are clearly visible.
Figure 11.
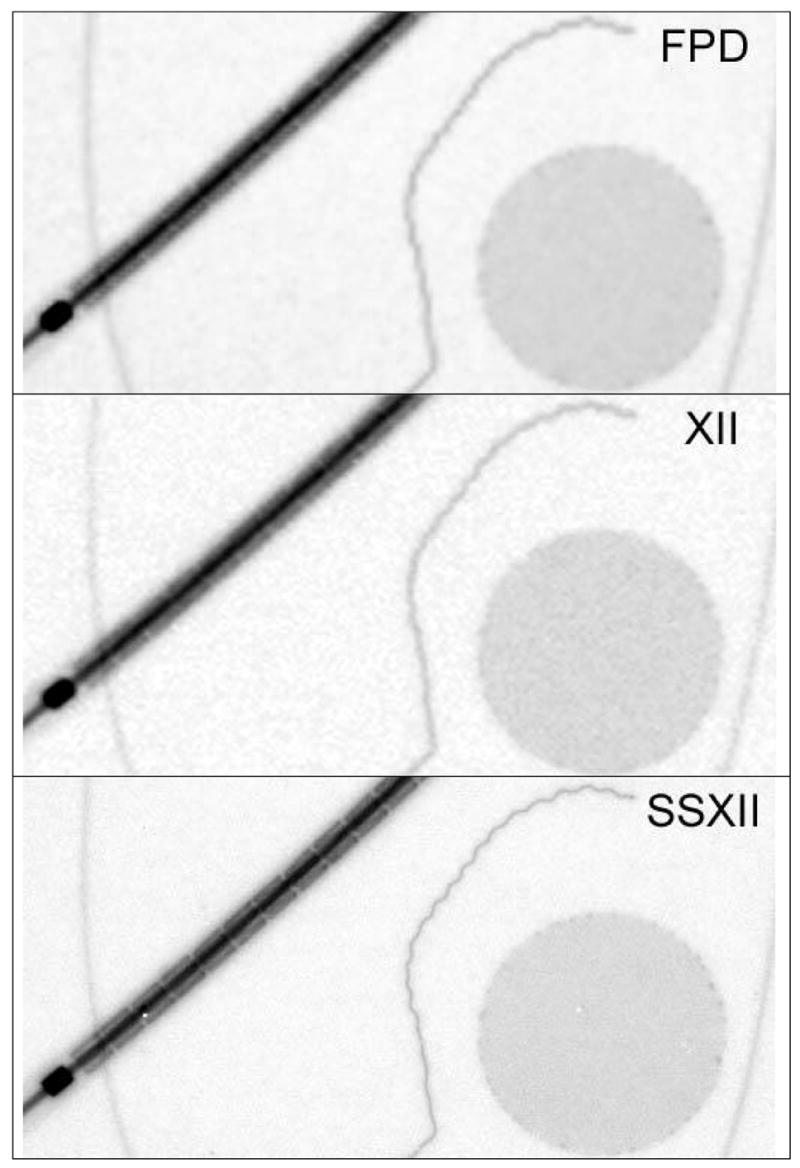
Images taken of a neurovascular device phantom with a FPD, an XII and the SSXII with 194, 120, and 32 μm pixel sizes respectively, further demonstrating the improved resolution of the SSXII.
4. SUMMARY AND CONCLUSION
Initial use of the solid-state x-ray image intensifier has shown that this technology has great promise to be the next-generation dynamic x-ray imager, overcoming the inherent limitations of x-ray image intensifiers and flat panel detectors. MTF measurements have indicated the ability to resolve upwards of 10 cycles/mm, a vast increase in resolution over current state-of-the-art x-ray image intensifiers and flat panel detectors. The measured DQE, taken over a range of clinically relevant exposure conditions has demonstrated quantum-limited, instrumentation-noise-free images over three orders of magnitude. The instrumentation-noise equivalent exposure was measured to be less than 0.2 μR, indicating the ability to operate with negligible instrumentation-noise well below even the lowest of fluoroscopic exposure levels. Comparative images taken between the different detector technologies, under the same exposure conditions, x-ray spectrum and imaging geometry reveal that this new solid-state imager has the capability of providing large improvements over current technologies.
Acknowledgments
This work was supported in part by the UB Interdisciplinary Research Development Fund (IRDF), NIH R01 Grants EB002873, NS43924 and an equipment grant from Toshiba Medical Systems Corp.
References
- 1.Rudin S, Bednarek DR, Hoffmann KR. VISION 20/20: “Endovascular image-guided interventions EIGIs) Med Phys. 2008;35(1):301–309. doi: 10.1118/1.2821702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudin S, Bednarek DR, Wong R. Accurate characterization of image intensifier distortion. Med Phys. 1991;18(6):1145–1151. doi: 10.1118/1.596623. [DOI] [PubMed] [Google Scholar]
- 3.Hunt DC, Tousignant O, Rowlands JA. Evaluation of the imaging properties of an amorphous selenium-based flat panel detector for digital fluoroscopy. Med Phys. 2004;31(5):1166–1175. doi: 10.1118/1.1707755. [DOI] [PubMed] [Google Scholar]
- 4.Maolinbay M, El-Mohri Y, Antonuk LE, Jee KW, Nassif S, Rong X, Zhao Q. Additive noise properties of active matrix flat-panel imagers. Med Phys. 2000;27(8):1841–1854. doi: 10.1118/1.1286721. [DOI] [PubMed] [Google Scholar]
- 5.Siewerdsen JH, Jaffray DA. A ghost story: Spatio-temporal response characteristics of an indirect-detection flat-panel imager. Med Phys. 1999;26(8):1530–1541. doi: 10.1118/1.598657. [DOI] [PubMed] [Google Scholar]
- 6.Siewerdsen JH, Antonuk LE, El-Mohri Y, Yorkston J, Huang W, Boudry JM, Cunningham IA. Empirical investigation of the noise performance of indirect detection, active matrix flat-panel imagers (AMFPIs) for diagnostic radiology. Med Phys. 1997;24(1):71–89. doi: 10.1118/1.597919. [DOI] [PubMed] [Google Scholar]
- 7.Rudin S, Kuhls AT, Yadava GK, Josan GC, Wu Y, Chityala RN, Rangwala HS, Ionita C, Hoffmann KR, Bednarek DR. New light-amplifier-based detector designs for high spatial resolution and high sensitivity CBCT mammography and fluoroscopy. Proc SPIE. 2006;6142:61421R. doi: 10.1117/12.649501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhls AT, Yadava G, Patel V, Bednarek DR, Rudin S. Progress in electron-multiplying CCD (EMCCD) based high-resolution high-sensitivity x-ray detector for fluoroscopy and radiography. Proc SPIE. 2007;6510:65101C. doi: 10.1117/12.713140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhls A, Yadava G, Patel V, Bednarek D, Rudin S. Linear Systems Analysis for a New Solid State X-Ray Image Intensifier (SSXII) Based on Electron-Multiplying Charge-Coupled Devices (EMCCDs) Med Phys. 2007;34:WE-C-L100J-06. [Google Scholar]
- 10.Rudin S, Kuhls A, Keleshis C, Kim D, Yadava G, Patel V, Ionita C, Hamwi H, Cartwright A, Verevkin A, Hoffmann K, Bednarek D. The Solid State X-Ray Image Intensifier (SSXII): A Next-Generation High-Resolution Fluoroscopic Detector System. Med Phys. 2007;34:WE-C-L100J-04. [Google Scholar]
- 11.Kuhls A, Yadava G, Bednarek D, Rudin S. The New Solid State X-Ray Image Intensifier (SSXII): A Demonstration of Operation Over a Range of Angiographic and Fluoroscopic Exposure Levels. Med Phys. 2007;34:WE-C-L100J-03. [Google Scholar]
- 12.Cunningham IA, Dobbins JT., III . Handbook of Medical Imaging Physics and Psychophysics. In: Beutel J, Kundel HL, Van Metter RL, editors. SPIE; Bellingham, WA: 2000. pp. 79–222. [Google Scholar]
- 13.IEC 62220-1:2003, Medical electrical equipment - Characteristics of digital X-ray imaging devices - Part 1: Determination of the detective quantum efficiency
- 14.Yadava G, Rudin S, Kuhls A, Patel V, Bednarek D, Hoffmann K. Instrumentation Noise Equivalent Exposure (INEE) Representation for High Sensitivity X-Ray Imaging Detectors. Med Phys. 2006;33:TU-FF-A4-01. [Google Scholar]
- 15.Yadava G, Rudin S, Kuhls A, Bednarek D. Instrumentation Noise Equivalent Exposure (INEE) and the Effect of Detecctor Blurring and Image Post-Process Smoothing: A Simulation Study. Med Phys. 2007;34:TH-C-L100F-02. [Google Scholar]
- 16.Yadava GK, Kuhls-Gilcrist AT, Rudin S, Patel VK, Bednarek DR, Hoffmann KR. A practical exposure-equivalent metric for instrumentation noise in x-ray imaging systems. In Submission to Physics in Medicine and Biology. 2008 doi: 10.1088/0031-9155/53/18/017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynecek J. Impactron - A New Solid State Image Intensifier. IEEE Trans Electron Devices. 2001;48:2238–2241. [Google Scholar]
- 18.Rudin S, Bednarek DR, Wong R. Region of interest fluoroscopy. Med Phys. 1992;19(5):1183–1189. doi: 10.1118/1.596792. [DOI] [PubMed] [Google Scholar]
- 19.Chityala R, Hoffmann KR, Bednarek DR, Rudin S. Region of interest (ROI) computer tomography (CT): comparison with full field of view (FFOV) and truncated CT for a human head phantom. Proc SPIE. 2005;5745:583–890. doi: 10.1117/12.595430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ionita CN, Keleshis C, Patel V, Yadava G, Hoffmann KR, Bednarek DR, Jain A, Rudin S. Proc SPIE. 2008. Implementation of high-sensitivity microangiographic fluoroscope (HS-MAF) for in-vivo endovascular image guided interventions (EIGI) and ROI-CT. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chityala RN. Region of Interest Cone Beam Computed Tomography (ROI-CBCT) Ph. D. Dissertation submitted to Department of Mechanical and Aerospace Engineering; 2007. [Google Scholar]
- 22.Chityala RN, Hoffmann KR, Rudin S, Bednarek DR. Region-of-interest (ROI) Computed Tomography: Combining dual resolution XRII images. Med Phys. 2005;32(6):1888. [Google Scholar]
- 23.Chityala RN, Hoffmann KR, Rudin S, Bednarek DR. High-Resolution Region-of-interest (ROI) Cone-Beam CT. Proc RSNA. 2005:392. [Google Scholar]
- 24.Chityala RN, Patel V, Hoffmann KR, Rudin S, Bednarek DR. Region of Interest (ROI) Cone-beam CT Using Dual Resolution, Dual Detector Image Acquisition: Evaluation of Off Centered ROIs. Proc RSNA. 2006:923. [Google Scholar]
- 25.Ranger NT, Samei E, Dobbins JT, III, Ravin CE. Measurement of the detective quantum efficiency in digital detectors consistent with the IEC 62220-1 standard: Practical considerations regarding the choice of filter material. Med Phys. 2005;32(7):2305–2311. doi: 10.1118/1.1929187. [DOI] [PubMed] [Google Scholar]
- 26.Reilly AJ, Sutton D. IPEM Report 78 - Spectrum processor. York: The Institute of Physics and Engineering in Medicine; 1997. [Google Scholar]
- 27.Kuhls AT, Patel V, Ionita C, Noel PB, Walczak AM, Rangwala HS, Hoffmann KR, Rudin S. New microangiography system development providing improved small vessel imaging, increased contrast to noise ratios, and multi-view 3D reconstructions. Proc SPIE. 2006;6142:61423M–1. doi: 10.1117/12.653654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samei E, Flynn MJ, Reimann DA. A method for measuring the presampled MTF of digital radiographic systems using an edge test device. Med Phys. 1998;25(1):102–113. doi: 10.1118/1.598165. [DOI] [PubMed] [Google Scholar]
- 29.Samei E. Image quality in two phosphor-based flat panel digital radiographic detectors. Med Phys. 2003;30(7):1747–1757. doi: 10.1118/1.1578772. [DOI] [PubMed] [Google Scholar]
- 30.Ionita CN, Rudin S, Hoffmann KR, Bednarek DR. Microangiographic Image Guided Localization of a New Asymmetric Stent for Treatment of Cerebral Aneurysms. Proc SPIE. 2005;5744:354–365. doi: 10.1117/12.594789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos PG, Colbeth RE, Mollov I, Munro P, Pavkovich J, Seppi EJ, Shapiro EG, Tognina CA, Virshup GF, Yu JM, Zentai G, Kaissl W, Matsinos E, Richters J, Riem H. Multiple gain ranging readout method to extend the dynamic range of amorphous silicon flat panel imagers. Proc SPIE. 2004;5368:139–149. [Google Scholar]
- 32.Keleshis CM, Ionita CN, Yadava GK, Bednarek DR, Hoffmann KR, Verevkin A, Rudin S. LabVIEW graphical user interface for a new high sensitivity, high resolution micro-angio-fluoroscopic system. Proc SPIE. doi: 10.1117/12.769630. In Press. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]