Abstract
Background
While symptom questionnaires provide a snapshot of bowel habits, they may not reflect day-to-day variations or the relationship between bowel symptoms and stool form.
Aim
To assess bowel habits by daily diaries in women with and without functional bowel disorders.
Method
From a community-based survey among Olmsted County, MN, women, 278 randomly selected subjects were interviewed by a gastroenterologist, who completed a bowel symptom questionnaire. Subjects also maintained bowel diaries for 2 wk.
Results
Among 278 subjects, questionnaires revealed diarrhea (26%), constipation (21%), or neither (53%). Asymptomatic subjects reported bowel symptoms (e.g., urgency) infrequently (i.e., <25% of the time) and generally for hard or loose stools. Urgency for soft, formed stools (i.e., Bristol form = 4) was more prevalent in subjects with diarrhea (31%) and constipation (27%) than in normals (16%). Stool form, straining to begin (odds ratio [OR] 4.1, 95% confidence interval [CI] 1.7–10.2) and end (OR 4.7, 95% CI 1.6–15.2) defecation increased the odds for constipation. Straining to end defecation (OR 3.7, 95% CI 1.2–12.0), increased stool frequency (OR 1.9, 95% CI 1.02–3.7), incomplete evacuation (OR 2.2, 95% CI 1.04–4.6), and rectal urgency (OR 3.1, 95% CI 1.4–6.6) increased the odds for diarrhea. In contrast, variations in stool frequency and form were not useful for discriminating between health and disease.
Conclusions
Bowel symptoms occur in association with, but are only partly explained by, stool form disturbances. These observations support a role for other pathophysiological mechanisms in functional bowel disorders.
Introduction
Symptom questionnaires are widely used to characterize bowel habits in epidemiological studies and in therapeutic trials for functional bowel disorders. These questionnaires have provided insights into the prevalence, risk factors, disease burden, and natural history of functional bowel disorders (1, 2). In these instruments, respondents are asked to focus on their predominant symptoms over a defined period, typically between 3 and 12 months. So framed, these questions may not adequately assess variability in bowel habits, and they are also prone to recall bias. Indeed, people tend to exaggerate their bowel habits and overestimate their bowel frequency (3, 4). Thus, a comparison of bowel habits by self-report and diaries suggested that 51% of the patients with self-reported constipation underestimated their stool frequency by three or more bowel movements per week, underscoring the utility of a bowel diary for accurately recording bowel habits (5).
Bowel diaries also suggest that several bowel symptoms are influenced by stool form, even among asymptomatic subjects. For example, less than 10% of a sample of healthy subjects from the United Kingdom reported urgency or straining to defecate with smooth and formed stools (i.e., a Bristol form of 4 on a scale of 1–7) (6). In contrast, 100% reported rectal urgency for watery stools (i.e., a form of 7) and over 50% reported straining for hard or lumpy stools (i.e., a form of 1 or 2). Therefore, straining to expel hard stools may not be abnormal. However, currently available bowel questionnaires do not ascertain the relationship between bowel symptoms (e.g., straining) and stool form. This is a significant lacuna, particularly in patients who have varying bowel habits, which is not uncommon in functional bowel disorders (7–10). Indeed, studies from primary care and referral practices suggest that a majority of patients with irritable bowel syndrome (IBS) have symptoms of constipation and diarrhea, termed mixed IBS (or IBS-M) (11, 12).
Our understanding of normal bowel patterns in the community is predominantly derived from the questionnaires. To our knowledge, there are no diary-based data on normal bowel habits or the relationships among bowel symptoms among community residents from the United States. Consequently, as acknowledged in the Rome criteria, the suggested symptom frequencies for defining functional bowel disorders are arbitrary (2). Moreover, some patients find it difficult to relate their symptom frequency to the criteria (e.g., >25% of the time), partly because their symptoms vary (13). An improved understanding of the relationships among bowel symptoms may not only facilitate the diagnosis of functional bowel disease, but also improve our understanding of symptoms in these disorders. To address these issues, we compared bowel habits evaluated by the questionnaires to the bowel diaries among women in the community.
Methods
Study Design
The institutional review board of Mayo Clinic approved this study. Using a validated mailed questionnaire, we identified 507 women with fecal incontinence (FI) among an age-stratified random sample of 5,300 women of Olmsted County, MN (14). Thereafter, 400 women (200 FI and 200 controls) selected at random were invited to participate in a case-control study. From this group, 154 women with FI (age 60 ± 1 yr [mean ± SEM]) and 124 controls without self-reported FI (age 61 ± 1 yr) participated. A single gastroenterologist interviewed subjects about their bowel habits over the past year using a validated bowel symptom questionnaire (Fecal Incontinence and Constipation Assessment [FICA]) (15), clarifying questions when necessary for subjects and completing the questionnaire during the interview. The questions in the FICA were framed to be consistent with Rome II diagnostic criteria for functional bowel disorders and abdominal pain. Responses to questions during the physician interview were used to characterize subjects as normal (i.e., no functional bowel disorder), functional diarrhea, functional constipation, and diarrhea- or constipation-predominant IBS as defined by Rome II criteria. Subsequently, subjects recorded the details of every (i.e., continent and incontinent) bowel movement in detailed diaries for 2 wk.
Statistical Analysis
For each subject, the data (e.g., stool frequency and form, proportion of bowel movements associated with rectal urgency, and straining to begin and to end defecation) were first averaged per day and then over the entire diary duration. Variability in stool frequency over the diary period was summarized by the coefficient of variation. Variability in stool form per subject was assessed by first calculating the proportion (%) of all bowel movements recorded over the diary period corresponding to each type (i.e., 1 [i.e., hard pellets] through 7 [i.e., watery diarrhea]) on the Bristol stool form score. The per-subject variation was then summarized by the highest proportion among the stool form score categories (i.e., the highest proportion over all stool form categories recorded by each subject); higher values (maximum = 100) indicated that a larger proportion of stools were of the same stool form, implying less variability.
A multiple logistic regression model ascertained if bowel symptoms recorded by diaries could predict functional bowel disorders, as defined by questionnaire-based Rome II criteria (13). Consistent with the objectives of this study, subjects were grouped into three categories (i.e., constipation, diarrhea, and “neither”) regardless of whether they did or did not have FI. For this analysis, functional constipation and constipation-predominant IBS were combined in one category, as were functional diarrhea and diarrhea-predominant IBS. The logistic model thus considered a three-category response (with “neither” as the comparison category) using a generalized logit link function.
The association between stool form and other quantitative parameters (e.g., the proportions of stools recorded with rectal urgency and with straining during defecation) was evaluated by comparing stool form scores for bowel movements associated with and without a symptom (e.g., urgency). First, the data for each subject (e.g., mean stool form scores over bowel movements with urgency recorded, and separately the bowel movements without urgency recorded) were summarized. Then, these values were compared by a paired t-test over all subjects with complete pairs, i.e., subjects who never reported a symptom (e.g., urgency) in their diaries were excluded from the analysis for that specific symptom. Clinical observations suggest that a sense of incomplete evacuation may be related to several bowel symptoms (e.g., stool form and straining during defecation). Therefore, a multiple linear regression model was used to assess whether bowel symptoms could predict the proportion of stools with a sense of incomplete evacuation.
Results
Of the 278 subjects, 146 (53%) did not have symptoms of a functional bowel disorder according to symptom-based (Rome II) criteria by the questionnaire (i.e., normals), 72 (26%) had diarrhea (i.e., functional diarrhea or diarrhea-predominant IBS), and 58 (21%) had constipation (i.e., functional constipation or constipation-predominant IBS). The functional bowel disorder status could not be characterized in two subjects.
Comparison of Bowel Habits in Normals and Functional Bowel Disorders
Among subjects without a functional bowel disorder or FI, 90th percentile values for stool frequency and the Bristol stool form were 1.7 and 3.5 stools/day, respectively (Table 1). The 90th percentile values for frequencies of rectal urgency, straining to begin and to end defecation, and the sense of incomplete evacuation were each ≤17%, which is lower than the 25% frequency cutoff for discriminating normal from abnormal in the Rome II criteria.
Table 1.
Upper Limit of Normal (90th Percentile) Values for Bowel Habit Parameters Among Women Without Rome II Symptom Criteria for Functional Bowel Disorders or Fecal Incontinence by Questionnaire
| Bowel Symptom | 10th Percentile | 90th Percentile |
|---|---|---|
| Stool frequency (per day) | 0.9 | 1.7 |
| Stool frequency (CV [%]) | 0 | 45 |
| Bristol stool form score | 2.0 | 3.5 |
| Maximum proportionate stool-form score (%) | 41 | 96 |
| Sense of incomplete evacuation (%) | 0 | 10 |
| Rectal urgency (%) | 0 | 17 |
| Straining to begin (%) | 0 | 7 |
| Straining to end (%) | 0 | 0 |
| Postprandial bowel movements (%) | 0 | 75 |
Data are derived from 124 subjects.
CV (%) = percent coefficient of variation.
Table 2 compares bowel diary summary parameters among women with or without constipation or diarrhea regardless of whether they had FI. Table 3 lists the results from corresponding multiple logistic regression models. Compared to women who did not have a functional bowel disorder, subjects with looser stools had decreased odds of constipation (odds ratio [OR] 0.6, 95% confidence interval [CI] 0.4–1.0). Conversely, straining to begin (OR 4.1, 95% CI 1.7–10.2) and to end (OR 4.7, 95% CI 1.6–15.2) defecation increased the odds for constipation. Straining to end defecation (OR 3.7, 95% CI 1.2–12.0), increased stool frequency (OR 1.9, 95% CI 1.03–3.7), a sense of incomplete evacuation (OR 2.2, 95% CI 1.04–4.6), and rectal urgency (OR 3.1, 95% CI 1.4–6.6) increased the odds for diarrhea. In contrast, variations in stool form or frequency were not independent risk factors of constipation or diarrhea in these multiple logistic regression models.
Table 2.
Comparison of Bowel Habits Recorded by Diaries Among Women With and Without Bowel Symptoms by Questionnaires in Olmsted County, Minnesota
| Bowel Habits by Rome II Criteria* | |||
|---|---|---|---|
| Parameter | Normal | Constipation | Diarrhea |
| Number of subjects | 146 | 58 | 72 |
| Stool frequency (per day) | 1.46 ± 0.04 | 1.70 ± 0.09 | 1.80 ± 0.09 |
| Stool form | 3.0 ± 0.06 | 2.74 ± 0.11 | 3.44 ± 0.12 |
| Maximum proportionate stool form score (%) | 61 ± 2 | 56 ± 0 | 49 ± 20 |
| Sense of incomplete evacuation (%) | 13 ± 2 | 28 ± 3 | 32 ± 4 |
| Rectal urgency (%) | 14 ± 2 | 20 ± 3 | 32 ± 3 |
| Straining to begin (%) | 5 ± 1 | 23 ± 3 | 9±2 |
| Straining to end (%) | 1 ± 1 | 4± 1 | 4±1 |
| Postprandial bowel movements (%) | 34 ± 2 | 25 ± 2 | 32 ± 2 |
Diarrhea and constipation comprise consolidated categories, i.e., the corresponding functional bowel disorder and the subtype of irritable bowel syndrome.
Table 3.
Do Bowel Symptoms Recorded by a Diary Predict the Rome Subtype Among Women in Olmsted County, Minnesota
| Bowel Symptom | Odds Ratios (95% CI) for Constipation Versus Normal | Odds Ratios (95% CI) for Diarrhea Versus Normal |
|---|---|---|
| Stool frequency | 1.54 (0.76–3.09) | 1.91 (1.03–3.66) |
| Stool frequency (CV [%]) | 1.01 (0.99–1.04) | 1.00 (0.98–1.02) |
| Stool form | 0.61 (0.37–0.98) | 1.19 (0.77–1.85) |
| Variation in stool form (Maximum proportionate stool form score [%]) | 0.70 (0.1–4.94) | 0.23 (0.03–1.49) |
| Sense of incomplete evacuation | 0.93 (0.39–2.14) | 2.18 (1.04–4.59) |
| Rectal urgency (%) | 1.82 (0.78–4.24) | 3.06 (1.42–6.63) |
| Straining to begin | 4.10 (1.69–10.20) | 1.07 (0.38–2.93) |
| Straining to end | 4.70 (1.59–15.18) | 3.67 (1.23–11.97) |
| Any fecal incontinence | 1.02 (0.44–2.33) | 0.82 (0.37–1.76) |
Model explained 35% of the variance between controls and subjects with functional bowel disorders.
CV (%) = percent coefficient of variation.
Relationships Among Bowel Symptoms
Figure 1 depicts the relationship between stool form and other bowel symptoms for individual bowel movements in normal subjects (upper panel) and those with constipation (middle panel) or diarrhea (lower panel). Figure 2 compares stool form scores for bowel movements which were, or were not, associated with bowel symptoms. Sixty-five percent of all bowel movements in the normal subjects and 48% of all bowel movements in the subjects with constipation and diarrhea had a stool form score of 3 or 4. Constipated subjects had a higher proportion (i.e., 41% vs 25% for controls) of hard bowel movements (i.e., a stool form of 1 or 2), while 31% of the bowel movements in the subjects with diarrhea were semiformed or loose (i.e., form of 5, 6, or 7) as compared to only 10% in the controls. In all three groups, stool form scores were higher (P = 0.0001), reflecting looser stools, for bowel movements associated with urgency (Fig. 2). However, urgency for soft, formed stools (i.e., form of 3 or 4) was twice as common in diarrhea and constipation.
Figure 1.
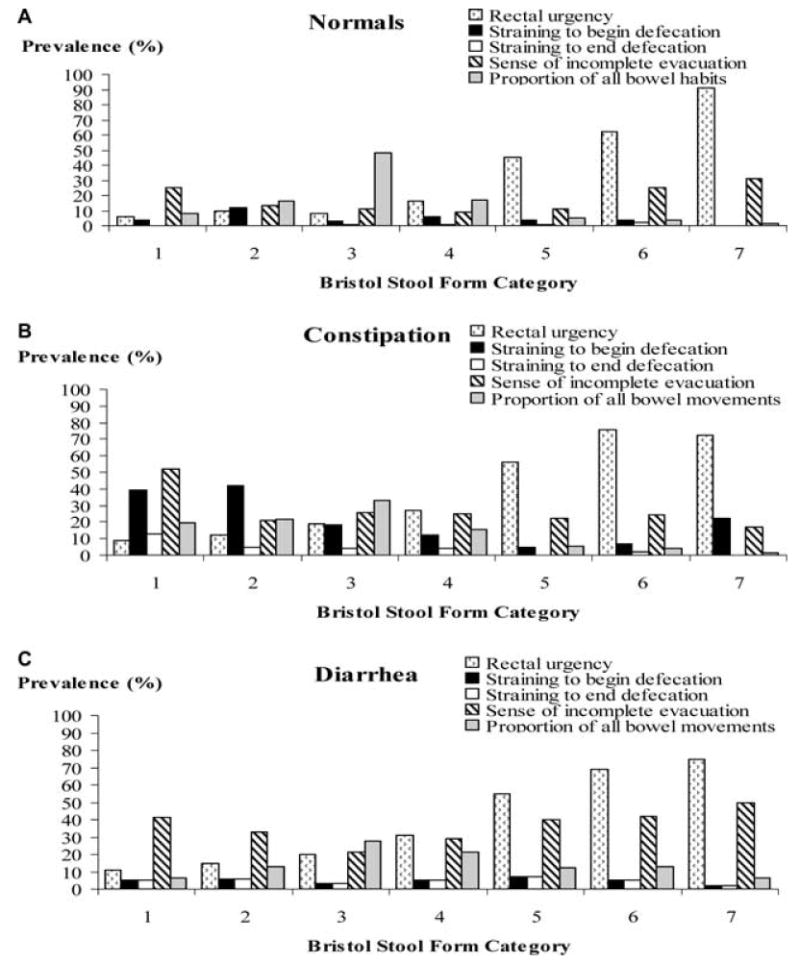
Distribution of bowel symptoms by stool forms in normals (A), constipation (B), and diarrhea (C) for all bowel movements. The grey bars represent the proportion of all bowel movements corresponding to specific stool forms. In general, the prevalence of symptoms was lowest for normal stools (i.e., a form of 4). All subjects reported more urgency and less straining for loose than for hard stools. In contrast, incomplete evacuation was most frequently reported for a stool form score of 1 or 7 and was lowest for a stool form score of 3. The data in this figure are not broken down by FI status.
Figure 2.
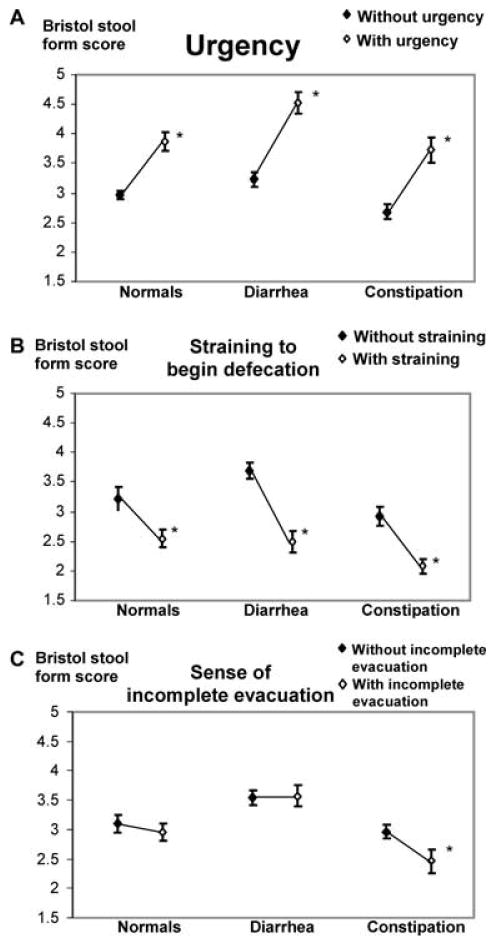
Relationship between Bristol stool form score and rectal urgency (A), straining to begin defecation (B), and sense of incomplete evacuation (C) in normals, diarrhea, and constipation. Stool form scores were higher and lower, respectively, for bowel movements associated with, compared to without, rectal urgency and straining to begin defecation. The data in this figure are not broken down by FI status. *P = 0.0001 versus without symptom (i.e., urgency, straining, or sense of incomplete evacuation) for subjects in that category (i.e., normals, diarrhea, or constipation).
For all subjects (i.e., normals, constipation, and diarrhea), stool form scores were lower (P = 0.0001), reflecting harder stools, for bowel movements associated with straining to begin defecation (Fig. 2). However, among normal subjects, straining to begin defecation was unusual and straining to end defecation was rare, even for hard stools (Fig. 1). In contrast, straining to begin evacuating hard stools (i.e., stool form of 1 or 2) was reported more frequently in subjects with constipation (i.e., 40%) and diarrhea (20%).
Normal subjects reported the sense of incomplete evacuation for stool forms at both extremes. Constipated subjects complained of incomplete evacuation not only after scybalous stools (i.e., 50%) but also after soft, formed stools (i.e., 25% for a stool form of 3 or 4). Subjects with diarrhea reported incomplete evacuation for 40–50% of the loose bowel movements. Among constipated subjects, stool form scores were lower (P = 0.0001), reflecting harder stools, for bowel movements associated with, compared to without, a sense of in-complete evacuation.
The bowel symptoms associated with a sense of incomplete evacuation were explored by a multiple linear regression model. In this model, the predictive variables (stool frequency, stool form, straining during defecation, and rectal urgency) explained 24% of the intersubject variation in the proportion of stools recorded with a sense of incomplete evacuation (Table 4). Rectal urgency was the strongest predictor and explained 10% of the intersubject variation for this symptom.
Table 4.
Risk Factors for the Sense of Incomplete Evacuation
| Predictor Variable | Squared Partial Correlation Coefficients |
|---|---|
| Stool form | <0.01 |
| Stool frequency | 0.01† |
| Straining to begin defecation | 0.01* |
| Straining to end defecation | 0.04‡ |
| Rectal urgency | 0.1‡ |
| Total variance explained (%) | 24 |
P = 0.07;
P = 0.05;
P ≤ 0.001.
Discussion
Our understanding of the epidemiology of functional bowel disorders is predominantly derived from the questionnaire-based Rome criteria. Because the natural history of these disorders is characterized by considerable variation in bowel symptoms and because questionnaires are susceptible to recall bias, daily bowel diaries are also used to document the response to treatment in trials. In this study, the 90th percentile values for the frequency of several bowel symptoms (i.e., sense of incomplete evacuation, rectal urgency, and straining to begin defecation) recorded by diaries from a random sample of community women were ≤17%. These observations support the cutoff (i.e., 25% of the time) for regarding symptoms as abnormal in the Rome II criteria (13).
Stool form is a more reliable marker of intestinal transit than frequency, and the Rome criteria classify bowel disturbances (i.e., constipation and diarrhea) by stool form rather than by frequency (2, 16). Our current concepts of “normal” stool form among asymptomatic people in the community primarily emanates from the detailed records of 12 consecutive bowel movements in 27 healthy subjects from the United Kingdom (6). The distribution of stool forms among normal subjects in that study was as follows: 11% had a form of 1, 17% (2), 19% (3), 23% (4), 14% (5), 15% (6), and 2% (7). Thus, 54% of the subjects (vs 27% in this study) had a stool form ≥4. Based on our observations (i.e., 17%, 48%, and 17% had stool forms of 2, 3, and 4), we propose that a stool form of 1 or ≥5 should be regarded as abnormal. Although constipation and diarrhea were associated with hard and loose stools, respectively, 20% of the stools in the subjects with diarrhea had a form of 1 or 2 while 10% of the stools in the subjects with constipation had a form ≥5. These observations may reflect variations in stool consistency and reinforce the utility of recording bowel habits by diaries.
Looser stools were associated with more rectal urgency and less straining during defecation, confirming previous observations (6). The relationship between stool form and bowel symptoms was influenced by disease status. Compared to normals, a higher proportion (i.e., approximately 20–30% vs 8–16%) of the subjects with constipation or diarrhea reported urgency when the stool form score was 3 or 4. Similarly, subjects with constipation reported straining for hard stools (i.e., stool form of 1 or 2) 40% of the time, compared to 4% (for a form of 1) or 12% (for a form of 2) of the time in normal subjects. A sense of incomplete evacuation was associated with harder stools only among subjects with constipation. Based on these observations, we infer that in addition to disturbed stool form, other factors such as pelvic floor dysfunction, visceral hypersensitivity, or even hypervigilance also contribute to the symptoms in constipation and diarrhea. Indeed in the multivariate model, these symptoms (i.e., rectal urgency, straining to begin and end defecation, and stool frequency) were more useful than stool form and explained 24% of the variation in the sense of incomplete evacuation. Rectal urgency is associated with rectal hypersensitivity (17, 18), while the sense of incomplete evacuation may be indicative of pelvic floor dysfunction (19). Further studies are required to understand if the symptoms not evaluated here (e.g., relief of abdominal discomfort or bloating) explain the residual variance (i.e., 76%) in the sense of incomplete evacuation. The relationship between the time spent during evacuation and the sense of incomplete evacuation also needs to be evaluated.
Similarly, the multiple logistic regression models only explained 35% of the variation between normal and functional bowel disorders, perhaps because diaries did not inquire about abdominal discomfort. To our surprise, straining to end defecation was a significant risk factor not only for constipation, but also for diarrhea. In addition, harder stools and straining to begin defecation were also risk factors for constipation, suggesting that constipated subjects strain because they have hard stools. In contrast, rectal urgency and the sense of incomplete evacuation, but not straining to begin defecation or stool form, were risk factors for diarrhea. Rectal urgency is a marker not only for loose stools, but also for rectal hyper-sensitivity (18, 20). Taken together, these data suggest that women with diarrhea strain to defecate because they have a sense of incomplete evacuation, perhaps related to rectal hypersensitivity rather than because they have hard stools.
The Rome III criteria recognize that bowel symptoms fluctuate over time (2). In addition to fluctuations over the medium (i.e., several months) or long (i.e., several years) term, a majority of patients randomized to placebo in therapeutic trials have rapidly fluctuating symptoms lasting from <1 h to <1 wk (11, 12). We observed that stool frequency was more variable in women with functional GI disorders, but variation in stool frequency and form did not add to the utility of other features for discriminating functional bowel disorders from asymptomatic subjects. Because bowel diaries were only maintained for 2 wk, fluctuations over a longer duration may have been missed. However, in one study of 59 subjects, episodes of altered stool form and passage lasted an average of 2.2 and 1.4 days, respectively (21).
Based on these data, it is conceivable that a simultaneous consideration of stool form with other bowel symptoms (e.g., straining to defecate and rectal urgency) may refine our ability to identify patients, grade the severity, and clarify the role of pathophysiological mechanisms other than the stool form in functional bowel disorders. We conceptualize a system wherein bowel symptoms (e.g., straining) are weighted by stool form. For example, straining to evacuate formed stools (i.e., form 3 or 4) may more strongly suggest a defecatory disorder than straining to evacuate form 1 or 2 stools.
In summary, these observations, from a random sample of predominantly white women in the community, confirm that individual bowel symptoms occur infrequently (i.e., <25% of the time) in otherwise healthy subjects. Stool form is useful for interpreting the significance of bowel symptoms such as straining to defecate and urgency. People with functional bowel disorders are more likely to have these symptoms even when stool form is normal, suggesting a role for other pathophysiological mechanisms. Straining to begin and end defecation and hard stools discriminated constipated subjects from normals, while straining to end defecation, a sense of incomplete evacuation, and rectal urgency were useful for discriminating diarrhea from normal. These data may be useful for refining symptom-based criteria and for explaining the mechanisms of symptoms in functional bowel disorders.
Study Highlights.
What Is Current Knowledge
Functional bowel disorders are defined by bowel symptoms, which can be evaluated by bowel questionnaires.
Questionnaires provide a limited assessment of the relationship between stool form and other bowel symptoms (e.g., urgency).
Questionnaires incompletely assess day-to-day variations in bowel symptoms.
What Is New Here
Stool form is useful for interpreting the significance of bowel symptoms such as straining to defecate and urgency.
People with functional bowel disorders are more likely to have symptoms even when stool form is normal, suggesting a role for other pathophysiological mechanisms.
Day-to-day variations in stool frequency and form do not augment the utility of other bowel symptoms (e.g., straining to defecate and stool frequency and form) for discriminating between health and functional bowel disorders.
Acknowledgments
Financial support: This work was supported in part by Grants R01 HD41129, RO1 AR30582, and General Clinical Research Center grant M01 RR00585 from the National Institutes of Health, U.S. Public Health Service. The study, design, collection, analysis and interpretation of data, and writing of the manuscript were done independently of these sponsors.
Footnotes
Guarantor of the article: Adil E. Bharucha, M.D.
Specific author contributions: Adil E. Bharucha: planning the experiment, conducting the study, and drafting the manuscript; Barbara Seide: conducting the study; Alan R. Zinsmeister: conducting the study and drafting the manuscript; and L. Joseph Melton III: planning the experiment and drafting the manuscript.
Potential competing interests: None.
References
- 1.Cremonini F, Talley NJ. Irritable bowel syndrome: Epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterology Clinics of North America. 2005;34:189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. erratum appears in Gastroenterology. 2006;131(2):688. [DOI] [PubMed] [Google Scholar]
- 3.Manning AP, Wyman JB, Heaton KW. How trustworthy are bowel histories? Comparison of recalled and recorded information. Br Med J. 1976;2:213–4. doi: 10.1136/bmj.2.6029.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaussade S, Khyari A, Roche H, et al. Determination of total and segmental colonic transit time in constipated patients. Results in 91 patients with a new simplified method. Dig Dis Sci. 1989;34:1168–72. doi: 10.1007/BF01537263. [DOI] [PubMed] [Google Scholar]
- 5.Ashraf W, Park F, Lof J, et al. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol. 1996;91:26–32. [PubMed] [Google Scholar]
- 6.Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73–9. doi: 10.1136/gut.32.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mearin F, Balboa A, Badia X, et al. Irritable bowel syndrome subtypes according to bowel habit: Revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15:165–72. doi: 10.1097/00042737-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Mearin F, Baro E, Roset M, et al. Clinical patterns over time in irritable bowel syndrome: Symptom instability and severity variability. Am J Gastroenterol. 2004;99:113–21. doi: 10.1046/j.1572-0241.2003.04023.x. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: Defining an alternator. Gastroenterology. 2005;128:580–9. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Mearin F, Badia X, Balboa A, et al. Predictive factors of irritable bowel syndrome improvement: 1-year prospective evaluation in 400 patients. Aliment Pharmacol Ther. 2006;23:815–26. doi: 10.1111/j.1365-2036.2006.02828.x. [DOI] [PubMed] [Google Scholar]
- 11.Guilera M, Balboa A, Mearin F. Bowel habit subtypes and temporal patterns in irritable bowel syndrome: Systematic review. Am J Gastroenterol. 2005;100:1174–84. doi: 10.1111/j.1572-0241.2005.40674.x. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Tillisch K, Labus JS, Naliboff BD, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896–904. doi: 10.1111/j.1572-0241.2005.41211.x. [DOI] [PubMed] [Google Scholar]
- 13.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:1143–1147. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population based study in women. Gastroenterology. 2005;129:42–9. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Locke GR, Seide B, et al. A New Questionnaire for Constipation and Fecal Incontinence. Aliment Pharmacol Ther. 2004;20:355–64. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 16.Heaton KW, O'Donnell LJ. An office guide to whole-gut transit time. Patients' recollection of their stool form. J Clin Gastroenterol. 1994;19:28–30. doi: 10.1097/00004836-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Chan CL, Lunniss PJ, Wang D, et al. Rectal sensorimotor dysfunction in patients with urge faecal incontinence: Evidence from prolonged manometric studies. Gut. 2005;54:1263–72. doi: 10.1136/gut.2005.071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan CL, Scott SM, Williams NS, et al. Rectal hypersensitivity worsens stool frequency, urgency, and lifestyle in patients with urge fecal incontinence. Diseases of the Colon & Rectum. 2005;48:134–40. doi: 10.1007/s10350-004-0774-x. [DOI] [PubMed] [Google Scholar]
- 19.Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol. 1999;94:131–8. doi: 10.1111/j.1572-0241.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 20.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn B, Watson M, Yan S, et al. Irritable bowel syndrome symptom patterns: Frequency, duration, and severity. Dig Dis Sci. 1998;43:2715–8. doi: 10.1023/a:1026663613695. [DOI] [PubMed] [Google Scholar]


