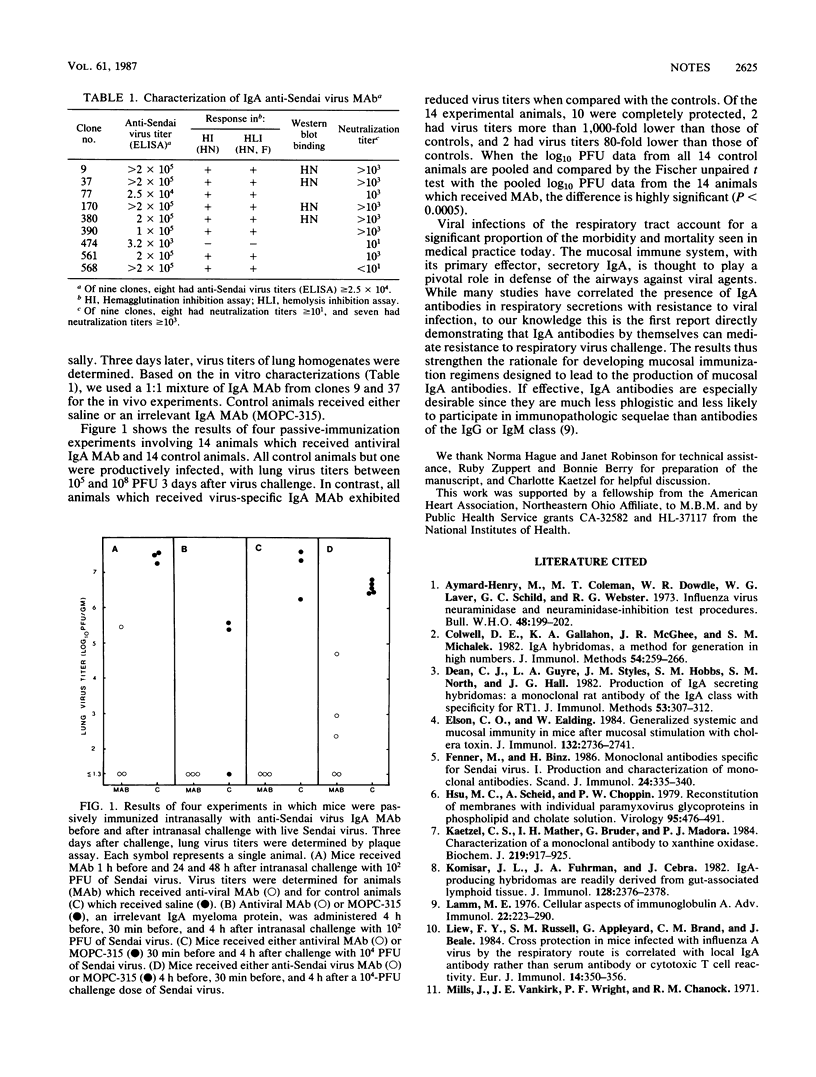
Abstract
Immunoglobulin A anti-Sendai virus HN protein monoclonal antibodies, generated via a mucosal immunization protocol, were shown to neutralize virus in vitro and, when passively administered to the mouse respiratory tract, to protect against Sendai virus in vivo. Thus, immunoglobulin A antibodies by themselves can protect against respiratory virus infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Colwell D. E., Gollahon K. A., McGhee J. R., Michalek S. M. IgA hybridomas: a method for generation in high numbers. J Immunol Methods. 1982 Oct 29;54(2):259–266. doi: 10.1016/0022-1759(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Gyure L. A., Styles J. M., Hobbs S. M., North S. M., Hall J. G. Production of IgA secreting hybridomas: a monoclonal rat antibody of the IgA class with specificity for RT1c. J Immunol Methods. 1982 Sep 30;53(3):307–312. doi: 10.1016/0022-1759(82)90177-6. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Fenner M., Binz H. Monoclonal antibodies specific for Sendai virus. I. Production and characterization of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):335–340. doi: 10.1111/j.1365-3083.1986.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Kaetzel C. S., Mather I. H., Bruder G., Madara P. J. Characterization of a monoclonal antibody to bovine xanthine oxidase. Biochem J. 1984 May 1;219(3):917–925. doi: 10.1042/bj2190917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisar J. L., Fuhrman J. A., Cebra J. J. IgA-producing hybridomas are readily derived from gut-associated lymphoid tissue. J Immunol. 1982 May;128(5):2376–2378. [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- Orvell C., Grandien M. The effects of monoclonal antibodies on biologic activities of structural proteins of Sendai virus. J Immunol. 1982 Dec;129(6):2779–2787. [PubMed] [Google Scholar]
- Orvell C., Norrby E. Immunologic properties of purified Sendai virus glycoproteins. J Immunol. 1977 Dec;119(6):1882–1887. [PubMed] [Google Scholar]
- Parker J. C., O'Beirne A. J., Collins M. J., Jr Sensitivity of enzyme-linked immunosorbent assay, complement fixation, and hemagglutination inhibition serological tests for detection of Sendai virus antibody in laboratory mice. J Clin Microbiol. 1979 Mar;9(3):444–447. doi: 10.1128/jcm.9.3.444-447.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Beffy P., Portner A. Restriction of cell surface expression of Sendai virus hemagglutinin-neuraminidase glycoprotein correlates with its higher instability in persistently and standard plus defective interfering virus infected BHK-21 cells. Virology. 1984 Oct 15;138(1):118–128. doi: 10.1016/0042-6822(84)90152-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J., Gerhard W. Delineation of four antigenic sites on a paramyxovirus glycoprotein via which monoclonal antibodies mediate distinct antiviral activities. J Immunol. 1982 Jun;128(6):2670–2675. [PubMed] [Google Scholar]


