Abstract
BACKGROUND
The Centers for Disease Control and Prevention (CDC) Guideline for Hand Hygiene in Health Care Settings was issued in 2002. In 2003, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) established complying with the CDC Guideline as a National Patient Safety Goal for 2004. This goal has been maintained through 2006. The CDC's emphasis on the use of alcohol-based hand rubs (ABHRs) rather than soap and water was an opportunity to improve compliance, but the Guideline contained over 40 specific recommendations to implement.
OBJECTIVE
To use the Six Sigma process to examine hand hygiene practices and increase compliance with the CDC hand hygiene recommendations required by JCAHO.
DESIGN
Six Sigma Project with pre-post design.
PARTICIPANTS
Physicians, nurses, and other staff working in 4 intensive care units at 3 hospitals.
MEASUREMENTS
Observed compliance with 10 required hand hygiene practices, mass of ABHR used per month per 100 patient-days, and staff attitudes and perceptions regarding hand hygiene reported by questionnaire.
RESULTS
Observed compliance increased from 47% to 80%, based on over 4,000 total observations. The mass of ABHR used per 100 patient-days in 3 intensive care units (ICUs) increased by 97%, 94%, and 70%; increases were sustained for 9 months. Self-reported compliance using the questionnaire did not change. Staff reported increased use of ABHR and increased satisfaction with hand hygiene practices and products.
CONCLUSIONS
The Six Sigma process was effective for organizing the knowledge, opinions, and actions of a group of professionals to implement the CDC's evidence-based hand hygiene practices in 4 ICUs. Several tools were developed for widespread use.
Keywords: hand hygiene, alcohol-based hand rub, JCAHO National Patient Safety Goals, Six Sigma, intensive care units
Hand decontamination has been shown to reduce the spread of infectious agents for more than 150 years.1 The Institute of Medicine has identified nosocomial infections as the most common complication for hospital inpatients,2 and hands are the most common mode of transmission for many important nosocomial pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).3,4 The 1991 Harvard Practice Study on adverse events in health care5 indicated that surgical site infections were the second most frequent type of adverse event for inpatients, constituting 13% of adverse events. This study did not include other hospital-acquired infections such as urinary tract infections or central line infections. A subsequent study by the Centers for Disease Control and Prevention (CDC) of 1992 to 1996 data6 indicated that surgical site infections constituted only 17% of all hospital-acquired infections. This suggests that only 1 in 6 hospital-acquired infections were counted in the Harvard Practice Study, and that hospital-acquired infections are almost certainly the most frequent adverse event for inpatients.
In 2002, the CDC issued a new Guideline for Hand Hygiene in Health Care Settings,7 which elucidated many points related to hospital-acquired infection. The 2 most basic findings were: (1) the hands of health care workers are regularly contaminated with pathogenic microorganisms; and (2) the hands of health care workers are a major route of transmission of pathogens throughout the hospital environment and from the body of one patient to another. These findings were already well known, but the primary recommendation was new: alcohol-based hand rub (ABHR) should be used for “routinely decontaminating hands.” The 2002 CDC Guideline noted that ABHRs are faster and easier to use than soap and water, more effective at killing most microorganisms, and are less likely to cause dermatitis. Recent studies have shown that use of ABHRs results in fewer infections.8–10 Using products other than soap is not new; the original hand decontamination process established by Semmelweis in the 1850s used a decontaminating rinse with chlorinated lime rather than soap.11
In total, the 2002 CDC Guideline provided over 40 recommendations, with 4 categories for the level of evidence and for 8 different aspects of practice. In 2003, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) added “comply with current CDC hand hygiene guidelines” to its list of National Patient Safety Goals for 2004. Although this goal was clear, how to achieve it in hospitals was not.
In 2003, the 3M Company approached the Department of Veterans Affairs (VA) and Veterans Health Administration (VHA) about conducting a joint project using the Six Sigma methodology. After discussions, VHA and 3M signed a memorandum of agreement to work together to develop methods to comply with JCAHO's required hand hygiene practices. We report on the implementation and results of this agreement.
METHODS AND DEVELOPMENT OF INTERVENTIONS USING THE SIX SIGMA PROCESS
The lead representative for VA was the National Center for Patient Safety. The first challenge was to understand which of the over 40 CDC recommendations were required by JCAHO. Discussions revealed that JCAHO would require CDC Category IA, IB, and IC recommendations, and VA staff composed a 1-page summary of the required CDC recommendations (Fig. 1). It contains 19 consolidated recommendations on 4 topics and was used to simplify the CDC Guideline, and specify what to address using the Six Sigma process.
FIGURE 1.
Summary of Centers for Disease Control and Prevention hand hygiene recommendations required by Joint Commission on Accreditation of Healthcare Organizations.
The Six Sigma process focuses on identifying critical points where changes should be made, making those changes, and ensuring that the changes are established as permanent practice.12,13 The version of the Six Sigma process developed and implemented by 3M has 5 steps, Define, Measure, Analyze, Improve, and Control, which are referred to as the acronym DMAIC.
In the Define step, a “project charter” is developed that explicitly defines the problem or opportunity using a standard 1-page format that is agreed upon by all participants. This ensures that everyone understands what is to be addressed.
The Measure step includes development of a “process map” that describes the way that things currently occur, a “cause and effect matrix” linking actions and missed actions to good and bad outcomes, and quantitative measurement of the baseline performance parameter(s) to develop the “initial capability,” i.e., initial performance level(s).
In the Analyze step, “Failure Modes and Effects Analysis (FMEA)” is performed, and the data collected previously in the Measure step is analyzed using appropriate statistical methods (“multi-vari studies”) to determine/confirm sources of variation and opportunities to improve.
The Improve step involves adjusting processes and implementing improvements that address, fix, and/or prevent problems. After implementation of the improvements, data are collected again and the improvements may be refined.
The final Control step (1) codifies the improvements into a “Control Plan” that describes the interventions, who is responsible for each, and how and when they will be monitored and/or measured; and (2) sustains the gains by assuring continued adherence to the Control Plan to maintain improved performance and results. The points of the Control Plan, translated out of the Six Sigma format and into a checklist format, are seen in the list of interventions (Fig. 2, more detailed version in the Appendix, and online at VA web site14).
FIGURE 2.
Hand hygiene interventions developed through Six Sigma process.
The Six Sigma process was used to organize and focus efforts to implement JCAHO's hand hygiene goal in 4 intensive care units (ICUs) at 3 VA medical centers. These ICUs are coded as Medical ICU-1 (MICU-1), Surgical ICU-1 (SICU-1), ICU-2, and ICU-3. MICU-1 and SICU-1 are in a Midwestern city, in a large hospital affiliated with a university medical school; MICU-1 is a “closed” ICU staffed by full-time intensivist physicians. Surgical ICU-1, ICU-2, and ICU-3 are “open” ICUs not staffed by full-time intensivists. Intensive care unit-2 is in a Southern city, in a small hospital not affiliated with a medical school. Intensive care unit-3 is in a Midwestern city, in a medium-sized hospital affiliated with a medical school.
3M provided Six Sigma training to VA staff, and assigned 2 team members and a program manager (a “Six Sigma Black Belt”) to the project. Weekly teleconferences with representatives from the hospitals, 3M, and VA Central Office took place over a 6-month period. Control charts were used to track the monthly grams of ABHR used per 100 patient-days, providing monthly performance data and reducing reliance on observation data, which are time consuming to collect, inherently intermittent, and have more potential for unintentional bias.
To track the mass of ABHR used, the number of containers of ABHR replaced at each ICU was recorded monthly by staff responsible for this duty as part of the project. This was challenging because it required scrupulous measurement of something not previously controlled, monitored, or measured. Each container held 198 g of ABHR, and ICU staff already recorded monthly patient-days of care provided in each ICU, so grams of ABHR used per 100 patient-days was easy to compute. Working with foam, gel, and liquid products, and based on a combination of information from manufacturers, the CDC Guideline, and empirical tests with the products, it was determined that an appropriate usage quantity of ABHR was approximately 1.4 g. A template for recording and calculating this data is shown in the Appendix.
To collect data on observed hand hygiene practices, a tool was developed to allow observers to record caregiver compliance with 10 required hand hygiene practices (see Appendix). Intensive care unit (ICU) staff used the tool to monitor the practices of their colleagues without notification as to when the observations would take place. A special instruction sheet was developed to accompany the tool and standardize its use. Data were collected over several days at different times by persons who would typically be in the ICU, i.e., ICU staff rather than Infection Control Staff. The observation method and tool allowed the recording of several hand hygiene opportunities during a single patient encounter. The objective was to record at least 400 opportunities per ICU. This sample size was sufficient to show statistically significant changes as small as 10%. Staff professions or titles were noted, but names were not. There were many categories of staff recorded, but these were later recategorized as MD, RN, or “Other.”
A questionnaire was developed to record ICU staff attitudes and perceptions (Appendix). This questionnaire contained a Hand Assessment Scale15 and questions developed during the Six Sigma process. The questionnaire was used before and after interventions. The questionnaires provided insights into staff attitudes as well as data for a Six Sigma “counterbalance.” The idea for a counterbalance is to measure a parameter that might get worse as the desired parameters get better. In this study, staff satisfaction with hand hygiene practices was the counterbalance. We did not want to increase observed compliance, or the use of ABHR, at the cost of making ICU staff dissatisfied. We believed that improvements were unlikely to be sustainable if the ICU staff were unhappy about the improvement mechanisms.
Reviewing hand hygiene processes systematically, it became clear that widespread and easy access to hand hygiene products such as ABHR, antimicrobial soap, and sterile and nonsterile gloves, must be provided before anything else. First, ABHRs must be available at the bedside and/or the entryway to all patient rooms, and antimicrobial soap at all sinks. In discussions, it became clear that health care workers had not established the frequently repeated hand hygiene practices required by JCAHO as part of their routine, and that they did not feel comfortable reminding each other to decontaminate their hands. Also, health care workers did not know the specific recommendations in the CDC Guideline, and many believed that they were practicing hand hygiene at an unrealistically high rate. The latter point has been previously reported.16 This meant that health care workers did not fully comprehend the need to increase hand hygiene practices. The most challenging improvement identified to improve staff hand hygiene practices was for patients and visitors to remind staff to decontaminate their hands, and for patients and visitors to also decontaminate their hands.
Of note, a major educational effort was not identified as a critical aspect of the intervention by the Six Sigma process. Education-oriented items focused only on updating and reorienting existing educational training materials and providing new posters presenting new information or perspectives (Fig. 2). The posters developed during the project and redesigned subsequently are online at a VA web site.17 There were no special training sessions, and no exams based on the CDC Guideline. Other educational interventions were relatively simple: a 1-page summary document (Fig. 1), and updating preexisting Power Point presentations. An 8-minute video on hand hygiene was also provided to each ICU and shown at an ordinary staff meeting.
RESULTS
Results are presented on (1) the mass of ABHR used per 100 patient-days (Fig. 3), 2) the observed rate of compliance with hand hygiene practices (Table 1, Fig. 4), and (3) the responses to items on the hand hygiene questionnaire before and after the interventions (Table 2).
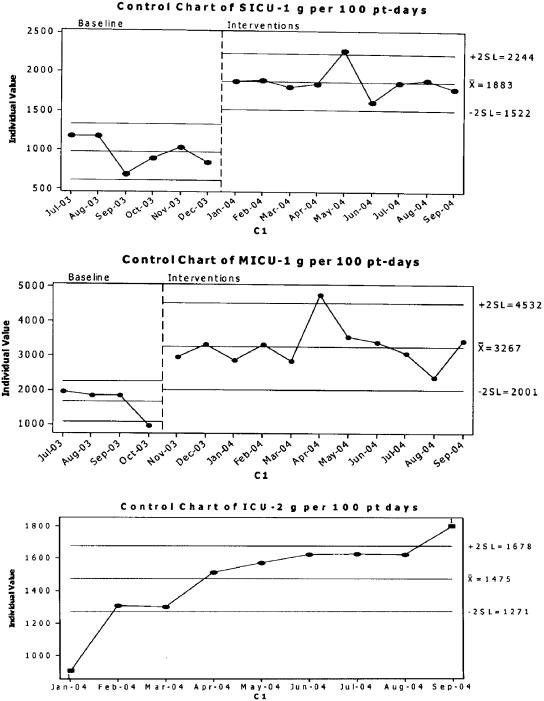
FIGURE 3.
Control chart showing baseline and final grams of alcohol-based hand rub used per 100 part-days per month for Medical Intensive Care unit-1, Surgical Intensive Care Unit (SICU)-1, and SICU-2. Control bars are set at 2 standard deviation intervals.
Table 1.
Observed Compliance, by ICU, by Healthcare Worker (HCW) Category, and Overall
| MICU-1 | SICU-1 | ICU-2 | ICU-3 | All Nurses | All MDs | All Other HCWs | Total | |
|---|---|---|---|---|---|---|---|---|
| Baseline observations | 509 | 511 | 407 | 911 | 1130 | 311 | 897 | 2,338 |
| Baseline Yes (%) | 51 | 51 | 52 | 39 | 53 | 47 | 39 | 47 |
| Time of initial observations | October 2003 | January/February 2004 | January 2004 | January 2004 | October 2003 to January 2004 | October 2003 to January 2004 | October 2003 to January 2004 | NA |
| Final observations | 338 | 320 | 405 | 699 | 864 | 187 | 711 | 1,762 |
| Final Yes (%) | 79 | 87 | 83 | 76 | 82 | 77 | 78 | 80 |
| P<.001 | P<.001 | P<.001 | P<.001 | P<.001 | P<.001 | P<.001 | P<.001 | |
| Time of final observations | May 2004 | May 2004 | April/May 2004 | May 2004 | April/May 2004 | April/May 2004 | April/May 2004 | NA |
| Relative (%) improvement in compliance | 55 | 71 | 60 | 95 | 54 | 64 | 100 | 72 |
ICU, intensive care unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; NA, not applicable.
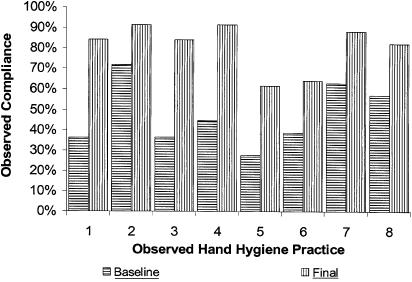
FIGURE 4.
Observed hand hygiene compliance, by practice measured in observation tool. Observed practices were as follows (Fig. 2 and Appendix for additional information): (1) Before clean and aseptic procedures, including medication prep and prior to prep, gown and glove for sterile procedures. (2) After contact with blood, body fluids, secretions or excretions, mucous membranes, nonintact skin. (3) After handling objects and devices such as soiled linen, trash, equipment. (4) After removing gloves or other PPE used for contact with body substances. (5) Before patient contact. (6) After patient contact upon exiting patient's room. (7) Upon entering patient's room before equipment contact. (8) After equipment contact upon exiting patient's room. PPE, personal protective equipment.
Table 2.
Baseline and Final Responses to Hand Hygiene Questionnaire
| Question | Initial Data (104) | Final Data (77) | P Value |
|---|---|---|---|
| Is there a protocol? | 90.4% | 93.5% | .166 |
| Self-compliance rate estimate | 87.8% (n=96) | 86.5% (n=73) | .207 |
| Hand self-assessment | |||
| Appearance (1 to 7) | 5.5 | 5.8 | .269 |
| Intactness (1 to 7) | 5.2 | 5.5 | .267 |
| Moisture (1 to 7) | 4.3 | 4.6 | .334 |
| Sensation (1 to 7) | 5.2 | 5.6 | .145 |
| Hand hygiene motivators | |||
| Relationship? (1 to 5) | 4.8 | 4.9 | .133 |
| Colleague reminds (1 to 5) | 2.1 | 2.1 | 1.0 |
| Remind others (1 to 5) | 2.8 | 2.9 | .661 |
| Patient reminds (1 to 5) | 1.2 | 1.2 | 1.0 |
| Products used | |||
| Soap and water (%) | 56.3 | 49.0 | .124 |
| Alcohol alone (%) | 39.1 | 52.4 | .002 |
| Satisfaction? | |||
| Satisfaction practices (1 to 5) | 4.0 | 4.3 | .065 |
| Satisfaction products (1 to 5) | 4.0 | 4.5 | .003 |
| Job function summary | RN—45 (43%) | RN—36 (47%) | |
| MD—6 (6%) | MD—8 (10%) | ||
| Other—51 (49%) | Other—31 (40%) | ||
| Blank—2 (2%) | Blank—2 (3%) | ||
Each ICU started interventions at a different time and MICU-1 and SICU-1 were tracking the containers of ABHR used per patient-day prior to the project (Fig. 3). Intensive care unit-2 started tracking later. Intensive care unit-3 tracked ABHR use, but partway through the project they discovered that the containers being counted included containers used in areas other than the ICU, so these data were not included in the study. Alcohol-based hand rub use nearly doubled at MICU-1 and SICU-1 (P<.001 for both pre-post comparisons), and increased by 70% at ICU-2 over the period of the study. Data on observed hand hygiene practices are based on the overall percent “yes” responses to observed hand hygiene opportunities noted using the Observation Tool (Table 1, Fig. 4; Appendix). All 4 ICUs showed statistically significant increases in observed compliance with hand hygiene practices, with relative improvement ranging from a 55% to 95%. All 3 provider groups (RNs, MDs, and others) demonstrated statistically significant improvements, with nurses starting and ending with the highest observed rate of compliance (53% and 82%, respectively). Of the 10 practices measured with the Observation Tool, those with the lowest average baseline and final compliance were numbers 5 and 6: “before patient contact” and “after patient contact upon exiting the room” (Fig. 4). The practices with the highest average baseline and final compliance were numbers 2 and 7: “after contact with blood, body fluids, secretions or excretions, mucous membranes, nonintact skin” and “upon entering the room before equipment contact.” Observation of practices 9 and 10 was added near the end of the project and the results of those questions are not shown.
Baseline and final responses to the hand hygiene questionnaire were similar for almost all questions (Table 2; Appendix). The measure selected as a Six Sigma counterbalance, staff satisfaction, increased slightly, a satisfactory result as the goal was to maintain a baseline level of staff satisfaction while ABHR use and observed compliance improved. Also, the reported use of ABHRs increased significantly. Table 3 shows the responses to “When you don't disinfect your hands (use soap or an alcohol hand rub to kill microbes) when you should, what is the reason why?” The reduction in the “too busy” response, and the increase in the “forget” response suggest that postintervention noncompliance was increasingly accidental rather than intentional. The mean estimated rate of self-compliance with hand hygiene practices (87%) was virtually unchanged. However, the distribution of responses changed; the final data contained fewer unrealistic self-responses of 99% to 100% and an increase in the more realistic response of 90% to 95% (Appendix).
Table 3.
Baseline and Final Reasons Reported for not Practicing Hand Hygiene
| Reasons Given for not Practicing Hand Hygiene as Required | Baseline Reasons (n=115 on 104 Questionnaires) (%) | Final Reasons (n=84 on 77 Questionnaires) (%) |
|---|---|---|
| Too busy | 41 | 33 |
| Forget | 26 | 44 |
| Not in convenient location | 11 | 5 |
| Damages skin | 9 | 9 |
| Out of product | 7 | 6 |
| Unsure of need | 4 | 4 |
| Always wear gloves | 3 | 0 |
| Emergency | 2 | 2 |
DISCUSSION AND CONCLUSIONS
We demonstrated that VA personnel working with a 3M project manager to employ the Six Sigma process were able to develop and implement effective interventions that improved hand hygiene practices. The Six Sigma process proved to be an effective tool for organizing the knowledge, opinions, and actions of health care professionals on an important clinical issue that has been historically intractable.
Many studies have reported that from 5% to 15% of inpatients acquire an infection while in the hospital6,18–21 or that about 2 million infections occur annually.6,22 One study established an excess mortality because of nosocomial infection of 44% in ICU patients.23 Despite the long-established, widespread, and injurious problem of hospital-acquired infections, numerous studies have shown that health care workers do not regularly perform hand hygiene practices consistent with the policies established by their professional groups, employers, etc.7,16,24 There is perhaps no aspect of inpatient care where the divergence between evidence and practice is so pervasive, damaging, long established, and well known.
Our observed 80% compliance after interventions was comparable with the most successful reports in the literature and superior to most published studies attempting to improve hand hygiene compliance.7 Furthermore, we observed statistically significant and sustained increases in the mass of ABHRs used in the observed ICUs per 100 patient-days, consistent with the compliance that we also observed. We validated monthly monitoring of ABHR use per 100 patient-days as a timesaving proxy for observed compliance with required hand hygiene practices. Data from the staff questionnaire indicated that improved compliance did not result in diminished staff satisfaction. Our finding that mean self-reported rate of compliance with hand hygiene practices was unchanged suggests that self-reported rates should not be used to indicate actual compliance rates, trends, or other changes in hand hygiene practices.
The Six Sigma process is different from the “Plan, Do, Study, Act” (PDSA) cycle used in many clinical quality improvement efforts. One important difference was evident in this study. The first 3 Six Sigma steps, “Define, Measure, and Analyze,” focus on studying the problem in depth using various prescribed methods and acquiring high-quality baseline data before doing anything else. These steps may make the Six Sigma process especially appropriate for patient safety improvement initiatives because they address a common problem in patient safety: the lack of reliable baseline data on the current or preexisting status.
Additional aspects of the Six Sigma process that were important to the success of our study included the focus on identifying the subset of potential interventions that will be especially effective, using control charts to separate normal variation from real performance changes, and using a control plan to “maintain the gains” achieved. The project generated several tools for widespread use in VA and elsewhere: a 1-page summary of the JCAHO-required CDC recommendations (which continue to be required through at least 2006), an observation tool, a staff questionnaire, a summary checklist of hand hygiene interventions, and a series of posters designed to remind staff of required hand hygiene practices and encourage compliance.
Acknowledgments
We thank Carol Meeter and Chris Hughes of 3M for help with planning data collection and data analysis, and staff at the 4 VA Medical Center Intensive Care Units for enthusiastic participation and help with data collection.
Supplementary Material
The following supplementary material is available for this article online at http://www.blackwell-synergy.com
Checklist of Hand Hygiene Interventions adapted from VA-3M Six Sigma Project.
Spreadsheet used to calculate alcohol-based hand-rub usage per 100 or 1,000 patient days.
Observation tool developed for project.
Questionaire for staff developed for project.
Self-reported compliance with required hand hygiene practices, baseline and final.
REFERENCES
- 1.Semmelweis I. In: Etiology, Concept, and Prophylaxis of Childbed Fever. 1. Carter KC, editor. Madison, WI: The University of Wisconsin Press; 1983. [Google Scholar]
- 2.Institute of Medicine. Priority Areas for National Action. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 3.Boyce JM. MRSA patients: proven methods to treat colonization. J Hosp Infect. 2001;48(suppl A):S9–14. doi: 10.1016/s0195-6701(01)90005-2. [DOI] [PubMed] [Google Scholar]
- 4.Larson E. Skin hygiene and infection prevention: more of the same or different approaches? Clin Infec Dis. 1999;29:1287–94. doi: 10.1086/313468. [DOI] [PubMed] [Google Scholar]
- 5.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 6.CDC NNIS System. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 to June 2002, issued August 2002. Am J Infect Control. 2002;30:458–75. doi: 10.1067/mic.2002.130032. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morb Mortal Wkly Rep. 2002;51 (No. RR-16) [PubMed] [Google Scholar]
- 8.MacDonald A, Dinah F, MacKenzie D, Wilson A. Performance feedback of hand hygiene, using alcohol gel as the skin decontaminant, reduces the number of inpatients newly affected by MRSA and antibiotic costs. J Hospital Infec. 2004;56:56–63. doi: 10.1016/s0195-6701(03)00293-7. [DOI] [PubMed] [Google Scholar]
- 9.Hilburn J, Hammond B, Fendler EJ, Groziak PA. Use of alcohol hand sanitizer as an infection control strategy in an acute care facility. Am J Infect Control. 2003;31:109–16. doi: 10.1067/mic.2003.15. [DOI] [PubMed] [Google Scholar]
- 10.Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30:226–33. doi: 10.1067/mic.2002.120129. [DOI] [PubMed] [Google Scholar]
- 11.Harbath S. Handwashing—The Semmelweis lesson misunderstood? Clin Infec Dis. 2000;30:990–1. doi: 10.1086/313793. [DOI] [PubMed] [Google Scholar]
- 12.Pande PS, Neuman RP, Cavanagh RR. The Six Sigma Way: How GE, Motorola, and Other Top Companies are Honing their Performance. New York, NY: McGraw Hill; 2000. [Google Scholar]
- 13.Rath T, Strong DO. Rath & Strong's Six Sigma Pocket Guide. Lexington, MA: AON Consulting Worldwide; 2002. [Google Scholar]
- 14.Department of Veterans Affairs, National Center for Patient Safety, Hand Hygiene Information and Tools. [August 10, 2005]. Available at http://vaww.ncps.med.va.gov/Hand_Hygiene/InfoTools/index.html.
- 15.Larson E. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404–12. doi: 10.1016/s0147-9563(97)90027-3. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein RA. Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerging Infec Dis. 2001;7:188–92. doi: 10.3201/eid0702.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Veterans Affairs. Office of Public Health and Environmental Hazards. [August 10, 2005]. Infection: Don't Pass it on. Available at http://www.publichealth.va.gov/infectiondontpassiton/
- 18.Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651–6. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel RP, Edmond MB. The impact of hospital-acquired bloodstream infections. Emerging Infec Dis. 2001;7:174–7. doi: 10.3201/eid0702.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein RA. Nosocomial infection update. Emerging Infec Dis. 1998;4:416–20. doi: 10.3201/eid0403.980320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059–93. doi: 10.1378/chest.120.6.2059. [DOI] [PubMed] [Google Scholar]
- 22.Hospital Infections Program, National Center for Infectious Diseases, CDC. Public health focus: surveillance, prevention, and control of nosocomial infections. Morb Mortal Wkly Rep. 1992;41:783–7. [PubMed] [Google Scholar]
- 23.Girou E, Stephan F, Novara A, Safar M, Fagon J-Y. Risk factors and outcome of nosocomial infections: results of a matched case-control study of ICU patients. Am J Respir Crit Care Med. 1998;157:1151–8. doi: 10.1164/ajrccm.157.4.9701129. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible alcohol-based hand antiseptic. Arch Intern Med. 2000;160:1017–21. doi: 10.1001/archinte.160.7.1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Checklist of Hand Hygiene Interventions adapted from VA-3M Six Sigma Project.
Spreadsheet used to calculate alcohol-based hand-rub usage per 100 or 1,000 patient days.
Observation tool developed for project.
Questionaire for staff developed for project.
Self-reported compliance with required hand hygiene practices, baseline and final.