Abstract
In Dictyostelium discoideum, a unique Gβ subunit is required for a G protein–coupled receptor system that mediates a variety of cellular responses. Binding of cAMP to cAR1, the receptor linked to the G protein G2, triggers a cascade of responses, including activation of adenylyl cyclase, gene induction, actin polymerization, and chemotaxis. Null mutations of the cAR1, Gα2, and Gβ genes completely impair all these responses. To dissect specificity in Gβγ signaling to downstream effectors in living cells, we screened a randomly mutagenized library of Gβ genes and isolated Gβ alleles that lacked the capacity to activate some effectors but retained the ability to regulate others. These mutant Gβ subunits were able to link cAR1 to G2, to support gene expression, and to mediate cAMP-induced actin polymerization, and some were able to mediate to chemotaxis toward cAMP. None was able to activate adenylyl cyclase, and some did not support chemotaxis. Thus, we separated in vivo functions of Gβγ by making point mutations on Gβ. Using the structure of the heterotrimeric G protein displayed in the computer program CHAIN, we examined the positions and the molecular interactions of the amino acids substituted in each of the mutant Gβs and analyzed the possible effects of each replacement. We identified several residues that are crucial for activation of the adenylyl cyclase. These residues formed an area that overlaps but is not identical to regions where bovine Gtβγ interacts with its regulators, Gα and phosducin.
INTRODUCTION
In eukaryotes, heterotrimeric G proteins coupled to seven transmembrane domain receptors mediate various cellular responses to extracellular stimuli, such as light, odorants, chemoattractants, and many hormones and neurotransmitters. Mammals contain multiple Gβ subunits, which form a large variety of heterotrimers with different Gα and Gγ subunits. Extensive biochemical studies in in vitro systems and overexpression of various inhibitors of Gβγ in tissue-cultured cells have provided information on roles for Gβγ signaling. Gβγ subunits can activate various effectors, including phospholipases, adenylyl cyclase, and ion channels (Birnbaumer, 1992; Clapham and Neer, 1993, 1997; Sunahara et al., 1996; Rhee and Bae, 1997; Schneider et al., 1997). Genetic evidence of in vivo functions of Gβγ signaling has been obtained from studies of Gβ null mutants. In Sacchromyces cerevisiae, null mutations of either STE4 (Gβ) or STE18 (Gγ) leads to defects in pheromone response and mating (Whiteway et al., 1989). In Caenorhabditis elegans, Gβ null embryos die at the blastula stage with abnormally distributed tissues (Zwaal et al., 1996). In Drosophila melanogaster, there are two cloned Gβ subunits. Mutants defective in an eye-specific Gβ subunit (Gβe) display severe defects in light response (Dolph et al., 1994). In Dictyostelium discoideum, there is a single Gβ gene that is essential for the organism’s developmental program. The Gβ null cells are viable but are unable to develop or differentiate because of an inability to regulate multiple signal transduction pathways (Lilly et al., 1993; Wu et al., 1995).
The same heterotrimeric G proteins coupled to one class of receptors can regulate multiple signal transduction pathways within a single cell. Signaling involves the sequential formation and dissociation of complexes between Gα and Gβγ subunits and between G proteins and receptors and effectors. This process is driven by the binding of ligands (extracellular stimuli) to receptors and by the binding and hydrolysis of GTP by the Gα subunit. Receptors catalyze exchange of GDP for GTP on Gα subunits; activated GTP-bound Gα then dissociates from the receptor and Gβγ, and both GTP-Gα and Gβγ regulate downstream effectors. This active stage is transient and decays because of the intrinsic GTPase activity of the Gα. GDP-bound Gα has a high affinity for Gβγ, and they reassociate to form a heterotrimer that is available for fresh stimulation by receptors (reviewed by Gilman, 1987; Birnbaumer, 1992). An analysis of functions of Gβγ and Gα in transducing signals to their effectors in vivo is complex. First, because Gβγ and Gα subunits must cooperate, deletion of either the Gβ or Gα gene eliminates signaling of a receptor–G protein system and impairs responses mediated by both Gβγ and Gα subunits. Second, signaling through that very same system often regulates expression of genes that encode effectors and components of the receptor–G protein system. To circumvent these difficulties and to dissect the functions of Gβγ in vivo, we used a random mutagenesis approach in D. discoideum to isolate Gβ alleles that are able to form heterotrimers with the Gα, to couple to receptors, and to regulate gene expression but selectively fail to activate one or more pathways.
D. discoideum is a useful system for studying signal transduction pathways regulated by heterotrimeric G proteins. G proteins coupled to seven transmembrane receptors play important regulatory functions during development. The life cycle of haploid cells consists of a vegetative growth stage and a developmental stage. Development is separated from growth and begins in the absence of a food source. Independent amoebae aggregate to form a multicellular mound by chemotaxis to pulsatile cAMP signals and then proceed through a series of morphological changes that lead to the formation of fruiting bodies within 24 h (Devreotes, 1994). There are four cAMP chemoattractant receptors (cAR1–cAR4), eight Gα subunits (Gα1–Gα8), and a single Gβ subunit (reviewed by Devreotes, 1994; Firtel, 1995). A Gγ gene has recently been cloned (Zhang and Devreotes, unpublished result). The unique Gβ and Gγ genes are expressed throughout growth and development, whereas the Gα subunits are transiently expressed at specific stages. Each of Gα subunits is thought to form a heterotrimeric G protein with the Gβ and Gγ and to couple to a specific receptor to regulate various cellular responses at different developmental stages.
The heterotrimeric G protein G2, containing Gα2 and Gβγ, coupled to the cAMP receptor cAR1, is essential in mediating various cellular responses to the chemoattractant cAMP during aggregation (reviewed by Devreotes 1994; Firtel, 1995; Parent and Devreotes, 1996). Activation of cAR1 results in several responses, including activation of the adenylyl cyclase (ACA), which produces cAMP signals, induction of gene expression, and regulation of actin polymerization and chemotaxis (Wu et al., 1995; Zigmond et al., 1997) (reviewed by Devreotes, 1994; Firtel, 1995). Cells lacking Gβ or Gα2 are defective in all these cAMP-mediated responses in vivo, because no functional heterotrimeric G2 is formed (reviewed by Firtel, 1995; Parent and Devreotes, 1996). Thus, the precise functions of Gβγ and Gα2 signaling in mediating these responses have been difficult to determine in vivo. In vitro experiments have shown that GTPγS stimulates ACA activity in membranes of Gα2 null cells but not Gβ null cells, suggesting that Gβγ rather than Gα2 mediates this activation (Wu et al., 1995). We reasoned that if ACA is specifically activated by Gβγ, then Gβ mutant alleles that are defective in ACA activation but not other cellular responses during aggregation should exist. Cells impaired in ACA activation because of deletion of the genes encoding the enzyme ACA or cytosolic proteins that are required for ACA activation, such as Aimless (a RasGEF), CRAC, and Pianissimo, are defective in producing cAMP signals and do not aggregate. However, unlike Gβ null cells, they can respond to cAMP signals generated by wild-type cells in a mixture and can differentiate into viable spores in chimeric fruiting bodies (Pitt et al., 1993; Insall et al., 1994, 1996; Chen et al., 1997). These mutants are designated as “synag.”
In this study, we screened a collection of cells carrying a library of random mutagenized Gβ genes to identify those that displayed a synag phenotype. We isolated five cell lines expressing Gβ alleles, SN1–SN5. Characterization of SN1–SN5 cells revealed that SN mutant Gβs could form a heterotrimeric G protein with Gα2, could couple to cAR1, and could mediate certain cellular responses, including gene expression during aggregation and cAMP-induced actin polymerization. Some of the mutants supported chemotaxis toward cAMP, in contrast to Gβ null cells, which are completely defective (Wu et al., 1995). In the membranes of SN1–SN5 cells, GTPγS failed to stimulate ACA activity, indicating that these SN Gβγ mutants are defective in signaling to downstream components that lead to ACA activation. We determined the mutations on each of these Gβ alleles. Using the structure displayed in the computer program CHAIN, we identified mutations that occurred at or affected residuals on the surface of Gβγ. These mutations are likely to cause the observed defects in connecting Gβγ to its downstream effectors. This study not only gives important insight into the specificity of complex Gβγ signaling in a well-defined genetic system but also sheds light onto the general mechanism of control of Gβγ functions by its regulators.
MATERIALS AND METHODS
Cell Growth and Development
Cells of AX3 (wild type) and LW6 (gβ−) were grown in HL5 axenic media, which, for transformed cell lines, contained 20 μg/ml G418 (Sigma, St. Louis, MO). To screen for developmental phenotypes, transformants were plated with Klebsiella aerogenes (∼40–50 D. discoideum cells per 10-cm bacterial plate) and incubated for 5–7 d until each cell formed a single plaque. For development on nonnutrient agar, cells were washed in DB (2 mM MgCl2 and 0.2 mM CaCl2 in 10 mM Na/K phosphate, pH 6.5) and then incubated on 1.5% agar in DB. For development in suspension, cells were harvested, washed twice in DB, resuspended in DB at 2 × 107 cells/ml, shaken at 120 rpm, and pulsed with 75 nM cAMP at 6-min intervals for 5 h.
Construction of the Library of Mutagenized Gβ Genes, Plasmid Recovery, and Sequence Analysis
The Gβ cDNA was randomly mutagenized by a low-fidelity PCR procedure. The 5′ primer (AGATCTATAAAAAATGTCATCAGATATTTCAG) corresponds to the sequence of a ribosomal binding site and the beginning of the coding region of the Gβ gene, flanked by a BglII site; the 3′ primer (GCGGCCGCTTAAGCCCAAATCTTGAGGAG) corresponds to the region around the stop codon of the Gβ gene, flanked by a NotI site. The Gβ cDNA was used as the template in the PCR reaction for mutagenesis in buffer (1 mM dTTP, dGTP, and dCTP, 0.2 mM dATP, 7 mM MgCl2, and 0.5 mM MnCl2). The PCR products were digested by BglII and NotI and subcloned into the BglII–NotI sites of the D. discoideum extrachromosomal expression vector pMC34 in which the inserted gene was driven by an actin 15 promoter and an actin 8 terminator. The ligation mixture was transformed into the Escherichia coli Sure strain (Stratagene, La Jolla, CA), and plasmids were isolated from mixture of ∼15,000 independent clones. To recover plasmid from transformants of D. discoideum, total DNA was isolated from 108 cells as described previously (Parent and Devreotes, 1995) and transformed into the XL1-blue E. coli strain (Stratagene).
Screening for SN Mutant Cells
The gβ− cell line LW6 (Wu et al., 1995) was transformed with the library of mutagenized Gβ genes and selected for 7 d in HL5 containing G418. The mixture of transformants was screened by two procedures to isolate SN mutants.
1) The mixture of transformants was plated out on SM/Ka plates for individual plaques, and agg− clones were isolated as SN mutant candidates and grown in HL5 containing G418. The cells of these candidates were mixed with wild-type cells (AX3) at a 1:1 ratio and allowed to develop. Spores were then collected from the fruiting bodies, resuspended in buffer containing 10 mM NaCl, 10 mM KCl, and 2.5 mM CaCl2, and treated at 45°C for 30 min twice with a 5-min intervening on ice to kill the possible contaminating nonspore cells. The treated spores were plated out on SM/Ka plates. The efficiency of synergy with wild-type cells was measured as the percentage of agg− plaques versus total plaques. SN4 and SN5 mutants were isolated using this procedure.
2) The mixture of the transformants (∼108 cells) was plated and allowed to develop on nonnutrient DB agar plates. The spores were collected from the fruiting bodies, resuspended, and treated as described above. The treated spores were then plated on SM/Ka plates, and the agg− plaques were isolated. The cells from these plaques were plated on a nonnutrient DB agar, and those that showed complete agg− phenotypes were collected as SN mutant candidates. The ability of these candidates to synergize with wild-type cells was further confirmed. SN1, SN2, and SN3 mutants were isolated using this procedure.
Immunoblot Analysis
Samples of 2 × 106 cells were solubilized in sample buffer, analyzed by a 10% regular or low-bis SDS gel, and then blotted onto membranes (Millipore, Bedford, MA). The membranes were probed with antibodies to Gβ and cAR1 as described previously (Wu et al., 1995). Bands were visualized using an enhanced chemiluminescence kit (Amersham, Arlington Heights, IL).
cAMP Binding to cAR1 Receptor and GTP Inhibition Effect of Its Binding
cAMP binding to the membranes in the absence or presence of 0.1 mM GTP was carried out as described (Van Haastert and Kien, 1983; Caterina et al., 1994), except that the membranes were resuspended at 108 cell equivalents/ml.
Adenylyl Cyclase Assay
Cells were developed in suspension with 50 nM cAMP additions at 6-min intervals for 5 h, lysed in 2× lysis buffer (2 mM MgSO4 and 20 mM Tris, pH 8.0) in the presence and absence of 40 μM GTPγS or in the presence of 5 mM MnSO4, and then rapidly mixed with 10× reaction mix (100 mM Tris, pH 8.0, 1 mM ATP, and 100 mM dithiothreitol and [α-32P]ATP). Reactions were stopped at 2 min and assayed for [32P]cAMP as described (Pupillo et al., 1992).
Chemotaxis Assays
Chemotaxis to cAMP was examined by two methods. The small population assay was performed as described previously (Konijn and Van Haastert 1987; Insall et al., 1996). Additionally, a quantitative assay was carried out by using Biocoat transwell tissue culture inserts (Collaborative Biomedical Products, Bedford, MA). The cells were washed with DB buffer and resuspended at 107/ml. The DB buffer containing various concentrations of cAMP was carefully placed into the wells avoiding any air bubbles. One hundred microliters of cells were then added to the top of the chamber and were incubated at 22°C. The cells were monitored closely under the microscope for cell migration to the bottom chamber, and the assay was stopped when the background well (no stimulus control) began to have cells migrating nonspecifically. The number of cells migrated to the lower chamber were counted by fluorescence-activating cell sorting using forward and side scatters.
Actin Polymerization Assay
Cells were developed in suspension for 6 h and then washed and resuspended at 2 × 107 cell/ml. After stimulation by 10 nM cAMP, F-actin levels in the cells were measured at various time points by methods previously described (Insall et al., 1996).
Molecular Interactions of the Amino Acids Substituted in the SN Gβ Subunit and Effects of Each Substitution
Using the structure of the heterotrimeric G protein Gαt chimera Gβ1γ1 displayed in the computer program CHAIN (Lambright et al., 1996), we examined the positions and the molecular interactions of each of the amino acids substituted in SN Gβ subunits and the possible effects of each substitution. Specific interactions were considered those <3.6 Å apart and were visually inspected for reasonable stereochemistry.
RESULTS
Isolation of SN Gβ Mutations
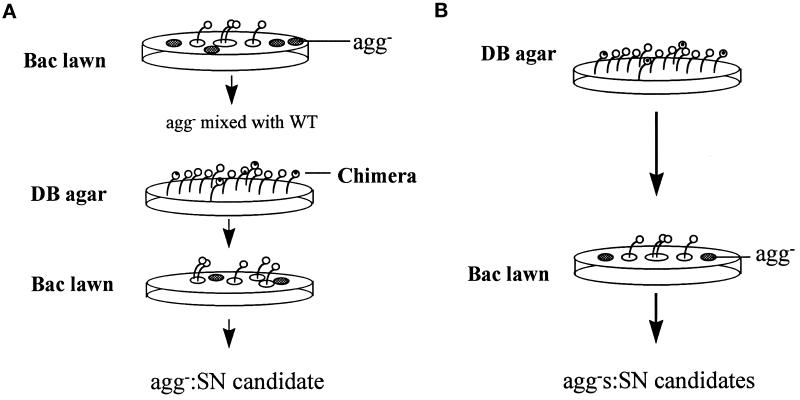
We devised a screen to sort through the huge number of mutant alleles of Gβ genes created by random mutagenesis and to isolate the alleles with a specific phenotype (synag). When plated on nonnutrient agar, wild-type cells aggregate and develop into fruiting bodies in 24 h, whereas Gβ null cells completely fail to aggregate and remain as individual cells in monolayers. When a mixture of Gβ null cells and wild-type cells is allowed to develop together, the spores from the resulting fruiting bodies are all derived from wild-type cells (Wu et al., 1995). In contrast, synag mutant cells, such as aca− and crac− cells, cannot aggregate alone but are able to form chimeric structures when mixed with wild-type cells (Pitt et al., 1993; Insall et al., 1994). To isolate Gβ alleles that resemble the phenotype seen in synag mutants, we transformed cells of a Gβ null parent strain with a library of randomly mutagenized Gβ cDNA and screened for transformants that failed to enter development in isolation but formed chimeric fruiting bodies when mixed with wild-type cells. We used two procedures to screen for such mutants, designated as SN. In the first procedure (Figure 1A), the transformants were plated on bacterial lawns and the aggregation-minus (agg−) plaques were collected. Each clone was then tested for its ability to differentiate into viable spore cells when mixed with wild-type cells, and the mutants that synergized were isolated as candidates. In the second procedure (Figure 1B), the mixture of the transformants (∼108 cells) was plated on nonnutrient agar and allowed to from chimeric fruiting bodies. The spores from these fruiting bodies were then plated on bacterial lawns, and the agg− plaques were isolated as candidates. Clones derived from both procedures were then retested on nonnutrient agar, and those that failed to aggregate were collected. Finally, the ability of each candidate to form chimeric fruiting bodies with wild-type cells was retested. The results are summarized in Table 1.
Figure 1.
Diagram of screening procedures for the SN mutant cells. The Gβ null cells were transformed with a library of randomly mutagenized gβ cDNA. The mixture of transformants, expressing Gβ alleles of wild type, SN, and nonfunction, was screened using two procedures. (A) The mixture of transformants was plated on a bacterial lawn, and cells expressing SN or nonfunctional Gβ alleles, which form aggregation minus plaques (agg−), were isolated. Clones that synergized and formed the chimeric fruiting bodies with wild-type cells were collected as SN candidates, whereas clones that did not form chimeric structures with wild-type cells were discarded. (B) The mixture of transformants was plated on DB agar, and the fruiting bodies containing spores were collected. These comprise cells only carrying SN or wild-type Gβ alleles. Spore cells were then plated on bacterial lawns, and cells from agg− plaques were isolated as SN candidates.
Table 1.
Ability of mutant cells to synergize wild-type cells to form chimeric fruiting bodies
| Cell line | agg+ plaques | agg− plaques | % of agg− |
|---|---|---|---|
| gβ− | 158 | 0 | 0 |
| SN1 | 122 | 20 | 14 |
| SN2 | 154 | 39 | 20 |
| SN3 | 104 | 44 | 29 |
| SN4 | 67 | 17 | 20 |
| SN5 | 202 | 40 | 16 |
| SN6 | 74 | 10 | 12 |
Each of the mutant cell lines was mixed with wild-type cells at a 1∶1 ratio and plated on nonnutrient agar. The collected spores from the fruiting bodies were plated on SM/Ka plates, and the individual plaques were counted and shown. The percentage of agg− plaques is designated as the number of agg− plaques versus the total number of plaques.
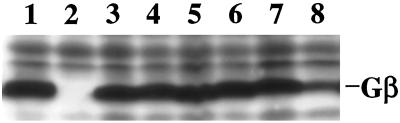
To prove that the SN phenotypes are plasmid dependent, we rescued the plasmids and retransformed them into fresh gβ− cells. The new transformants showed the same characteristics as did the original mutants. To rule out the possibility that the phenotypes of the SN mutants were caused by an inadequate expression of the mutant Gβ subunits, we carried out immunoblot analysis using specific Gβ antiserum (Lilly et al., 1993). As shown in Figure 2, the SN1–SN5 cells expressed Gβ at the same level as wild-type cells, whereas SN6 cells expressed Gβ at a lower level. Therefore, SN1–SN5 but not SN6 were used for further study.
Figure 2.
Protein levels of various Gβ alleles. Cells (2 × 106) of wild type (lane 1), gβ− (lane 2), SN1 (lane 3), SN2 (lane 4), SN3 (lane 5), SN4 (lane 6), SN5 (lane 7), and SN6 (lane 8) were harvested, solubilized in a gel loading buffer, analyzed by SDS-PAGE, and subjected to immunoblot using the specific Gβ antiserum.
Developmental Phenotypes of SN Mutant Cells after Treatment with Extracellular cAMP
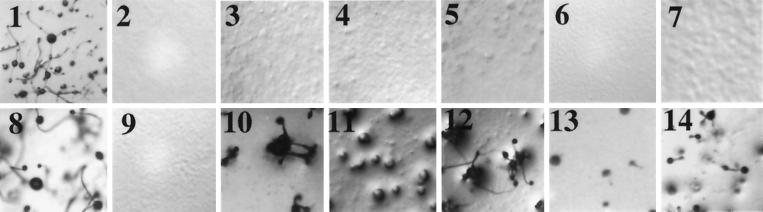
Cells specifically impaired in the ACA pathway, for example aca− cells, cannot enter development and remain as monolayers on nonnutrient agar, because they are unable to produce cAMP waves. However, these cells can respond to cAMP signals: after repeated treatment with extracellular cAMP, the cells will form aggregates and multicellular structures (Pitt et al., 1993). To determine whether such treatment with extracellular stimuli can rescue the SN mutants, the cells were starved in suspension, provided with exogenous cAMP at 6-min intervals for 5 h, and then plated on nonnutrient agar. As shown in Figure 3, after treatment with cAMP, each of the SN cell lines formed multicellular structures, whereas without treatment all remained as a monolayer. The SN1, SN3, and SN5 cells aggregated, completed development, and formed abnormal fruiting bodies. The SN2 and SN4 cells aggregated, formed mounds, and then arrested. As controls, gβ− cells remained as a monolayer, whereas wild-type cells formed fruiting bodies with or without cAMP treatment. These results suggested that cells carrying SN Gβ alleles cannot produce cAMP oscillatory signals but can respond to external cAMP signals; therefore, each of the SN Gβ subunits can couple to cAR1 and can carry out some functions of the Gβ subunit.
Figure 3.
Developmental phenotypes without or with extracellular cAMP stimuli. Cells were harvested from shaking cultures and allowed to develop on nonnutrient DB agar before or after the treatment with exogenous cAMP pulses at 6-min intervals for 5 h. The terminal developmental phenotypes, 36 h after harvesting, of cells without the treatment (1, wild type; 2, gβ−; 3, SN1; 4, SN2; 5, SN3; 6, SN4; 7, SN5) and with the treatment (8, wild type; 9, gβ−; 10, SN1; 11, SN2; 12, SN3; 13, SN4; 14, SN5) are shown.
Ability of SN Gβ Subunits to Mediate Induction of cAR1 and Form Heterotrimers That Couple to cAR1
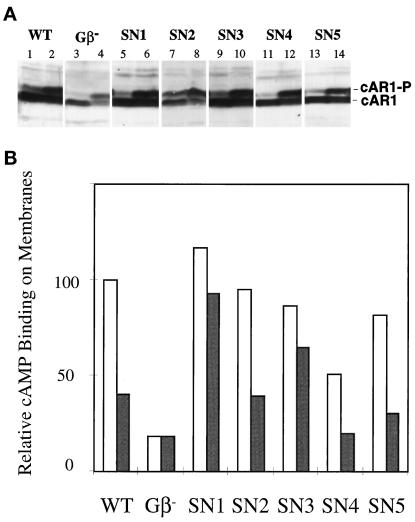
The expression levels of several components required for aggregation increase significantly during early development. Initially, cells accumulate sufficient levels of cAR1, Gα2, and ACA to support cAMP oscillations. The repeated cAMP stimuli resulting from the oscillations then further induce levels of several proteins, including cAR1 itself and csA/gp80, a cell adhesion glycoprotein (Firtel, 1995). The Gβ is required for induction of these components by cAMP (Milne et al., 1995; Jin et al., 1998). To examine whether SN Gβ subunits can mediate full induction of these genes, we allowed cells to develop in suspension with repeated cAMP stimuli for 5 h and then carried out an immunoblot using cAR1 antiserum. As shown in Figure 4A, each of the SN mutant cells expressed cAR1 at a level comparable to that of wild-type cells, whereas gβ− cells expressed cAR1 at a considerably lower level. These results indicate that the SN Gβ subunits can support cAR1-mediated induction of aggregation stage genes. cAMP induces phosphorylation of cAR1 in wild-type and Gβ null cells. cAMP elicited this response in all SN mutant cells (Figure 4).
Figure 4.
(A) cAMP-induced cAR1 expression and cAMP-induced cAR1 phosphorylation in SN cells. Cells were allowed to develop in suspension with addition of exogenous cAMP at 6-min intervals for 5 h. The developed cells were incubated with 0 nM (odd lanes) or 5 nM (even lanes) cAMP, and then the samples of 106 cells were analyzed by SDS-PAGE and detected using a cAR1 antiserum. (B) Analysis of cAMP binding to membranes in the absence or presence of GTP. Membranes were prepared from developed cells, and the binding assay was carried out in the absence (open bars) or presence (shaded bars) of GTP. The amount of [3H]cAMP binding in the absence of GTP in wild-type cells is used as a standard, designated as 100. Means of an experiment performed in triplicate are shown. Similar results were obtained form an independent experiment.
Agonist binding studies have demonstrated that cAR1 is linked to the heterotrimeric G protein containing Gα2 and Gβ. Membranes of wild-type cells exhibit both high- and low-affinity binding sites for cAMP. As for other G protein–coupled receptors, the high-affinity binding sites represent cAR1 coupled with a heterotrimeric G protein; GTP or GDP releases the G protein from the receptor and eliminates these sites. This process is conveniently assayed at 20 nM [3H]cAMP, a concentration at which addition of GTP or GDP causes a significant inhibition of binding (Kesbeke et al., 1988). Membranes of gβ− and gα2− cells display only low-affinity sites, insensitive to GTP inhibition, indicating that both Gβ and Gα2 are essential for maintaining the appropriate coupling between cAR1 and the G protein (Wu et al., 1995). Disruptions of other Gα genes do not affect cAMP binding in this assay (reviewed by Parent and Devreotes, 1996). To directly assess the ability of each SN Gβ subunit to form a heterotrimeric G protein capable of linking to cAR1, we examined effects of GTP on cAMP binding in isolated membranes. As shown in Figure 4B, in membranes prepared from wild-type cells, GTP reduced binding of [3H]cAMP to cAR1. In contrast, membranes prepared from gβ− cells displayed low, GTP-insensitive binding. Membranes from each of the SN mutant cells exhibited different cAMP binding affinities in the absence or presence of GTP. The percentage inhibition of cAMP binding by GTP varied among the SN Gβ mutants and was 20% in SN1, 53% in SN2, 33% in SN3, 62% in SN4, and 64% in SN5. These data indicate that each of SN Gβ subunits participates in formation a heterotrimer that couples to cAR1. The efficiency of forming a heterotrimer or coupling to cAR1 may be reduced in SN1 and SN3.
Chemoattractant-stimulated Actin Polymerization and Chemotaxis to cAMP in the SN Mutant Cells
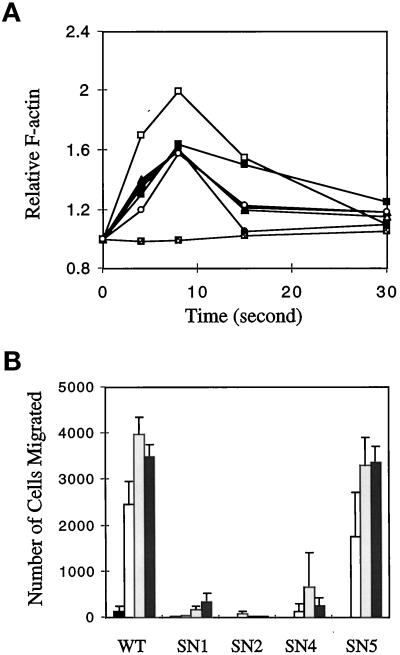
Stimulation of D. discoideum cells with chemoattractants causes a rapid and transient polymerization of actin (Devreotes and Zigmond, 1988; Hall et al., 1988; Zigmond et al., 1997). Gα2 or Gα4 null cells are specifically defective in this response to cAMP or folic acid, respectively, whereas Gβ null cells are completely unable to trigger actin polymerization in response to any chemoattractants, suggesting that two different heterotrimeric G proteins are linked to two classes of receptors and that Gβ is essential for each G protein to mediate an actin response (Zigmond et al., 1997). To test whether the SN Gβ subunits can mediate this response to a chemoattractant, we measured cAMP-stimulated actin polymerization in SN cells. As shown in Figure 5A, all the SN mutant cells (SN1–SN5) showed a clear transient actin polymerization response, with the peak in F-actin levels occurring ∼8 s after stimulation. The gβ− cells did not show any response, and the F-actin level remained at the resting level after the stimulation. This result indicates that these SN Gβ subunits retain the function of regulating actin polymerization.
Figure 5.
(A) Chemoattractant-stimulated alterations in actin polymerization. Wild-type (□), gβ− (░⃞), SN1 (▵), SN2 (▴), SN3 (•), SN4 (▪), and SN5 (○) cells were developed in shaken suspension and then washed in DB buffer, resuspended at 2 × 107 cells/ml. At time zero, 10 nM cAMP was added, and the amount of F-actin at different times was measured and expressed relative to the amount at time zero. Means of an assay performed in duplicate are shown. Independent experiments were done and yielded similar results. (B) Chemotaxis to cAMP. The cells were allowed to develop in suspension for 5 h and then were subjected to the transwell chemotaxis assay to cAMP at concentrations of 0 mM (black bars), 0.1 mM (open bars), 1 mM (light shaded bars), and 10 mM (heavy shaded bars). The number of cells that migrated toward cAMP is indicated. Means and SEMs of at least three duplicates are shown. Two independent experiments were done and yielded similar results.
Wild-type cells exhibit chemotaxis to a variety of attractants, whereas Gβ null cells fail completely to move toward any stimulus (Devreotes and Zigmond, 1988; Wu et al., 1995). To investigate the ability of cells expressing the SN Gβ alleles to carry out chemotaxis, we allowed the cells to develop to the aggregation stage and then measured chemotaxis to cAMP by both a small population assay and a transwell assay (details in MATERIALS AND METHODS). In the small population assay, SN4 and SN5 moved toward cAMP as well as did the wild-type cells. SN1–SN3 showed very weak chemotactic responses, whereas the control gβ− showed no response. In the more quantitative transwell chemotaxis assay, wild-type and SN5 cells displayed a similar sensitivity toward cAMP, and SN4 showed reduced sensitivity, whereas SN1 and SN2 displayed very weak and not detectable responses, respectively (Figure 5B).
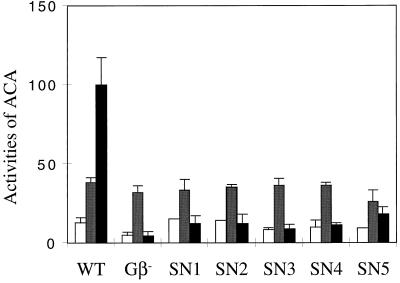
Activation of the Adenylyl Cyclase in SN Gβ Cells
The phenotype of the SN cells suggested that they are primarily defective in establishing cAMP oscillations. One possible reason could be inefficient activation of G2 by cAR1 in vivo, because some of the SN Gβ alleles have reduced affinity in coupling to the receptor. To assess whether free Gβγ containing an SN Gβ subunit is able to activate the adenylyl cyclase, we measured ACA activity in vitro after stimulation of GTPγS, which directly activates G proteins, bypassing the need for cAR1 (Soede et al., 1994). We stimulated cells with exogenous cAMP for 5 h to allow them to express aggregative genes and then examined ACA activity in lysates. As shown in Figure 6, GTPγS significantly stimulated ACA activity in the lysates of wild-type cells but did not stimulate activity in lysates of gβ− cells. The ratios of stimulated to basal were about 10 and 1 in wild-type and gβ− cells, respectively. In all SN mutant cells, GTPγS showed no or little stimulation of ACA activity. The ratios of stimulated to basal activity in the lysates of the SN cells were 0.9 in SN1, 0.8 in SN2, 1.1 in SN3, 1.1 in SN4, and 2.0 in SN5. In the presence of Mn2+ ions, which directly stimulate activity, lysates of wild-type, gβ−, and SN mutant cells showed comparable levels of activity, and immunoblot analyses showed that the SN mutant cells expressed ACA protein at the same level as wild-type cells (our unpublished results), demonstrating that the defect in GTPγS-stimulated activity in SN mutant cells is not due to a low level of ACA. These results indicated that Gβγ dimers containing each of the SN Gβ subunits are unable to transduce signals to downstream effectors that lead to activation of ACA.
Figure 6.
Adenylyl cyclase activity of wild-type, gβ−, and SN mutant cells. Cells were developed to the aggregation stage, and adenylyl cyclase activities in the lysates of wild-type, gβ−, SN1, SN2, SN3, SN4, and SN5 cells were measured in buffer (open bars), in the presence of Mn2+ (shaded bars), and in the presence of GTPγS (black bars). Means and SEMs of an experiment done in duplicate are presented. At least one independent experiment was done for each cell line and yielded similar results.
Determination of Mutations in the SN Gβ Alleles
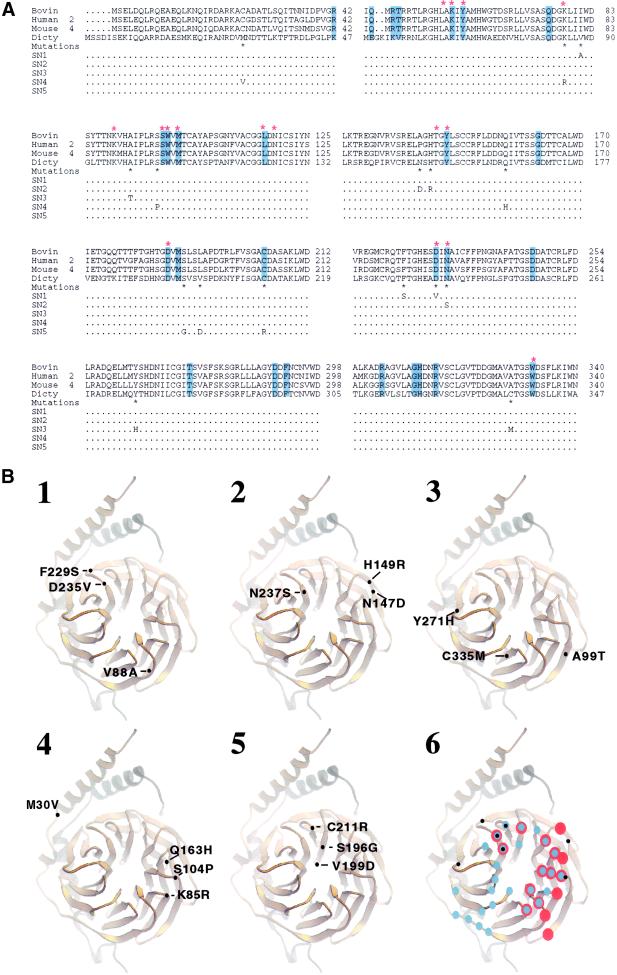
Each of the SN Gβ subunits retains some of functions of wild-type Gβ, strongly suggesting that mutations do not disrupt the global structure of the Gβγ dimer. It is likely the mutations cause local changes, which manifest most significantly in defects in activation of ACA. We sequenced the SN Gβ alleles and found that SN1, SN2, SN3, and SN5 have three mutations and SN4 has four (Figure 7). The Gβ subunit of D. discoideum is highly homologous to its counterparts in mammals, D. melanogaster, and C. elegans. The crystal structures of G proteins demonstrated that different Gβγ dimers display almost identical structures and that GDP-Gα and phosducin, negative regulators of Gβγ, inhibit signaling of Gβγ by binding to the “hub” in the propeller structure of Gβγ. The structures of Gαβγ, free Gβγ, and the complex of Gβγ with phosducin have also revealed that Gβγ does not undergo significant conformational changes whether free or in a complex with other protein Gβγ (Wall et al., 1995; Lambright et al., 1996; Sondek et al., 1996; Gaudet et al., 1996). Thus, if a mutation causes a defect in interaction with an effector, it is likely either to be on the surface or to affect residues on the surface of the Gβγ dimer. To understand how the SN mutations affect Gβγ functions, we examined the positions and the molecular interactions of the amino acids that were substituted, using the three-dimensional structure of the heterotrimeric G protein (Lambright et al., 1996) displayed in the computer program CHAIN.
Figure 7.
(A) Amino acid sequence alignment of four Gβ subunits and the substitutions in the SN Gβ subunits. Residues that contact phosducin are highlighted in blue. Red-stared residues interact with Gα. Black-starred residues are substituted in the SN Gβ subunits, and the substituted amino acids in SN1–SN5 are indicated. (B) Ribbon diagram showing the positions of the substitutions in each of the SN Gβ subunits. Gβ is gold; Gγ is silver. The numbers are placed directly on the ribbons according to the Gβ of D. discoideum (A), and positions are placed to the corresponding positions of the transducin Gβγ subunit (Sondek et al., 1996). (1) SN1; (2) SN2; (3) SN3; (4) SN4; (5) SN5. (6) Corresponding residues that contact Gα (red dots) and phosducin (blue dots) and the positions of the substitutions (black dots) that are predicated to cause defects in SN Gβ subunits.
In SN1, the substitutions are V88A, F229S, and D235V (Figure 7, A and B, 1). The F229S substitution creates a hole in a pocket of the Gβγ dimer, and will probably affect residues on the surface of the Gβγ. The D235V substitution is at the surface; its corresponding residue (D228) in bovine Gtβ makes direct contacts with both Gα and phosducin (Gaudet et al., 1996; Lambright et al., 1996). The substitution of the negatively charged D235 with a nonpolar V is very likely to affect positive–negative charge interactions of Gβγ with other proteins. In contrast, V88 is not on the surface, and A is a conservative substitution. This substitution is not expected to cause significant structural changes and thus it is not likely to cause a defect in SN1. We therefore propose that the mutations F229S and D235V cause the defects in SN1.
In SN2, the substitutions are N147D, H149R, and N237S (Figure 7, A and B, 2). The side chain of N147 is at the surface of Gβγ, and the substitution of N147 with a negatively charged D is likely to affect interactions with other proteins. The N237 residue is also at the surface, an its corresponding residue (N230) in the bovine Gtβ subunit interacts with both Gα and phosducin (Gaudet et al., 1996; Lambright et al., 1996). In contrast, the side chain of H149, pointing toward the inside of Gβγ, is not accessible to interact with other proteins. The H149R substitution is unlikely to cause a defect in SN2. We therefore propose that the mutations N147D and N237S cause the defects in SN2.
In SN3, the substitutions are A99T, Y271H, and C335 M (Figure 7, A and B, 3). The substitution of Y271H is likely to affect interactions of other amino acids and to reduce the stability of a region of the Gβγ dimer. The side chains of A99 and C335 are in internal pockets. These two substitutions are not expected to cause significant structural changes. We therefore propose that the mutation Y271H causes the defects in SN3.
In SN4, the substitutions are M30V, K85R, S104P, and Q163H (Figure 7, A and B, 4). S104 is at a surface loop of Gβγ. The S104P mutation will drastically change the positions of S105 and W106, because values of φ and ψ for S104 are −116 and −80, respectively, which are incompatible with a P substitution. The corresponding residues of S105 and W106 in bovine Gtβ, S98, and W99 are involved in direct interactions with both Gα and phosducin. Therefore, the S104P mutation is very likely to affect interactions between Gβγ and other proteins. Although the residue M30 is close to Gγ, the M30V mutation is not expected to cause structural changes and is probably neutral. K85 is located at the surface of Gβγ, and its corresponding residue in bovine Gtβ (K78) interacts with Gα through a hydrogen bond. However, the substitution of K85 with R, another positively charged residue, is not likely to affect interaction with Gα. Residue Q163 is conserved among some Gβs (Figure 7A) but is replaced by an H at the corresponding position of Gβ2 of D. melanogaster and the Gβ of S. cerevisiae (Sondek et al., 1996). Thus, the substitution Q163H is likely neutral. We propose that the mutation S104P causes the defects in SN4.
In SN5, the substitutions are S196G, V199D, and C211R (Figure 7, A and B, 5). C211 is on the surface of Gβγ, and its corresponding residue (C204) in bovine Gtβ is involved in interactions with phosducin. The C211R mutation will probably cause a simple rearrangement of side chains in this region and interfere with interactions with other proteins. In contrast, S196 is located in the central channel of Gβγ and is not accessible to interact with associated proteins. The S196G substitution will not have structural effects. Residue V199 is also not on the surface of Gβγ and will probably not affect local structure. Therefore S196G and V199D are unlikely to cause the defect in SN5. We propose the mutation C211R causes the defects in SN5.
The mutations on the surface of the Gβγ are predicated to probably cause the defects in SN Gβ alleles. The positions of these mutations (black dots) and residues of bovine Gtβ that interact with Gα (red dots) or phosducin (blue dots) are superimposed in a ribbon representation of a Gβγ (Figure 7B, 6). It is apparent that the relevant SN mutations are on the same face of the Gβγ dimer that interacts with both Gα and phosducin. These mutations occurred at the residues that are involved in interaction with both Gα and phosducin (D235, N237, S105, and W106, which were affected by the S104P substitution), only with phosducin (C211), or with neither of them (F229, Y271, and N147).
DISCUSSION
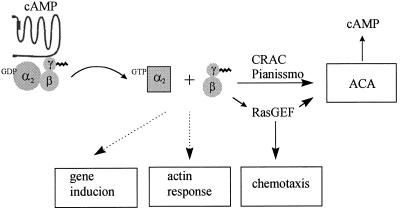
The Gβ subunit is essential for many steps in the mechanism of signaling by a G protein–coupled receptor system, including formation of functional heterotrimeric G proteins linked to receptors and transduction of signals to downstream effectors. In D. discoideum, the same Gβ subunits can form various G proteins with different Gα subunits in a single cell. Deletion of the Gβ gene results in the absence of functional G proteins linked to receptors. Therefore, the phenotypes displayed by the null mutant may be caused by impairment of cellular responses normally regulated by either Gβγ or Gα subunits. Furthermore, expression of genes encoding effectors and other components required for signaling are also affected in this mutant. To dissect the complex functions of Gβ and to study the specificity of Gβγ signaling in vivo, we randomly mutagenized the Gβ gene and isolated mutations on the Gβ subunit that impaired specific responses. We designed a screening procedure to identify a class of Gβ alleles whose phenotypes are similar to those displayed by synag mutant cells, which are specifically impaired in G protein–mediated activation of ACA, the pathway required for cell–cell cAMP signaling. Clonal populations of cells carrying these Gβ alleles (SN1–SN5) cannot enter development in isolation. However, unlike Gβ null cells, these mutants can complete development by forming chimeric fruiting bodies when mixed with wild-type cells or can form multicellular structures after treatment with exogenous cAMP that mimics oscillatory cAMP signals. The developmental phenotypes indicate that these Gβ alleles can form functional G proteins coupled to cAR1, respond to extracellular cAMP, and mediate some cellular responses. We used biochemical approaches to further assess the functions of SN Gβ alleles in signaling mediated by cAR1. The results are summarized in Table 2. Figure 8 shows a model of the cAR1–G2 signaling events that mediate cellular responses.
Table 2.
The characteristics of SN mutant cells
| Cell line | Developmental phenotypes on DB
|
Inhibition of cAMP binding (%) | Stimulation of ACA by GTPγS (fold) | Actin response | Chemotaxis to cAMP | |
|---|---|---|---|---|---|---|
| −cAMP | +cAMP | |||||
| WT | Fruit | Fruit | 60 | 10 | Yes | +++ |
| gβ− | agg− | agg− | 0 | 1 | No | − |
| SN1 | agg− | Fruit | 20 | 0.9 | Yes | +/− |
| SN2 | agg− | Mound | 53 | 0.8 | Yes | +/− |
| SN3 | agg− | Fruit | 33 | 1.1 | Yes | +/ND |
| SN4 | agg− | Mound | 62 | 1.1 | Yes | ++ |
| SN5 | agg− | Fruit | 63 | 2.0 | Yes | +++ |
ND, not determined; +/−, cells showed very weak chemotaxis in the small drop assay but displayed no chemotaxis in the transwell assay.
Figure 8.
Model of cellular responses mediated by Gβγ and Gα2 subunits upon cAMP stimulation. Excited cAR1 receptor activates G2 to generate GTP-Gα2 and Gβγ. Gβγ sends signals to regulate ACA activation and chemotaxis. Gene induction and actin response are activated by G2. It is not yet known whether GTP-Gα or Gβγ links to these two cellular responses.
Our results indicate that SN Gβ subunits can form heterotrimeric G proteins with Gα2 and couple to cAR1. Stable expression of a Gβ subunit has been shown to require Gγ to form a Gβγ dimer (Schmidt and Neer, 1991). Previous studies have demonstrated that the protein level of the D. discoideum Gβ does not change even when its mRNA is overexpressed >10 fold, presumably because excess Gβ proteins that do not form dimers with Gγ subunits are unstable (Lilly et al., 1993). We demonstrated that each Gβ subunit (SN1–SN5 alleles) was expressed at same level as a wild-type Gβ, suggesting that each SN Gβ subunit can associate with a Gγ to form a stable Gβγ dimer. This result is consistent with results from sequencing of the SN Gβ alleles, which revealed that no mutations occurred at the residues that have been implicated in interaction with a Gγ (Sondek et al., 1996). Our results from GTP inhibition of cAMP binding to membranes suggested that Gβγ dimers containing SN2, SN4, and SN5 have comparable affinities in forming G protein with Gα2 and coupling to cAR1, whereas those containing SN1 and SN3 have somewhat reduced affinities.
Two cellular responses that require the functional G protein G2, cAMP-induced gene expression and actin polymerization, are normal in SN cells. We demonstrated that SN cells can reach the aggregation stage of development, can form multicellular structures, and can regulate gene expression after repeated cAMP treatment, in contrast to Gβ null cells, which cannot respond to cAMP. Furthermore, we observed a clear actin polymerization response in all SN cells, indicating that the SN Gβ subunits are fully functional to regulate this pathway in response to cAMP. The precise target protein that links the heterotrimeric G protein to the actin polymerization response has not yet been identified in D. discoideum or other eukaryotic cells. In vitro studies suggest that activation of a small G protein is involved in the pathway that leads to actin polymerization (Zigmond et al., 1997). A recent study in S. cerevisiae indicated that the association of the Gβγ and Cdc24, a GDP–GTP exchange factor (GEF) for a small G protein Cdc42, is an essential step in mediating actin response (Nern and Arkowitz; 1998). It is possible that free Gβγ subunits generated from activation of G2 by cAR1 interact with a GEF for a small G protein that triggers this actin response.
SN cells displayed differential chemotactic abilities; SN1 and SN2 could hardly carry out chemotaxis, whereas SN4 and SN5 displayed chemotaxis. Because mutations on the Gβ subunit that do not impair interaction with Gα2 cause impairment of this response, Gβγ may directly signal to downstream effectors that lead to chemotaxis. Different chemotactic behaviors have been reported in mutants lacking downstream components that are required for ACA activation. The null mutations in CRAC, Pianissimo, and Aimless (a RasGEF) display weak chemotaxis to cAMP (Insall et al., 1994, 1996; Chen et al., 1997). Nonchemotactic mutants KI8 and KI10 isolated from chemical mutagenesis are defective in cAMP-stimulated activation of guanylyl cyclase but not the activation of adenylyl cyclase (Kuwayama et al., 1993); Gβ null cells are nonchemotactic and defective in the activation of both enzymes (Wu et al., 1995). These observations suggest that Gβγ regulates multiple pathways leading to chemotaxis.
SN cells are all defective in ACA activation. Both the developmental phenotypes and the failure of GTPγS to activate ACA activity in lysates demonstrated this defect. Because the SN Gβ subunits could form G proteins that were linked to cAR1 and could participate in signaling events that regulate other responses, the most likely explanation is an impairment in the linkage of free SN Gβγ dimer to the downstream effectors that lead to ACA activation. The pathway linking receptor–G protein to the activation of ACA involves several components, including four cytosolic proteins, CRAC, Pianissimo, Aimless (RasGEF), and ERK2 (MAP kinase) (Insall et al., 1994, 1996; Segall et al., 1995; Chen et al., 1997). It is not yet clear how these components act in a process that leads to ACA activation. Our study indicated that Gβγ directly sends signals to mediate this process. The differences in chemotaxis in the SN cells and cells lacking each of the cytosolic proteins suggest a possible model in which Gβγ links to at least two pathways. Each pathway is essential but not sufficient for activation of ACA. Further studies to identify proteins that interact with Gβγ should help us understand this process.
We have identified Gβ alleles that are defective in mediating specific cellular responses in vivo. It is conceivable that SN Gβs are just weak alleles, and these mutations generally reduce all functions of Gβ rather than directly affect the linkage between Gβγ and specific effectors, and that certain responses merely display differential sensitivities to nonspecific defects of Gβ subunits. We consider this possibility unlikely for the following reasons. First, our assay of ACA activation directly examined the linkage between Gβγ and downstream effectors by activating G proteins with GTPγS. This activity did not require the coupling of G2 to receptors. Second, there was no change in the threshold for triggering actin polymerization: 2 nM cAMP, the minimal concentration required for maximal actin responses in wild-type cells, could also trigger this response in each of the SN cells. Third, the study of inhibition of cAMP binding by GTP showed that each of the SN Gβ subunits did form functional heterotrimers linked to cAR1, and SN2, SN4, and SN5 Gβ subunits displayed the efficiency of wild-type Gβ. Fourth, cAR1-regulated gene expression was normal in the SN cells.
Two molecular genetic approaches can be used to identify the residues on Gβ that are crucial for interaction between Gβγ and effectors: site-directed mutagenesis to target certain residues and random mutagenesis and phenotypic screening to localize these residues. In this study, we screened a library of >15,000 randomly mutagenized Gβ genes using a developmental phenotype as a readout. The mutations in five SN Gβ alleles provide a rough map on Gβ. Our analysis of these SN Gβ alleles, using the crystal structure of bovine G proteins, allowed us to propose the residues that are important for signaling to downstream effectors. Interestingly, these mutations all mapped on the Gα binding face, or the hub of the propeller, and included residues that are involved in interactions with Gα and phosducin as well as residues that are not likely to contact these regulators. No mutations were found on the other surfaces of Gβγ, such as the blades and the back of the propeller. This study indicates that the Gα binding face of the Gβγ dimer directly participates in the interaction between the Gβγ and effectors leading to ACA activation. Two studies using site-directed mutagenesis to examine the mammalian Gβ residues that contact GDP-Gα have recently been reported (Ford et al., 1998; Li et al., 1998). They found that a single mutation on these residues differentially affects Gβγ signaling to its effectors. Although mutation of N237 (in Gβ of D. discoideum) has not been carried out in mammalian Gβ subunits, the other two identified mutations, D235V and S104P (which affects S105 and W106) in D. discoideum Gβ, have been created in corresponding residues, D228A and W99A (Ford et al., 1998) and D228R (Li et al., 1998), of mammalian Gβ. These mutations impaired the ability of mammalian Gβγ subunits to activate adenylyl cyclase II, which is a mammalian homologue of ACA. These results are consistent with our conclusion. The random mutagenesis approach allowed us to identify several residues in the region that are likely needed for effector interaction but not Gα binding sites, which have yet to be examined in other systems. Our study along with these two studies suggests that binding of Gα to Gβγ covers a part of the region that is required for interaction between Gβγ and its effectors—likely a general mechanism of regulation of Gβγ signaling by its regulators in all eukaryotic systems.
ACKNOWLEDGMENTS
We thank Dr. R. Gaudet of the P. Sigler laboratory for sending the ribbon diagram of the Gβγ subunit and Dr. J. Sondek of the Sigler laboratory for providing coordinates of the heterotrimeric G proteins; J. Borleis for assistance in the assay of actin polymerization response; and Dr. C. Parent, Dr. Z. Xiao, W.M. Froehlich, and other members in the Devreotes laboratory for valuable advice and discussions. This work was supported by National Institutes of Health grant GM34933 (to P.N.D.). J.T. is supported by a National Institutes of Health fellowship.
REFERENCES
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Milne JL, Devreotes PN. Mutation of the third intracellular loop of the cAMP receptor, cAR1, of Dictyosteliumyields mutants impaired in multiple signaling pathways. J Biol Chem. 1994;269:1523–1532. [PubMed] [Google Scholar]
- Chen MY, Long Y, Devreotes PN. A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G-protein mediated activation of the twelve transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 1997;11:3218–3230. doi: 10.1101/gad.11.23.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. New roles for G-protein βγ-dimers in transmembrane signaling. Nature. 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. G protein βγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Devreotes PN. G protein-linked signaling pathways control the developmental program of Dictyostelium. Neuron. 1994;12:235–241. doi: 10.1016/0896-6273(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Man-Son-Hing H, Yarfitz S, Colley NJ, Deer JR, Spencer M, Hurley JB, Zuker CS. An eye-specific Gβ subunit essential for termination of the phototransduction cascade. Nature. 1994;370:59–61. doi: 10.1038/370059a0. [DOI] [PubMed] [Google Scholar]
- Firtel RA. Integration of signaling information in controlling cell-fate decisions in Dictyostelium. Genes Dev. 1995;9:1427–1444. doi: 10.1101/gad.9.12.1427. [DOI] [PubMed] [Google Scholar]
- Ford CE, et al. Molecular basis for interaction of G protein βγ subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hall AL, Schlein A, Condeelis J. Relationship of pseudopod extension to chemotactic hormone-induced actin polymerization in amoeboid cells. J Cell Biochem. 1988;37:285–299. doi: 10.1002/jcb.240370304. [DOI] [PubMed] [Google Scholar]
- Insall RH, Borleis J, Devreotes PN. The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Curr Biol. 1996;6:719–729. doi: 10.1016/s0960-9822(09)00453-9. [DOI] [PubMed] [Google Scholar]
- Insall RH, Kuspa A, Lilly PJ, Shaulsky G, Levin LR, Loomis WF, Devreotes PN. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1994;126:1537–1545. doi: 10.1083/jcb.126.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, T., Soede, R.D., Liu, J., Kimmel, A.R., Devreotes, P.N., and Schaap, P. (1998). Temperature-sensitive Gβ mutants discriminate between G-protein depend and G-protein independent signaling mediated by serpentine receptors. EMBO J. (in press). [DOI] [PMC free article] [PubMed]
- Kesbeke F, Snaar-Jagalska BE, Van Haastert PJM. Signal transduction in Dictyostelium fgdA mutants with a defective interaction between surface cAMP receptors and GTP-binding regulatory protein. J Cell Biol. 1988;107:521–528. doi: 10.1083/jcb.107.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn TM, Van Haastert PJM. Measurement of chemotaxis in Dictyostelium. Methods Cell Biol. 1987;28:283–298. doi: 10.1016/s0091-679x(08)61652-0. [DOI] [PubMed] [Google Scholar]
- Kuwayama H, Ishida S, Van Haastert PJ. Non-chemotactic Dictyostelium discoideummutants with altered cGMP signal transduction. J Cell Biol. 1993;123:1453–1462. doi: 10.1083/jcb.123.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. Sites for Gα binding on the G protein β subunit overlap with sites for regulation of phospholipase Cβ and adenylyl cyclase. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- Lilly PJ, Wu L, Welker DL, Devreotes PN. A G-protein β-subunit is essential for Dictyosteliumdevelopment. Genes Dev. 1993;7:986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- Milne JLS, Wu L, Caterina MJ, Devreotes PN. Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J Biol Chem. 1995;270:5926–5931. doi: 10.1074/jbc.270.11.5926. [DOI] [PubMed] [Google Scholar]
- Nern A, Arkowitz RA. A GTP-exchange factor required for cell orientation. Nature. 1998;391:195–198. doi: 10.1038/34458. [DOI] [PubMed] [Google Scholar]
- Parent C, Devreotes PN. Isolation of inactive and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J Biol Chem. 1995;270:22693–22696. doi: 10.1074/jbc.270.39.22693. [DOI] [PubMed] [Google Scholar]
- Parent C, Devreotes PN. Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem. 1996;65:411–440. doi: 10.1146/annurev.bi.65.070196.002211. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Brandt R, Lin KC, Devreotes PN, Schaap P. Extracellular cAMP is sufficient to restore developmental gene expression and morphogenesis in Dictyosteliumcells lacking the aggregation adenylyl cyclase (ACA) Genes Dev. 1993;7:2172–2180. doi: 10.1101/gad.7.11.2172. [DOI] [PubMed] [Google Scholar]
- Pupillo M, Insall RH, Pitt GS, Devreotes PN. Multiple cyclic AMP receptors are linked to adenylyl cyclase in Dictyostelium. Mol Biol Cell. 1992;3:1229–1234. doi: 10.1091/mbc.3.11.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15058. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Neer EJ. In vitro synthesis of G protein βγ dimers. J Biol Chem. 1991;266:4538–4544. [PubMed] [Google Scholar]
- Schneider T, Igelmund P, Hescheler J. G protein interaction with K+ and Ca2+channels. Trends Pharmacol Sci. 1997;18:8–11. doi: 10.1016/s0165-6147(96)01001-2. [DOI] [PubMed] [Google Scholar]
- Segall J, Kuspa A, Shaulsky M, Ecke M, Maeda M, Gaskins C, Firtel R, Loomis W. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128:405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soede RD, Insall RH, Devreotes PN, Schaap P. Extracellular cAMP can restore development in Dictyosteliumcells lacking one, but not two subtypes of early cAMP receptors (cARs). Evidence for involvement of cAR1 in aggregative gene expression. Development. 1994;120:1997–2002. doi: 10.1242/dev.120.7.1997. [DOI] [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G protein βγ dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Kien E. Binding of cAMP derivatives to Dictyostelium discoideumcells: activation mechanism of the cell surface cAMP receptor. J Biol Chem. 1983;258:9636–9642. [PubMed] [Google Scholar]
- Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, Grant FJ, O’Hara P, MacKay VL. The STE4 and STE18 genes of yeast encode potential β and γ subunits of the mating factor receptor-coupled G protein. Cell. 1989;56:467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Wu L, Valkema R, Van Haastert PJ, Devreotes PN. The G protein β subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–1675. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Joyce M, Borleis J, Bokoch GM, Devreotes PN. Regulation of actin polymerization in cell-free systems by GTPγS and Cdc42. J Cell Biol. 1997;138:363–374. doi: 10.1083/jcb.138.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RR, Ahringer J, van Luenen HG, Rushforth A, Anderson P, Plasterk RH. G proteins are required for spatial orientation of early cell cleavages in C. elegansembryos. Cell. 1996;86:619–629. doi: 10.1016/s0092-8674(00)80135-x. [DOI] [PubMed] [Google Scholar]