Abstract
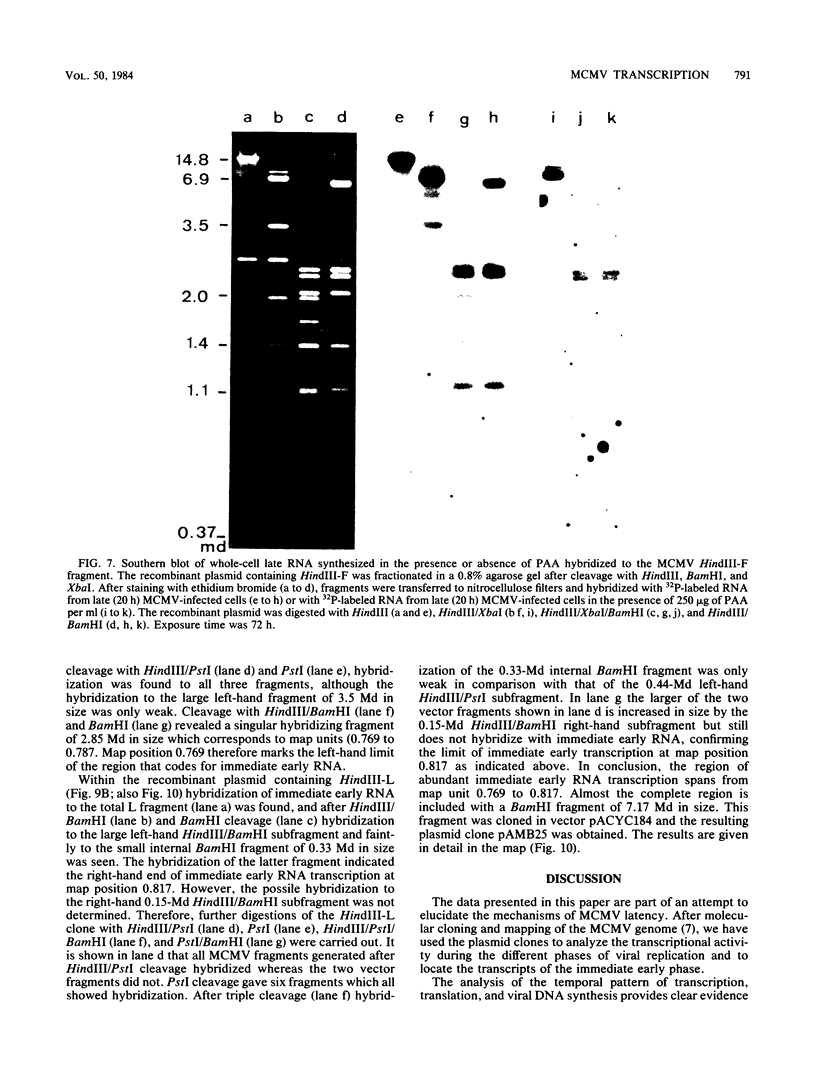
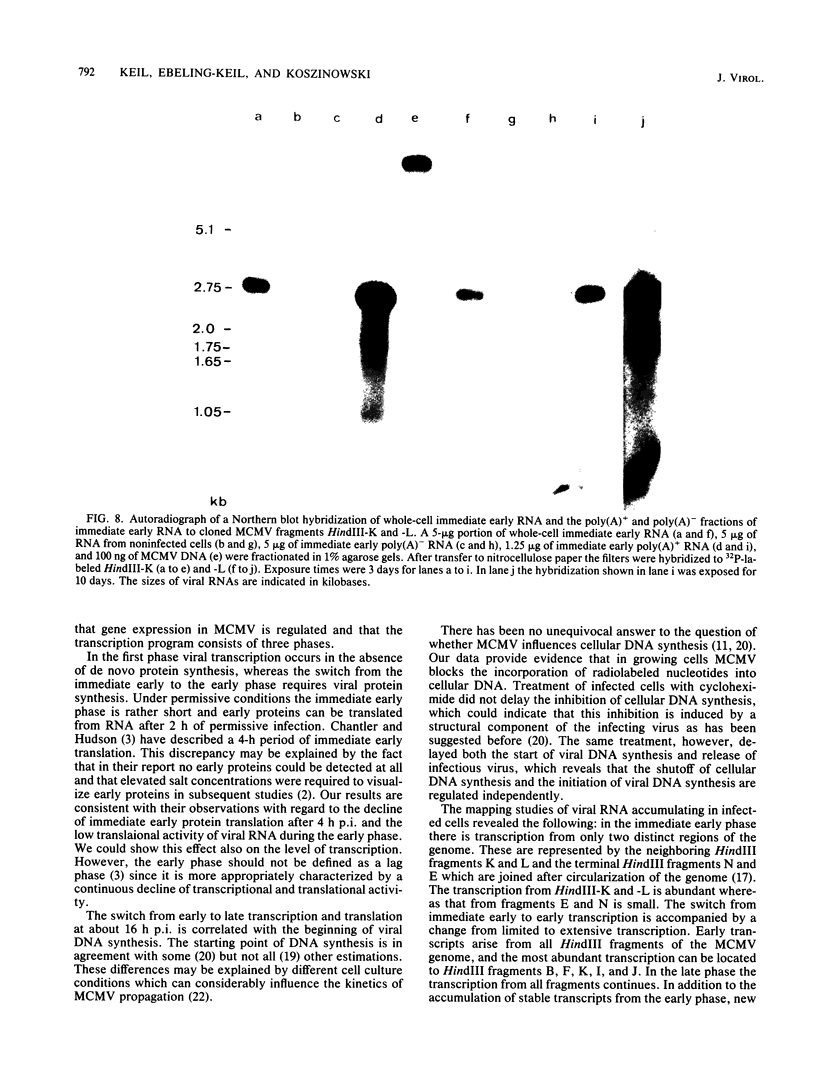
The replication of murine cytomegalovirus strain Smith in murine embryonic fibroblasts was investigated at immediate early, early, and late times after infection. Cloned subgenomic HindIII fragments of murine cytomegalovirus DNA served to define the regions of transcription. At immediate early times viral RNA classes ranging in size from 5.1 to 1.05 kilobases (kb) were transcribed mainly from the fragments HindIII-K and -L, whereas low levels of transcription were detected from the two termini HindIII-E and HindIII-N. A characteristic pattern of proteins could be translated from immediate early RNA in vitro. At early and late times after infection transcription from all HindIII fragments occurred, but different patterns of transcripts and proteins could be identified. Inhibitors of DNA synthesis induced differences in the late transcription pattern, located in the HindIII-F fragment. The coding region for abundant immediate early transcription could be located at between 0.769 and 0.817 map units. A plasmic clone containing the main part (0.769 to 0.815 map units) of this region was constructed. This region coded for six polyadenylated immediate early RNA species of 5.1, 2.75, 2.0, 1.75, 1.65, and 1.05 kb in size. Only the 1.75-kb RNA originated entirely from the HindIII-L fragment. The 5.1- and 2.75-kb RNA species were encoded by both the HindIII-L and HindIII-K fragments, and the 2.0-, 1.65-, and 1.05-kb RNA species were entirely transcribed within HindIII-K.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chantler J. K., Hudson J. B. Proteins of murine cytomegalovirus: identification of structural and nonstructural antigens in infected cells. Virology. 1978 May 1;86(1):22–36. doi: 10.1016/0042-6822(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Chantler J. K. The use of hypertonicity to selectively inhibit host translation in murine cytomegalovirus-infected cells. Virology. 1978 Oct 1;90(1):166–169. doi: 10.1016/0042-6822(78)90346-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Ebeling A., Keil G. M., Knust E., Koszinowski U. H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983 Sep;47(3):421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A., Keil G., Nowak B., Fleckenstein B., Berthelot N., Sheldrick P. Genome structure and virion polypeptides of the primate herpesviruses Herpesvirus aotus types 1 and 3: comparison with human cytomegalovirus. J Virol. 1983 Feb;45(2):715–726. doi: 10.1128/jvi.45.2.715-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976 Jul 1;72(1):235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- Hudson J. B. The problem of host DNA synthesis in murine cytomegalovirus-infected cells. Virology. 1980 Mar;101(2):545–548. doi: 10.1016/0042-6822(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Keil G., Fleckenstein B., Bodemer W. Structural proteins of Herpesvirus saimiri. J Virol. 1983 Sep;47(3):463–470. doi: 10.1128/jvi.47.3.463-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Marks J. R., Spector D. H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology. 1983 Aug;129(1):94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- Misra V., Muller M. T., Chantler J. K., Hudson J. B. Regulation of murine cytomegalovirus gene expression. I. Transcription during productive infection. J Virol. 1978 Aug;27(2):263–268. doi: 10.1128/jvi.27.2.263-268.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V., Muller M. T., Hudson J. B. The enumeration of viral genomes in murine cytomegalovirus-infected cells. Virology. 1977 Dec;83(2):458–461. doi: 10.1016/0042-6822(77)90195-7. [DOI] [PubMed] [Google Scholar]
- Moon H. M., Sapienza V. J., Carp R. I., Kim K. S. DNA synthesis in mouse embryo fibroblast cells infected with murine cytomegalovirus. Virology. 1976 Dec;75(2):376–383. doi: 10.1016/0042-6822(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Cell cycle dependency of murine cytomegalovirus replication in synchronized 3T3 cells. J Virol. 1977 May;22(2):267–272. doi: 10.1128/jvi.22.2.267-272.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. I. Distinct maturation stages of cytolytic T lymphocytes constitute the cellular immune response during acute infection of mice with the murine cytomegalovirus. J Immunol. 1984 Jan;132(1):482–489. [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984 Jan;14(1):56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]