Abstract
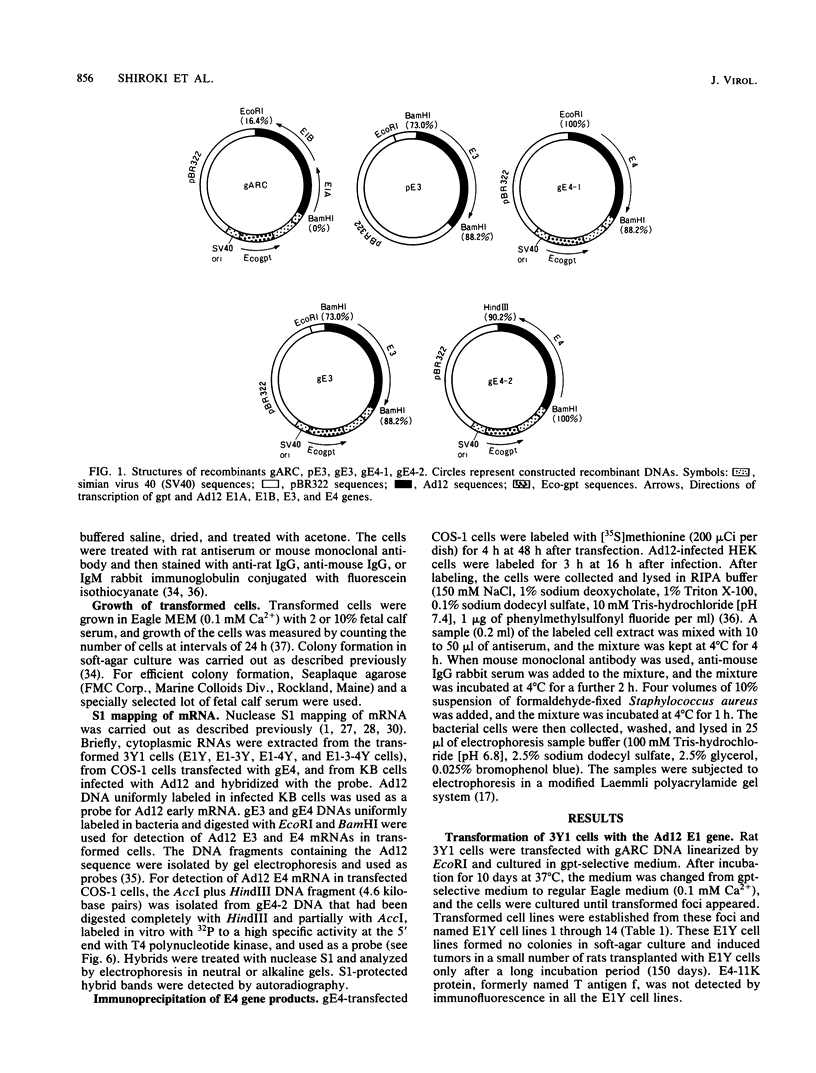
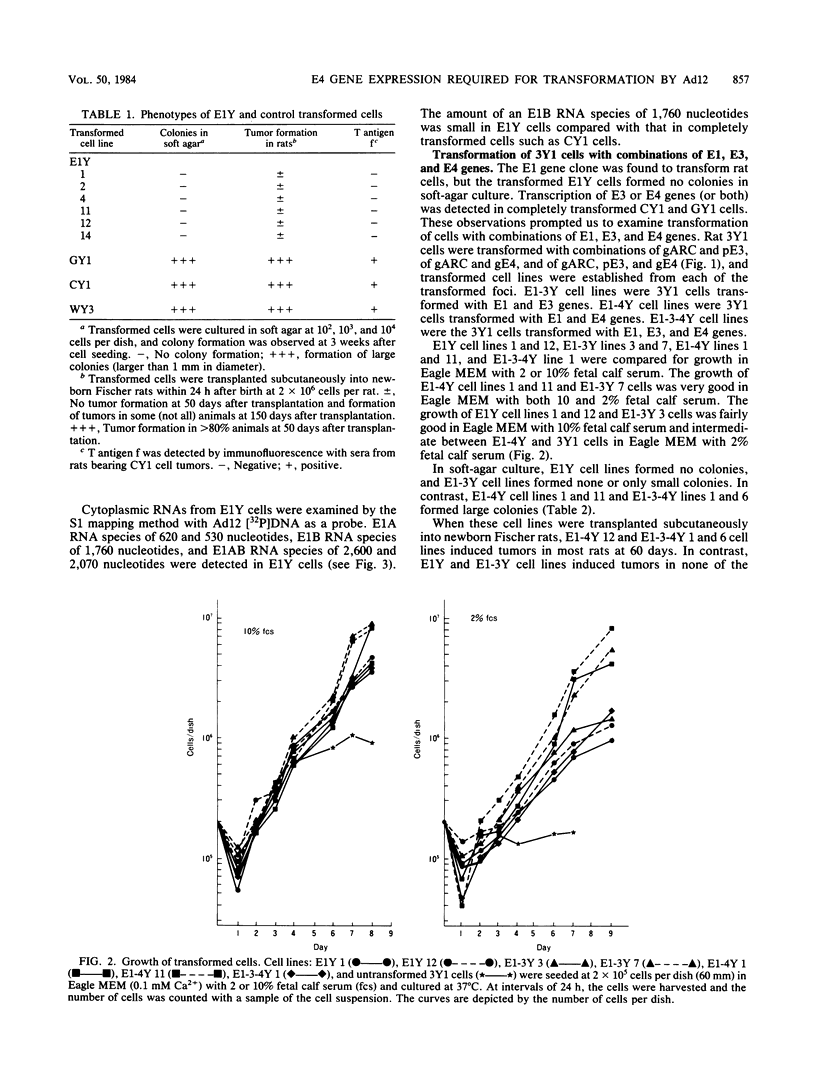
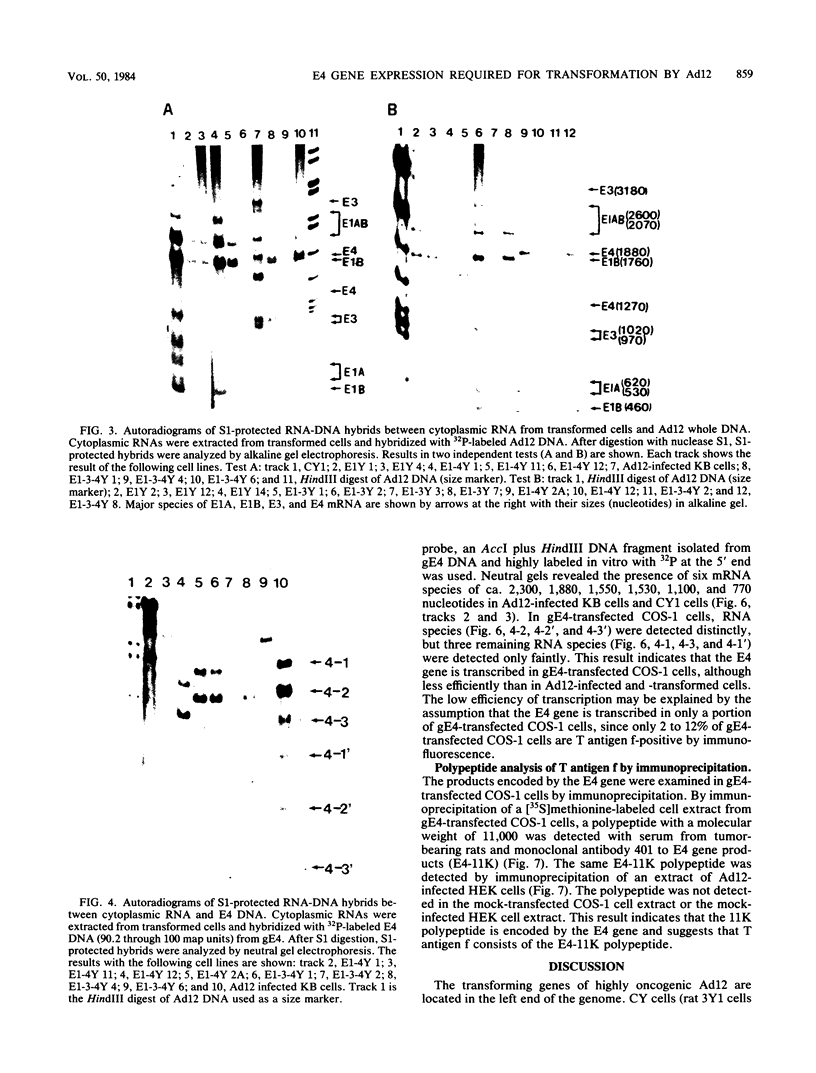
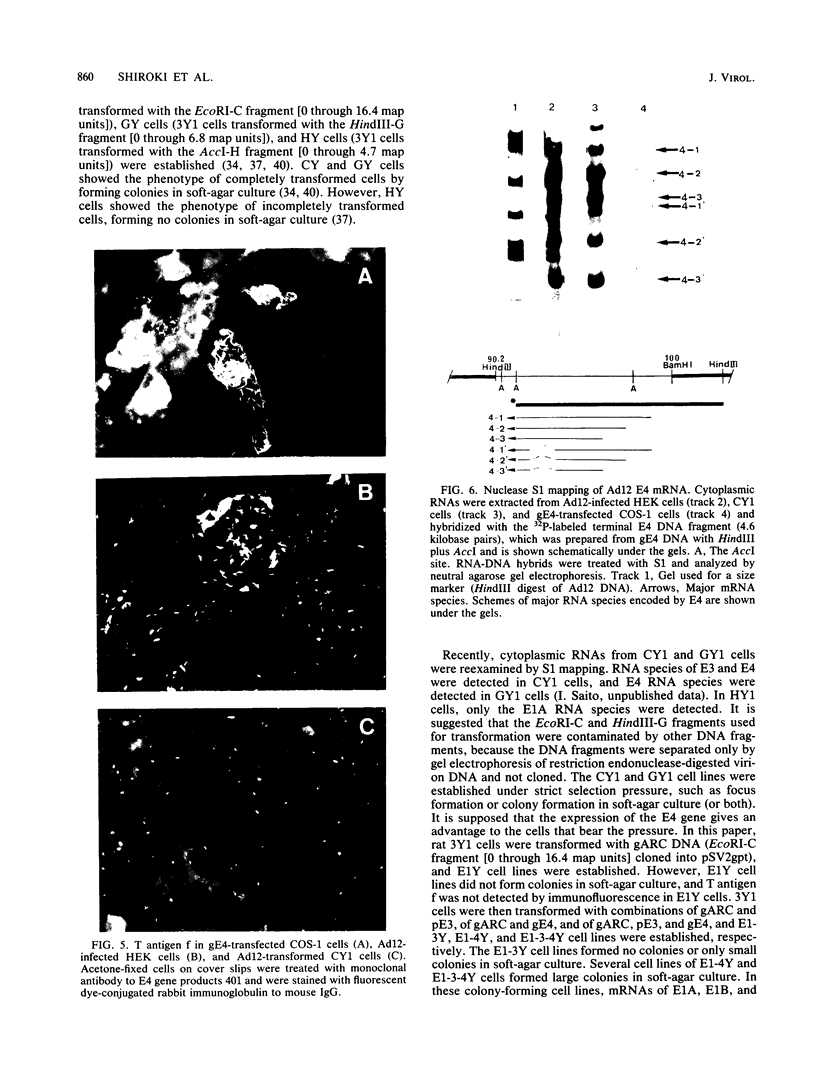
Rat 3Y1 cells were transfected with recombinant gARC ( pSV2gpt carrying the adenovirus 12 early region 1 [E1] gene), and focus formation was observed in monolayer cultures after culture of cells in gpt-selective medium (Eagle medium containing 10% fetal calf serum, xanthine, thymidine, aminopterin, and mycophenolic acid) for 10 days, followed by focus formation. Transformed E1Y cell lines were then established from these foci. The E1Y cells were transformed morphologically similarly to cells transformed with intact adenovirus 12 DNA but formed no colonies in soft-agar culture and induced tumors in transplanted rats only after a long incubation period. For the establishment of completely transformed cells, 3Y1 cells were transformed with combinations of gARC , pE3 (pBR322 carrying the adenovirus 12 E3 gene), and gE4 ( pSV2gpt carrying the adenovirus 12 E4 gene) DNA. E1- 3Y cells (3Y1 cells transformed with gARC and pE3 DNA), E1- 4Y cells (3Y1 cells transformed with gARC and gE4 DNA), and E1-3- 4Y cells (3Y1 cells transformed with gARC , pE3 , and gE4 DNA) were established. These transformed cell lines were compared for growth in Eagle medium with 2 or 10% fetal calf serum, colony formation in soft-agar culture, and tumor growth in rats transplanted with the transformed cells. Several transformed cell lines of E1- 4Y and E1-3- 4Y cells showed colony formation in soft-agar culture and abundant expression of the E1B gene. T antigen f was seen by immunofluorescence as flecks in these cells, in which the E4 gene was transcribed, but was not seen in E1Y cells, suggesting that T antigen f was encoded by the E4 gene. The suggestion was confirmed by the observation that T antigen f was detected in COS-1 cells transfected singly with gE4 DNA by immunofluorescence with polyclonal and monoclonal antibodies. Transcription of the E4 gene was confirmed in gE4 -transfected COS-1 cells. T antigen f, one of the E4 gene products, was identified as a polypeptide of molecular weight 11,000 (E4- 11K ) by immunoprecipitation with monoclonal antibodies. The above results also suggest that expression of the E4 gene gives cells the advantage of forming colonies in soft-agar culture. A tendency was noticed for E1B gene expression to be enhanced by E4 gene expression. The relationship between enhancement of colony formation in soft-agar culture and enhancement of E1B gene expression is discussed.
Full text
PDF


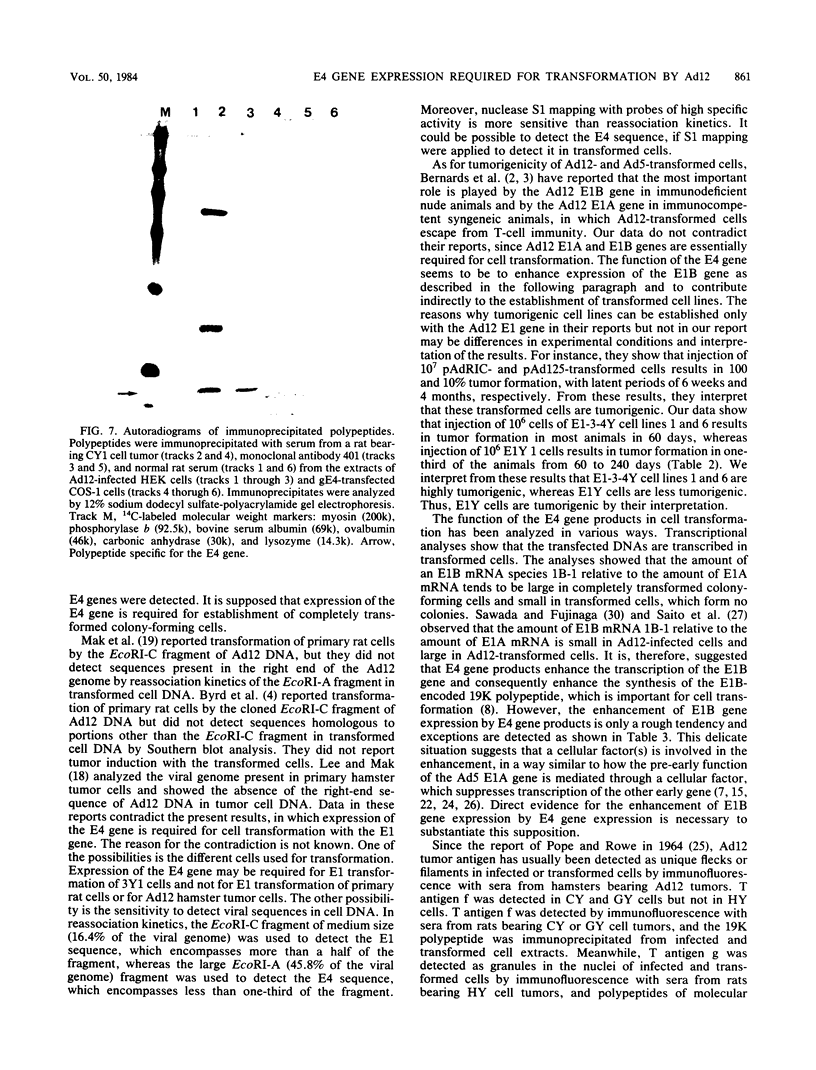
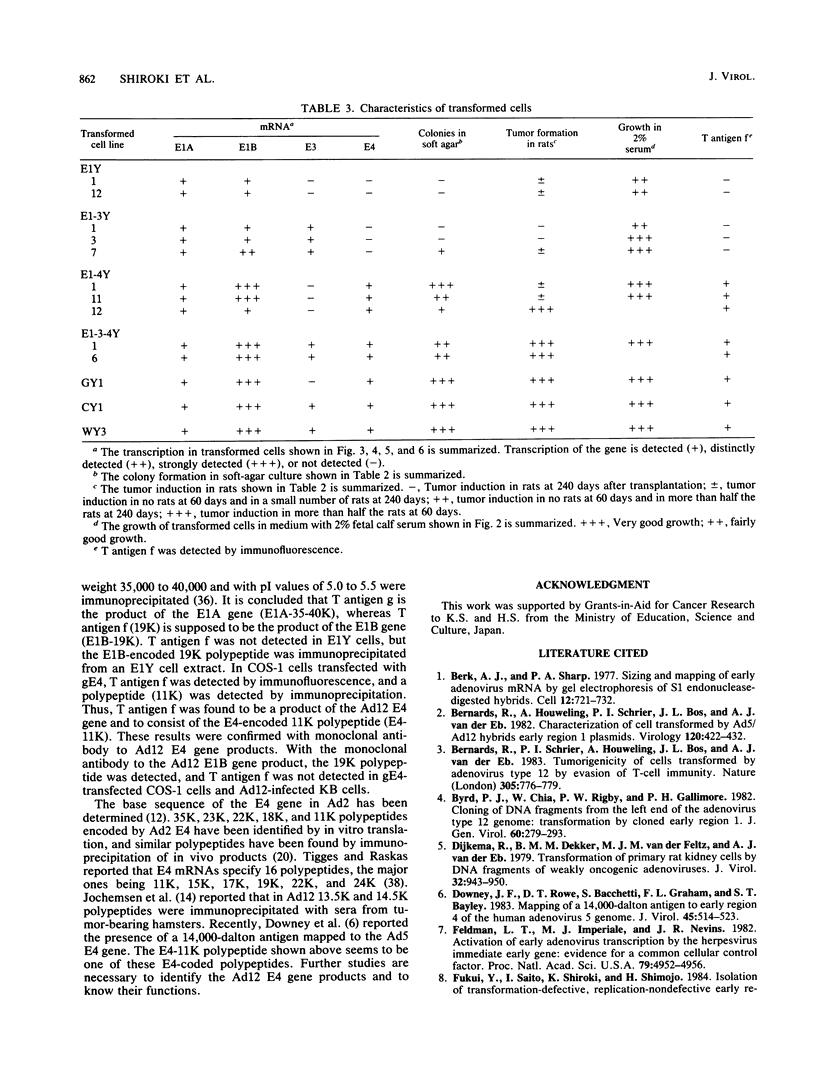

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Houweling A., Schrier P. I., Bos J. L., Van der Eb A. J. Characterization of cells transformed by Ad5/Ad12 hybrid early region I plasmids. Virology. 1982 Jul 30;120(2):422–432. doi: 10.1016/0042-6822(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Byrd P. J., Chia W., Rigby P. W., Gallimore P. H. Cloning of DNA fragments from the left end of the adenovirus type 12 genome: transformation by cloned early region 1. J Gen Virol. 1982 Jun;60(Pt 2):279–293. doi: 10.1099/0022-1317-60-2-279. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van der Feltz M. J., van der Eb A. J. Transformation of primary rat kidney cells by DNA fragments of weakly oncogenic adenoviruses. J Virol. 1979 Dec;32(3):943–950. doi: 10.1128/jvi.32.3.943-950.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey J. F., Rowe D. T., Bacchetti S., Graham F. L., Bayley S. T. Mapping of a 14,000-dalton antigen to early region 4 of the human adenovirus 5 genome. J Virol. 1983 Feb;45(2):514–523. doi: 10.1128/jvi.45.2.514-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Rigolet M., de Dinechin S. D., Galibert F. Nucleotide sequence of adenovirus 2 DNA fragment encoding for the carboxylic region of the fiber protein and the entire E4 region. Nucleic Acids Res. 1981 Aug 25;9(16):4023–4042. doi: 10.1093/nar/9.16.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen H., Daniëls G. S., Hertoghs J. J., Schrier P. I., van den Elsen P. J., van der EB A. J. Identification of adenovirus-type 12 gene products involved in transformation and oncogenesis. Virology. 1982 Oct 15;122(1):15–28. doi: 10.1016/0042-6822(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Persson H., Johansson B. M., Philipson L. Control of adenovirus gene expression: cellular gene products restrict expression of adenovirus host range mutants in nonpermissive cells. J Virol. 1983 Apr;46(1):50–59. doi: 10.1128/jvi.46.1.50-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Mak S. Adenovirus type 12 DNA sequences in primary hamster tumors. J Virol. 1977 Oct;24(1):408–411. doi: 10.1128/jvi.24.1.408-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S., Mak I., Smiley J. R., Graham F. L. Tumorigenicity and viral gene expression in rat cells transformed by Ad 12 virions or by the EcoRI c fragment of Ad 12 DNA. Virology. 1979 Oct 30;98(2):456–460. doi: 10.1016/0042-6822(79)90568-3. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Hashimoto S., Wold W. S., Symington J., Rankin A., Green M. Identification of adenovirus 2 early region 4 polypeptides by in vitro translation and tryptic peptide map analysis. J Virol. 1982 Jan;41(1):334–339. doi: 10.1128/jvi.41.1.334-339.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Control of adenovirus early gene expression: accumulation of viral mRNA after infection of transformed cells. J Virol. 1981 Nov;40(2):358–366. doi: 10.1128/jvi.40.2.358-366.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Sato J., Handa H., Shiroki K., Shimojo H. Mapping of RNAs transcribed from adenovirus type 12 early and VA RNA regions. Virology. 1981 Oct 30;114(2):379–398. doi: 10.1016/0042-6822(81)90219-1. [DOI] [PubMed] [Google Scholar]
- Saito I., Shiroki K., Shimojo H. mRNA species and proteins of adenovirus type 12 transforming regions: identification of proteins translated from multiple coding stretches in 2.2 kb region 1B mRNA in vitro and in vivo. Virology. 1983 Jun;127(2):272–289. doi: 10.1016/0042-6822(83)90143-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Fujinaga K. Mapping of adenovirus 12 mRNA's transcribed from the transforming region. J Virol. 1980 Dec;36(3):639–651. doi: 10.1128/jvi.36.3.639-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Saito I., Shiroki K., Shimojo H. In vitro translation of adenovirus type 12-specific mRNA complementary to the transforming gene. Virology. 1980 Nov;107(1):61–70. doi: 10.1016/0042-6822(80)90272-x. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Shiroki K., Shimojo H., Ojima S., Fujinaga K. Transformation of a rat cell line by an adenovirus 7 DNA fragment. Virology. 1978 Jul 1;88(1):1–7. doi: 10.1016/0042-6822(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Shimojo H., Yamamoto H., Abe C. Differentiation of adenovirus 12 antigens in cultured cells with immunofluorescent analysis. Virology. 1967 Apr;31(4):748–752. doi: 10.1016/0042-6822(67)90213-9. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Saito I., Maruyama K., Fukui Y., Imatani Y., Oda K. I., Shimojo H. Expression of adenovirus type 12 early region 1 in KB cells transformed by recombinants containing the gene. J Virol. 1983 Mar;45(3):1074–1082. doi: 10.1128/jvi.45.3.1074-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Segawa K., Shimojo H. Two tumor antigens and their polypeptides in adenovirus type 12-infected and transformed cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2274–2278. doi: 10.1073/pnas.77.4.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Sawada Y., Uemizu Y., Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. doi: 10.1016/0042-6822(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Tigges M. A., Raskas H. J. Expression of adenovirus-2 early region 4: assignment of the early region 4 polypeptides to their respective mRNAs, using in vitro translation. J Virol. 1982 Dec;44(3):907–921. doi: 10.1128/jvi.44.3.907-921.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Yano S., Ojima S., Fujinaga K., Shiroki K., Shimojo H. Transformation of a rat cell line by an adenovirus type 12 DNA fragment. Virology. 1977 Oct 1;82(1):214–220. doi: 10.1016/0042-6822(77)90044-7. [DOI] [PubMed] [Google Scholar]







