Abstract
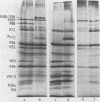
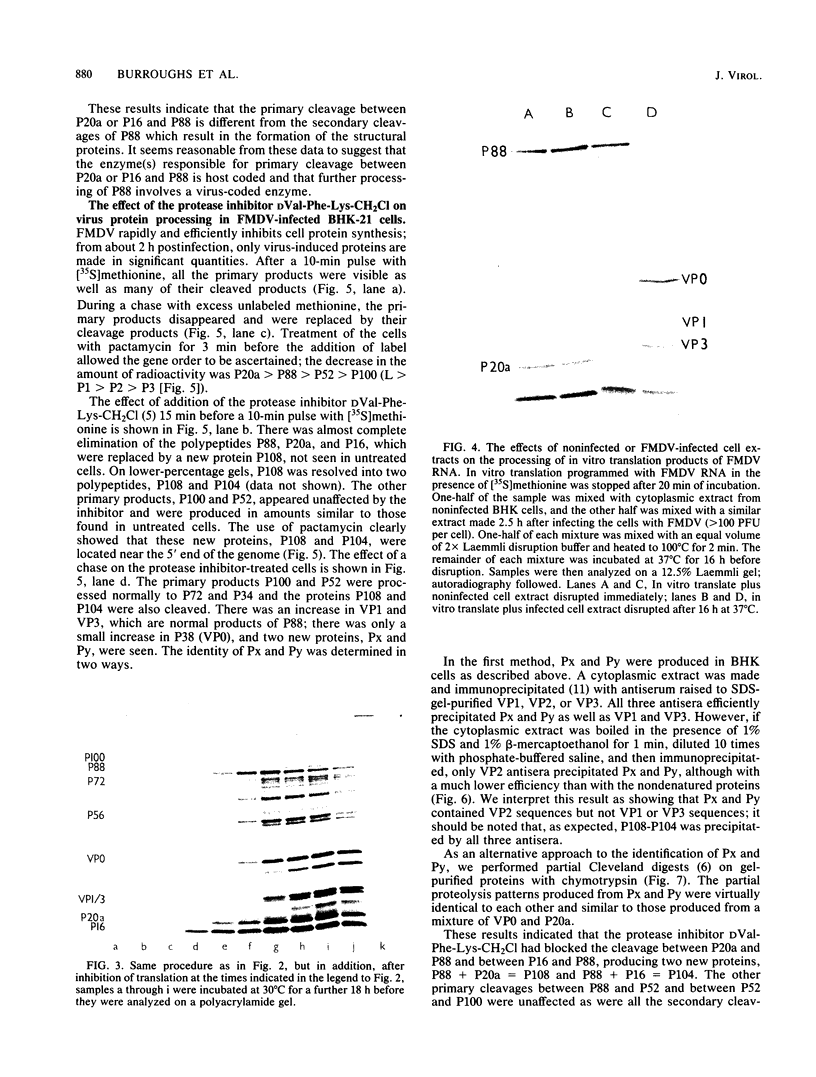
Translation of foot-and-mouth disease virus RNA in a rabbit reticulocyte lysate for short time intervals resulted in the production of the peptides P20a , P16, and P88 (Lab, Lb, and P1) (R. R. Rueckert , Recommendations of the 3rd European Study Group on Molecular Biology of Picornavirus, Urbino , Italy, 1983). If further translation was prevented, the structural protein precursor P88 was not cleaved, even after prolonged incubation. This result indicates that the mechanism of the cleavage between P20a -P16 and P88 and of that between P88 and P52 (P2) differs from the mechanism of the secondary cleavages which produce the structural proteins. Furthermore, treatment of foot-and-mouth disease virus-infected cells with the protease inhibitor D-valyl phenylalanyl lysyl chloromethyl ketone prevented the in vivo cleavage between P20a -P16 and P88 but had no effect on any of the other cleavage events. These results suggest that the cleavage of the foot-and-mouth disease virus polyprotein utilizes two different host proteases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothroyd J. C., Highfield P. E., Cross G. A., Rowlands D. J., Lowe P. A., Brown F., Harris T. J. Molecular cloning of foot and mouth disease virus genome and nucleotide sequences in the structural protein genes. Nature. 1981 Apr 30;290(5809):800–802. doi: 10.1038/290800a0. [DOI] [PubMed] [Google Scholar]
- Brown F., Newman J., Stott J., Porter A., Frisby D., Newton C., Carey N., Fellner P. Poly(C) in animal viral RNAs. Nature. 1974 Sep 27;251(5473):342–344. doi: 10.1038/251342a0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. A., Jackson R. J. Processing of the encephalomyocarditis virus capsid precursor protein studied in rabbit reticulocyte lysates incubated with N-formyl-[35S]methionine-tRNAfMet. J Virol. 1983 Jan;45(1):439–441. doi: 10.1128/jvi.45.1.439-441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collen D., Lijnen H. R., De Cock F., Durieux J. P., Loffet A. Kinetic properties of tripeptide lysyl chloromethyl ketone and lysyl p-nitroanilide derivatives towards trypsin-like serine proteinases. Biochim Biophys Acta. 1980 Sep 9;615(1):158–166. doi: 10.1016/0005-2744(80)90019-4. [DOI] [PubMed] [Google Scholar]
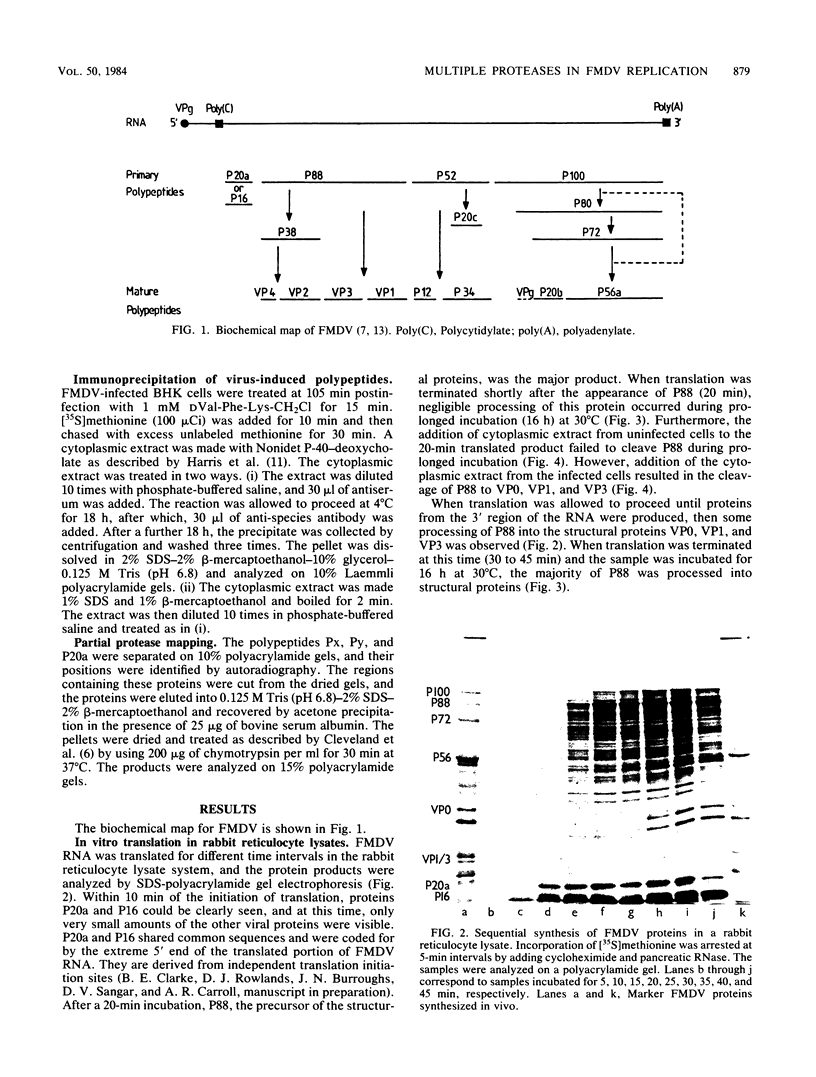
- Doel T. R., Sangar D. V., Rowlands D. J., Brown F. A re-appraisal of the biochemical map of foot-and-mouth disease virus RNA. J Gen Virol. 1978 Nov;41(2):395–404. doi: 10.1099/0022-1317-41-2-395. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin Y. V., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 is involved in the processing of the viral precursor polypeptides. FEBS Lett. 1979 Dec 1;108(1):1–5. doi: 10.1016/0014-5793(79)81164-3. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Brown F., Sangar D. V. Differential precipitation of foot and mouth disease virus proteins made in vivo and in vitro by hyperimmune and virus particle guinea pig antisera. Virology. 1981 Jul 15;112(1):91–98. doi: 10.1016/0042-6822(81)90615-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of poliovirus-specific polypeptide aggregates. J Virol. 1973 Sep;12(3):556–563. doi: 10.1128/jvi.12.3.556-563.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Brown F. Isolation of a soluble and template-dependent foot-and-mouth disease virus RNA polymerase. Virology. 1981 May;111(1):23–32. doi: 10.1016/0042-6822(81)90650-4. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Harris T. J., Brown F. Location of the initiation site for protein synthesis on foot-and-mouth disease virus RNA by in vitro translation of defined fragments of the RNA. J Virol. 1980 Jan;33(1):59–68. doi: 10.1128/jvi.33.1.59-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]