Abstract
Although silencing is a significant form of transcriptional regulation, the functional and mechanistic limits of its conservation have not yet been established. We have identified the Schizosaccharomyces pombe hst4+ gene as a member of the SIR2/HST silencing gene family that is defined in organisms ranging from bacteria to humans. hst4Δ mutants grow more slowly than wild-type cells and have abnormal morphology and fragmented DNA. Mutant strains show decreased silencing of reporter genes at both telomeres and centromeres. hst4+ appears to be important for centromere function as well because mutants have elevated chromosome-loss rates and are sensitive to a microtubule-destabilizing drug. Consistent with a role in chromatin structure, Hst4p localizes to the nucleus and appears concentrated in the nucleolus. hst4Δ mutant phenotypes, including growth and silencing phenotypes, are similar to those of the Saccharomyces cerevisiae HSTs, and at a molecular level, hst4+ is most similar to HST4. Furthermore, hst4+ is a functional homologue of S. cerevisiae HST3 and HST4 in that overexpression of hst4+ rescues the temperature-sensitivity and telomeric silencing defects of an hst3Δ hst4Δ double mutant. These results together demonstrate that a SIR-like silencing mechanism is conserved in the distantly related yeasts and is likely to be found in other organisms from prokaryotes to mammals.
INTRODUCTION
Transcriptional silencing is a general mechanism for regulating the genome that can occur through alterations of large regions of chromatin structure. This phenomenon has been studied extensively in Drosophila melanogaster, in which placement of a gene adjacent to heterochromatic regions can result in its variegated expression, and in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe (Weiler and Wakimoto, 1995). In S. cerevisiae, at least three loci are known to be silenced: the silent mating-type loci, the telomeres, and the rDNA repeats (reviewed by Loo and Rine, 1995; Sherman and Pillus, 1997; Lowell and Pillus, 1998). These silenced regions are inaccessible to DNA-modifying enzymes and have a special chromatin structure that is also inaccessible to transcriptional machinery (Nasmyth, 1982; Gottschling, 1992; Singh and Klar, 1992; Kyrion et al., 1993; Loo and Rine, 1994; Fritze et al., 1997; Smith and Boeke, 1997; Singh et al., 1998). Several cis- and trans-acting factors are required for transcriptional silencing in S. cerevisiae, including the four Silent Information Regulator (SIR) genes (Loo and Rine, 1995). SIR2 is unique among the SIR genes in that it is required for silencing at all three loci (Shore et al., 1984; Ivy et al., 1986; Aparicio et al., 1991; Bryk et al., 1997; Fritze et al., 1997; Smith and Boeke, 1997; reviewed by Sherman and Pillus, 1997). It has been proposed that SIR2 participates in regulating the level of histone deacetylation and thus the structural compactness of chromatin at these loci (Braunstein et al., 1993), although it has not been established if this regulation is direct. Indeed, histones can be modified not only through acetylation but also by means of methylation, phosphorylation, ubiquitination, and ADP ribosylation (Van Holde, 1989; Wolffe, 1992). Thus, it is possible that SIR2’s role may be catalytic or may regulate one or more of these modifications.
A family of SIR2-related HST (Homologues of SIR2) genes has been identified in organisms ranging from bacteria to humans, including four additional genes in S. cerevisiae (Chen and Clark-Walker, 1994; Brachmann et al., 1995; Derbyshire et al., 1996; Yahiaoui et al., 1996; Tsang and Escalante-Semerena, 1998; Zemzoumi et al., 1998; Frye, 1999; Perez-Martin et al., 1999). Members of this gene family share between 26 and 63% identity over the full lengths of their ORFs and contain a central core domain of ∼155 amino acids (Figure 1, bounded by arrowheads) where identity is as high as 89% (Brachmann et al., 1995). The core domain contains two conserved sequence motifs of unknown function (Figure 1, solid line) and two pairs of cysteines (Figure 1, dots) that may form a noncanonical zinc finger. The core domain and each of the conserved sequence motifs and cysteines are essential for Sir2p silencing activity (Sherman et al., 1999). Furthermore, the core domain specifies a conserved silencing function, because a chimeric protein substituting the core domain of a human homologue within Sir2p functions in transcriptional silencing in S. cerevisiae (Sherman et al., 1999).
Figure 1.
Alignment of full-length S. pombe Hst4 protein with full-length S. cerevisiae Hst3p and Hst4p. (●) Conserved cysteine residues that form a putative zinc finger; (▾) boundaries of the core domain. Solid bold lines mark conserved sequence motifs diagnostic of the SIR2 gene family: GAG(I/V)SxxxG(I/V)PDFRS and (Y/I)TQNID. Sequence motif residues in parentheses have additional variations in published sequences, and further differences are observed in incomplete database sequences. Identical and similar residues are shaded. The consensus line lists residues that are identical in at least two of the three proteins. The homology of hst4+ and HST3 extends through the C-terminal tail of HST3. The alignment was performed with the use of the PILEUP program of the Genetics Computer Group.
The HSTs are divided into three subfamilies based on the overall sequence identities of their encoded proteins (Brachmann et al., 1995). The first contains S. cerevisiae, Kluyveromyces lactis, and Candida albicans SIR2 homologues and HST1 from S. cerevisiae (Chen and Clark-Walker, 1994; Brachmann et al., 1995; Derbyshire et al., 1996; Perez-Martin et al., 1999). The second contains HST3 and HST4 of S. cerevisiae as well as hst4+, the S. pombe homologue described here. The third subfamily contains HST2 and homologues from many other organisms. Homo sapiens has at least five homologues (Brachmann et al., 1995; Frye, 1999). Members from the first and second subfamilies have demonstrated roles in transcriptional silencing. As noted above, SIR2 is required at each of the silenced loci in S. cerevisiae, and sir2Δ phenotypes can be partially rescued by overexpressed HST1 or K. lactis or C. albicans SIR2 homologues (Chen and Clark-Walker, 1994; Brachmann et al., 1995; Derbyshire et al., 1996; Perez-Martin et al., 1999). Mutants in the second subfamily also have silencing defects. Although neither the hst3Δ nor the hst4Δ single mutant has a strong phenotype individually, an hst3Δ hst4Δ double mutant has a variety of defects, including loss of telomeric silencing. The double mutant is also temperature sensitive for growth, is hypersensitive to UV radiation in combination with a rad9Δ mutant, and displays defects in chromosome segregation (Brachmann et al., 1995). Thus, in addition to their role in transcriptional silencing, HST3 and HST4 contribute to cell cycle control, DNA damage, and genomic stability.
Here we report the identification of a S. pombe SIR2 homologue, thereby extending the SIR2 family to fission yeast. In S. pombe as in S. cerevisiae, genes placed adjacent to the silent mating-type loci (Thon and Klar, 1992; Thon et al., 1994) or telomeres (Nimmo et al., 1994) are silenced. Reporter genes are also silenced when placed within the relatively complex centromeres (Allshire et al., 1994, 1995), a regulation that is not found in budding yeast. Many silencing genes have been discovered in S. pombe, predominantly through screens focused on regulation of the silent mating loci. These genes include clr1+, clr2+, clr3+, clr4+, clr6+, rik1+, and swi6+, a homologue of the D. melanogaster heterochromatin protein HP1 (Egel et al., 1989; Lorentz et al., 1992, 1994; Thon and Klar, 1992; Ekwall and Ruusala, 1994; Thon et al., 1994; Grewal et al., 1998). Swi6 protein localizes to all three silenced loci in S. pombe and is important for transcriptional silencing at all three sites (Lorentz et al., 1992, 1994; Allshire et al., 1995; Ekwall et al., 1995; Grewal and Klar, 1997). Four of the silencing genes, rik1+, swi6+, clr4+, and clr6+, are also important for chromosome segregation (Allshire et al., 1995; Ekwall et al., 1995, 1996; Grewal et al., 1998). Furthermore, all but clr6+ have been demonstrated to repress meiotic recombination at the mating loci (Egel et al., 1989; Klar and Bonaduce, 1991; Lorentz et al., 1992; Thon and Klar, 1992; Thon et al., 1994). Although extensive genetic screens have been carried out to identify additional silencing genes in fission yeast, no homologues of the SIR silencing genes have been previously identified.
This study describes the cloning and phenotypic characterization of the S. pombe hst4+ gene. S. pombe hst4Δ mutants have phenotypes pointing to defects in chromatin structure and function. hst4+ is not an essential gene, but mutants display growth and morphological defects as well as defects in transcriptional silencing, centromeric function, and genomic stability. Thus, members of the SIR2/HST subfamilies are likely to be similar in both molecular detail and organismal function.
MATERIALS AND METHODS
Yeast Strains and Culture Conditions
Genotypes of the strains used are presented in Table 1. S. pombe strains were grown in yeast extract (YE), yeast extract plus supplements (YES), or Edinburgh Minimal Medium (EMM) (Moreno et al., 1991). Malt extract plates were used for sporulation (BIO 101, Vista, CA). 5-FOA plates are EMM supplemented with 1 g/l 5-FOA (Toronto Research Chemicals, North York, Ontario, Canada). For scoring of sectored colonies, YE medium was supplemented with 12 mg/l adenine. S. pombe cells were grown at 32°C on plates and at 30°C in liquid culture unless otherwise noted. Crosses were carried out by mixing fresh cultures on malt extract plates and either selecting for diploids after 18 h or sporulating for 3 d before dissecting tetrads. Transformations were performed with the use of a lithium acetate protocol (Moreno et al., 1991), with the following modifications. A 50-ml culture was grown to an optical density (A600) of ∼0.6. The cells were resuspended in 10 ml of 0.1 M lithium acetate and incubated at 32°C for 45 min before resuspending in 0.5 ml of 0.1 M lithium acetate. After adding DNA and polyethylene glycol, the cells were incubated at 32°C for 40 min followed by 20 min at 45°C. Cells were resuspended in 100 μl of water and plated on EMM plates supplemented with appropriate amino acids.
Table 1.
Yeast strains used in this study
| Strain | ura4+ or his3+ insertion | hst locus | mat | Auxotrophic markers/Ch16a | Plasmid | Source |
|---|---|---|---|---|---|---|
| S. pombe strains | ||||||
| LPY3277 | hst4Δ∷his3+ | h− | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | This study | ||
| LPY3278 | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4-D18 | This study | ||
| LPY3279 | h− | ade6-210 arg3-D4 his3-D1 leu1-32 ura4-D18 | This study | |||
| LPY3280 | h+ | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | This study | |||
| LPY3537 | h− | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | |||
| Ch16 ade6-216 m23∷ura4∷TEL | ||||||
| LPY3551 | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | ||
| Ch16 ade6-216 m23∷ura4∷TEL | ||||||
| LPY3552 | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | |||
| Ch16 ade6-216 m23∷ura4∷TEL | ||||||
| LPY3555 | h− | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | |||
| Ch16 ade6-216 m23∷ura4∷TEL | ||||||
| LPY3556 | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | ||
| Ch16 ade6-216 m23∷ura4∷TEL | ||||||
| LPY3562 | hst4Δ∷his3+ | h− | ade6 arg3-D4 his3-D1 leu1-32 ura4 | This study | ||
| LPY3563 | h+ | ade6 arg3-D4 his3-D1 leu1-32 ura4 | This study | |||
| LPY3564 | otr1R(SphI)∷ura4+ | h+ | ade6 arg3-D4 his3-D1 leu1-32 ura4 | This study | ||
| LPY3565 | otr1R(SphI)∷ura4+ | hst4Δ∷his3+ | h− | ade6 arg3-D4 his3-D1 leu1-32 ura4 | This study | |
| LPY3566 | otr1R(SphI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | |
| LPY3570 | otr1R(SphI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4 | This study | |
| LPY3590 | his3∷TEL1L | h90 | ade6-216 his3-D1 leu1-32 ura4-D18 | This study | ||
| otr1R(SphI)∷ade6+ | ||||||
| LPY3591 | his3∷TEL1L | hst4Δ∷ura4+ | h90 | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | This study | |
| LPY3977 | cnt1/TM1(NcoI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 leu1-32 ura4 | This study | |
| LPY3978 | cnt1/TM1(NcoI)∷ura4+ | h+ | ade6-216 his3-D1 leu1-32 ura4 | This study | ||
| LPY3979 | h− | ade6-216 his3-D1 leu1-32 ura4 | This study | |||
| LPY3980 | hst4Δ∷his3+ | h− | ade6-210 arg3-D4 leu1-32 ura4 | This study | ||
| LPY3991 | imr1R(NcoI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6-210 arg3-D4 leu1-32 ura4 | This study | |
| LPY3992 | hst4Δ∷his3+ | h− | ade6-216 leu1-32 ura4 | This study | ||
| LPY3993 | h+ | ade6-216 arg3-D4 his3-D1 leu1-32 ura4 | This study | |||
| LPY3994 | imr1R(NcoI)∷ura4+ | h− | ade6-210 his3-D1 leu1-32 ura4 | This study | ||
| LPY4011 | hst4Δ∷his3+ | h− | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | pSK248 | This study | |
| LPY4012 | hst4Δ∷his3+ | h− | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | pLP1093 | This study | |
| LPY4019 | h+ | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | pSK248 | This study | ||
| LPY4020 | h+ | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | pLP1093 | This study | ||
| LPY4379 | cnt3/TM3(NcoI)∷ura4+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4-D18 | pSK248 | This study | |
| cnt1/TM1(NcoI)∷ade6+ | ||||||
| LPY4380 | cnt3/TM3(NcoI)∷ura4+ | h+ | ade6-210 arg3-D4 his3-D1 leu1-32 ura4-D18 | pLP1093 | This study | |
| cnt1/TM1(NcoI)∷ade6+ | ||||||
| LPY4381 | cnt3/TM3(NcoI)∷ura4+ | h− | ade6 arg3-D4 his3-D1 leu1-32 ura4-D18 | pSK248 | This study | |
| cnt1/TM1(NcoI)∷ade6+ | ||||||
| LPY4384 | cnt3/TM3(NcoI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6 arg3-D4 his3-D1 leu1-32 ura4-D18 | pSK248 | This study |
| cnt1/TM1(NcoI)∷ade6+ | ||||||
| LPY4385 | cnt3/TM3(NcoI)∷ura4+ | hst4Δ∷his3+ | h+ | ade6 arg3-D4 his3-D1 leu1-32 ura4-D18 | pLP1093 | This study |
| cnt1/TM1(NcoI)∷ade6+ | ||||||
| LPY4387 | cnt1/TM1(NcoI)∷ura4+ | hst4Δ∷his3+ | h− | ade6 arg3-D4 leu1-32 ura4 | This study | |
| FY336 | cnt1/TM1(NcoI)∷ura4+ | h+ | ade6-210 leu1-32 ura4-DS/E | Allshire lab | ||
| FY498 | imr1R(NcoI)∷ura4+ | h+ | ade6-210 leu1-32 ura4-DS/E | Allshire lab | ||
| FY691 | imr1R(NcoI)∷ura4+ | hA | ade6-210 leu1-32 ura4-DS/E clr2-E22 | Allshire lab | ||
| FY695 | imr1R(NcoI)∷ura4+ | hA | ade6-210 leu1-32 ura4-DS/E clr4-S5 | Allshire lab | ||
| FY973 | otr1R(SphI)∷ura4+ | h− | ade6-210 his1-102 leu1-32 ura4-DS/E | Allshire lab | ||
| FY1180 | h+ | ade6-210 leu1-32 ura4-D18 | Allshire lab | |||
| otr1R(SphI)∷ade6+ | ||||||
| FY1648 | h− | ade6-216 arg3-D4 his3-D1 leu1-32 ura4-D18 | Allshire lab | |||
| FY1863 | his3∷TEL1L | h90 | ade6-210 his3-D1 leu1-32 ura4-D18 | Allshire lab | ||
| otr1R(SphI)∷ade6+ | ||||||
| S. cerevisiae strains | ||||||
| LPY4361 | ADH4∷URA3-TEL | hst3Δ3∷TRP1 | a | his3Δ200 leu2Δ1 lys2Δ202 trp1Δ63 ura3-52 | pLP0371 | This study |
| hst4Δ1∷TRP1 | ||||||
| LPY4364 | ADH4∷URA3-TEL | hst3Δ3∷TRP1 | a | his3Δ200 leu2Δ1 lys2Δ202 trp1Δ63 ura3-52 | pLP0610 | This study |
| hst4Δ1∷TRP1 | ||||||
| LPY4366 | ADH4∷URA3-TEL | hst3Δ3∷TRP1 | a | his3Δ200 leu2Δ1 lys2Δ202 trp1Δ63 ura3-52 | pLP0748 | This study |
| hst4Δ1∷TRP1 | ||||||
| LPY4367 | ADH4∷URA3-TEL | hst3Δ3∷TRP1 | a | his3Δ200 leu2Δ1 lys2Δ202 trp1Δ63 ura3-52 | pLP0749 | This study |
| hst4Δ1∷TRP1 | ||||||
| LPY4377 | ADH4∷URA3-TEL | a | his3Δ200 leu2Δ∷TRP1 lys2Δ202 trp1Δ63 ura3-52 | pLP0548 | This study | |
| LPY4378 | ADH4∷URA3-TEL | hst3Δ3∷TRP1 | a | his3Δ200 leu2Δ1 lys2Δ202 trp1Δ63 ura3-52 | pLP0548 | This study |
| hst4Δ1∷TRP1 | ||||||
The ura4 allele, when not designated, is either DS/E or D18; the ade6 allele, when not designated, is either 210 or 216.
S. cerevisiae were grown in YPD or minimal medium lacking the appropriate nutrient for plasmid selection (Sherman, 1991). 5-FOA plates were made by adding 1 g/l 5-FOA to the supplemented minimal medium. Cells were grown at 30°C unless otherwise specified. Transformations were carried out with the use of a lithium acetate protocol (Schiestl and Gietz, 1989). The GAL10 galactose inducible promoter was induced in 2% galactose. These cultures were then plated onto rich or minimal plates with 2% galactose as the carbon source.
Oligonucleotide Primers Used in This Study
Primers (5′–3′) used in this study were as follows: JB708, RTCDATRTTYTGNGTRTA; JB710, GGNRTNCCNGAYTTYMG; 5′EagI, ATATACTGCAGATGGGCGGCCGTAAAGTGGAGGAGCACGTC; 3′XhoI, CCGTGAAGAGGAATCGTG; T7, TAATACGACTCACTATAGGG; promoter, AAACTGCAGCTACGTAAATTTGGAATTTC; 5′start, ACCGCGTACCGTCAATCAC; 5′us, GTTTACTATCCCATAACGT; his3A, GTAAGAAGAAAGCGATCGATGAG; 5′hst4-ura4, GGATTTTAATATATTTTATTAAGGCACTCAACTTTTTGGATTTAAGAAATTCCAAATTTACGTAGCTTAGTAAGCTTAGCTACAAATCCCACTG; 3′hst4-ura4, CAGGCATTATTTAAACCTCATTTGGGGTAAGAAGGATAAATTGATATTTTTAACATGGTTTATTGAAGCAAGCTTGTGATATTGACGAAAC; ko check, GCAATTAACCAACGCGAC; and ura4B, GATTGGTGTTGGAACAGA.
hst4+ Identification and Plasmid Construction
We used degenerate primers JB708 and JB710 and a touchdown molecular amplification protocol as described (Brachmann et al., 1995) to probe genomic DNA and genomic and cDNA libraries. We obtained evidence for multiple members of the HST family in S. pombe (Freeman-Cook and Pillus, unpublished results) and focused our analysis on an ∼250-base pair (bp) fragment from a S. pombe cDNA library (a generous gift of F. LaCroute, Centre de Genetique Moleculare, Gif-sur-Yvette, France). The band was purified, labeled, and used to probe a genomic library (Weaver et al., 1993). A full-length clone was isolated, and the gene was cloned from the library vector into Bluescript pKS+ (Stratagene, La Jolla, CA) and pUC19 (New England Biolabs, Beverly, MA) in two steps: a 1.7-kilobase (kb) EcoRI fragment containing the 3′ end of the gene was added to a 1.3-kb PstI/EcoRI fragment containing the promoter and the 5′ end of the gene (pLP554 in pKS+ and pLP553 in pUC19). These clones contain a 125-bp intron in the 5′ end of the gene after nucleotide 193 that was removed with the use of a molecular amplification strategy while simultaneously creating an EagI site directly downstream of the start codon to facilitate epitope tagging. Primers 5′EagI and 3′XhoI were used to amplify a spliced version of the 5′ end of the gene from a cDNA library. This 0.5-kb molecular amplification product was sequenced, digested with PstI/BglII, and cloned into pLP554 at PstI/BglII to create pLP591. This clone has the 5′ intron and the promoter deleted. A three-amino acid insertion (GGR) was created directly after the starting methionine to create the EagI restriction site. The genetic map position of hst4+ was determined by probing an ordered S. pombe cosmid filter (Hoheisel et al., 1993).
Epitope Tagging and Expression
hst4+ was epitope tagged by inserting triple HA- (influenza virus hemagglutinin, from pLP8 [Wilson et al., 1984]) or protein A (proA) (from pLP580 [Aitchison et al., 1995]) tags as NotI fragments into the EagI site of pLP591 to create pLP607 or pLP744, respectively.
Plasmids for hst4+ expression in S. cerevisiae were constructed with the use of pLP548 in which the GAL1/GAL10 divergent promoter was cloned into pRS313 (Sikorski and Hieter, 1989) as a 0.7-kb EcoRI/BamHI fragment. SmaI/EcoRV fragments from pLP607 and pLP744, containing the entire hst4+ ORF, were inserted into the EcoRV site of pLP548. The resulting plasmids have N-terminal HA- (pLP610) and proA- (pLP748 and pLP749) tagged hst4+ under the control of the GAL10 inducible promoter.
For expression studies in S. pombe with the endogenous hst4+ promoter, primers T7 and promoter were used to amplify a 1-kb fragment from pLP554, which was then cut with PstI and inserted into the PstI site of pLP744 to create pLP935 (proA). A SacI fragment from pLP935 was then cloned into the S. pombe LEU2 vector pSK248 (Stone et al., 1993) to create pLP1093 for the proA tag.
Null Mutant Construction
The hst4Δ::his3+ null mutant was created by replacing the core domain of hst4+ from amino acids 75–162 with a 1.9-kb fragment containing the his3+ gene. To use the 5′ EcoRI site of hst4+ to insert his3+, we deleted the 3′ EcoRI site of hst4+. A PstI/ClaI fragment with the ClaI site filled with VENT polymerase (New England Biolabs) prepared from pLP553 was ligated into Bluescript pKS+ at PstI/XhoI with the XhoI site filled to create pLP1077. This plasmid was digested with EcoRI/XhoI and filled in, and a 1.9-kb filled-in BglII his3+ fragment from pAF1 (Ohi et al., 1996) was inserted to create pLP862. Approximately 2 μg of a 3.3-kb BsaAI fragment from pLP862 was transformed into LPY3170. Among candidate his+ transformants, the correct disruption was verified by molecular amplification with the use of primers 5′start, 3′XhoI, 5′us, and his3A and confirmed by Southern blot analysis. The hst4Δ::ura4+ complete null mutant, replacing the entire ORF with a 1.8-kb ura4+ gene fragment, was created with the use of primers 5′hst4-ura4 and 3′hst4-ura4. Approximately 3 μg of the molecular amplification product was transformed into LPY3277, and colonies were selected that could grow on medium lacking uracil but could no longer grow on medium lacking histidine. The null mutant was confirmed by molecular amplification with the use of primers 5′us, his3A, ko check, and ura4B. The two null alleles were created with different markers to facilitate silencing assays with either a ura4+ or a his3+ reporter gene. Both alleles have the same growth and morphology phenotypes (our unpublished results), and thus both appear to be complete-loss-of-function alleles.
Sequence Analysis
Alignments were generated with the use of the PILEUP program (Wisconsin Package Version 10.0, Genetics Computer Group, Madison, WI) with homologue sequences obtained from GenBank. Pairwise comparisons were performed with the use of the BESTFIT program from the Genetics Computer Group. Default settings were used for PILEUP, BESTFIT, and BLAST analysis. Database searches were performed with the use of the National Center for Biotechnology Information BLAST network service (www.ncbi.nlm.nih.gov) and the Sanger Center S. pombe database search service (www.sanger.ac.uk). The hst4+ nucleotide sequence has been submitted to GenBank under accession number AF173939.
Cytological Techniques
S. pombe cells were prepared for DAPI staining by fixing 1 ml of a saturated culture in 1 ml of 30% methanol (MeOH)/70% acetone for at least 20 min at −20°C. Cells were rehydrated with 5-min washes in 100 μl of 75, 50, and 25% MeOH in PBS. Cells were then resuspended in 100 μl of PBS, and 6 μl of cells was mixed with 1 μl of DAPI (0.25 μg/ml; Boehringer Mannheim, Indianapolis, IN) on a slide immediately before viewing. For immunofluorescence, 30 ml of cells was grown to an optical density of 0.3–0.5, centrifuged, and resuspended in 10 ml of MeOH at −20°C for 15 min. Cells were then washed in 1 ml of 60% MeOH/40% PBS, washed in 1 ml of PBS, and resuspended in 1 ml of PBS plus 1.2 M sorbitol with 0.25 mg/ml zymolyase (70,000 U/g, ICN, Costa Mesa, CA) at 37°C for 30 min. Cells were then resuspended in 1 ml of 1% Triton X-100 in PBS for 1 min, washed three times in 1 ml of PBS, and stored in 500 μl of PBS plus 1% BSA plus 100 mM lysine-HCl (PBSBL) at 4°C overnight. Multiple-well slides for immunofluorescence were prepared by adding 10 μl of 0.1% poly-l-lysine to each well for 30 min, washing six times with 10 μl of water, and air drying. Ten microliters of cell suspension was added to each well for 30 min. After aspirating the remaining liquid, the cells were dried onto the slide. All antibodies were diluted in PBSBL. Primary antibody incubation was at room temperature for 16 h in a moist chamber (1:2000 dilution of rabbit immunoglobulin G [Sigma, St. Louis, MO] for proA or 1:100 dilution of anti-Nop1 D77 [Aris and Blobel, 1988]). Cells were then washed six times in PBSBL, followed by the addition of secondary antibody for 20 h at room temperature (1:200 fluorescein-conjugated donkey anti-rabbit antibody [Amersham, Piscataway, NJ] or 1:300 Texas Red–conjugated goat anti-mouse antibody [Jackson Immunoresearch, West Grove, PA]). Cells were then washed four times with 10 μl of PBSBL, incubated in 10 μl of 0.25 μg/ml DAPI for 5 min, and washed six times with 10 μl of PBSBL. The cells were then dried on the slide and were mounted with Citifluor (Ted Pella, Redding, CA). Staining was visualized with a Leica (Heidelberg, Germany) DMRXA microscope with a Cooke (Tonawanda, NY) Sensicam charge-coupled device camera. Images were captured and manipulated with the use of SlideBook software (Intelligent Imaging Innovations, Denver, CO).
Pedigree Analysis
Individual cells from populations grown to stationary phase in liquid YES were micromanipulated onto YES plates and grown at 32°C. Three classes of cells were identified for micromanipulation: wild-type cells, hst4Δ cells that appeared to be normal in cell size, and hst4Δ cells that were at least three times as long as wild-type cells. These cells were then monitored at intervals to determine subsequent divisions. The number of cells examined reflects the number that could be readily micromanipulated and monitored on three plates.
UV Sensitivity Assay
Saturated cultures from a series of fivefold dilutions in a 96-well plate were plated onto YES plates with the use of a pin replicator. The plates were treated with 80 J/m2 UV irradiation with the use of a Stratalinker (Stratagene) immediately after plating and again after 12 h. Control plates were left untreated. Plates were placed in a dark box to prevent photoreactivation and incubated at 30°C.
Silencing Assays
Assays were performed to evaluate the expression level of reporter genes placed at seven silenced loci: within cnt1, cnt3, otr1, and imr1, at the telomere of the linear minichromosome Ch16, at the endogenous telomere of chromosome I, and adjacent to the silent mating loci (Thon and Klar, 1992; Allshire et al., 1994, 1995; Nimmo et al., 1994, 1998). Saturated cultures from a series of fivefold dilutions were plated onto YES, supplemented EMM, and 5-FOA plates. Plates were incubated at 32°C or room temperature (∼20°C) until full growth was achieved (3–10 d). Plates were incubated for extended periods to ensure that any differences seen were not due simply to the slow growth of the hst4Δ mutant strains.
Minichromosome-Loss Assay
LPY3552 and its hst4Δ derivative, LPY3551, containing the linear minichromosome Ch16 (Nimmo et al., 1994) were used to determine chromosome stability. Ch16 carries the ade6-216 mutation that complements the ade6-210 allele present at the chromosomal locus. Loss of the minichromosome in an ade6-210 background results in red color formation on YE plates supplemented with minimal adenine (12 mg/l). The frequency of chromosome loss is calculated from the frequency of half-sectored colonies, as described by Allshire et al. (1995). Three separate isolates were examined for the wild-type strain and four isolates were examined for the hst4Δ strain, for a total of 14,529 and 18,059 colonies, respectively. The chromosome-loss experiments were all performed at 30°C, although it has been noted in other studies that chromosome loss is further elevated at decreased temperatures (Ekwall et al., 1996). The results reported here likely represent a conservative estimate of the severity of the chromosome-loss phenotype.
RESULTS
Identification of a S. pombe Homologue of SIR2
We used degenerate primers designed from two highly conserved sequence motifs (Brachmann et al., 1995) to amplify a 250-bp fragment from a S. pombe cDNA library that was then used to isolate a full-length genomic clone of the gene that we have named hst4+.
hst4+ encodes a 416-amino acid predicted ORF that maps to chromosome I between ras1+ and cdc3+ (see MATERIALS AND METHODS). hst4+ contains both sequence motifs and the two pairs of cysteines characteristic of the HSTs (Figure 1). Comparison of cDNA and genomic sequences revealed that hst4+ contains a single 125-bp intron that splits the first of the conserved sequence motifs. Table 2 shows expect (E) values from BLAST analysis for S. pombe Hst4p relative to Sir2p and each of the other S. cerevisiae homologues. The table also shows percent identity and similarity from BESTFIT analysis of full-length S. pombe Hst4p to each of the homologues as well as a comparison of the core domains. The sequence analysis reveals that hst4+ is a member of the subfamily represented by HST3 and HST4 and is most closely related to HST4. Figure 1 shows an alignment of the full-length S. pombe Hst4p with S. cerevisiae Hst3p and Hst4p, illustrating the high level of similarity present throughout the proteins.
Table 2.
S. pombe Hst4 protein is most similar to S. cerevisiae Hst4p
| S. pombe Hst4 BLAST E-value | S. pombe Hst4 BESTFIT (gaps)a | S. pombe Hst4 BESTFIT coreb | |
|---|---|---|---|
| Hst4p | 4 × 10−59 | 49 /59% 285 aa (6) | 55 /62% |
| Hst3p | 1 × 10−41 | 40 /50% 271 aa (5) | 49 /62% |
| Hst1p | 4 × 10−15 | 39 /50% 127 aa (4) | 40 /51% |
| Sir2p | 5 × 10−13 | 36 /51% 110 aa (3) | 36 /50% |
| Hst2p | 3 × 10−8 | 34 /43% 101 aa (3) | 36 /41% |
Percent identity/similarity of S. pombe Hst4 protein to the S. cerevisiae proteins over the indicated lengths; aa, amino acids.
Percent identity/similarity in the core domain from the GAGIS motif to the YTQNID motif (see Figure 1).
hst4Δ Mutants Have Multiple Growth and Morphological Defects
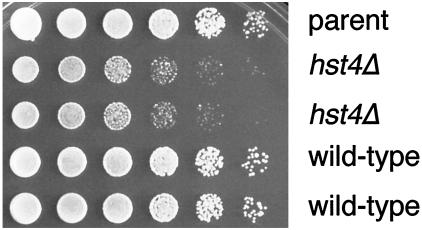
To better understand the function of hst4+, we created heterozygous null alleles in diploid cells by replacing a large portion of the core domain containing both conserved sequence motifs with the his3+ gene or by replacing the entire ORF with ura4+. The region of the core deleted in the his3+ allele is essential for SIR2 function in S. cerevisiae (Sherman et al., 1999). After sporulating the heterozygous diploids, tetrads were dissected and germinated to reveal four viable spore products. Thus, hst4+ is not an essential gene. When hst4Δ mutant and wild-type cells were compared by flow cytometry, both strains looked comparable, indicating that there were no major defects in cell cycle progression (our unpublished results). Although viable, hst4Δ haploid cells have growth defects. The two hst4Δ spores produced smaller colonies than the wild-type spores. Figure 2 shows a dilution assay in which fivefold dilutions of four spore products from the same tetrad were plated onto rich medium at 32°C. The two null strains grew to the same dilution as their wild-type sister strains, but the colony size was much smaller. This phenotype was observed at all temperatures tested (14–37°C) (Figure 2 and our unpublished results). After prolonged growth, mutant and wild-type colonies eventually reached the same size.
Figure 2.
S. pombe hst4Δ mutants exhibit growth defects. A wild-type parent (FY1648) and four sister spore products from an hst4Δ heterozygous diploid (LPY3277 and LPY3278 are the hst4Δ strains, LPY3279 and LPY3280 are the isogenic wild-type strains) were grown to saturation in rich (YES) liquid medium at 30°C. Fivefold serial dilutions were plated onto YES plates that were incubated at 14, 16, 20, 25, 30, 32, 35, and 37°C (32°C plate is shown).
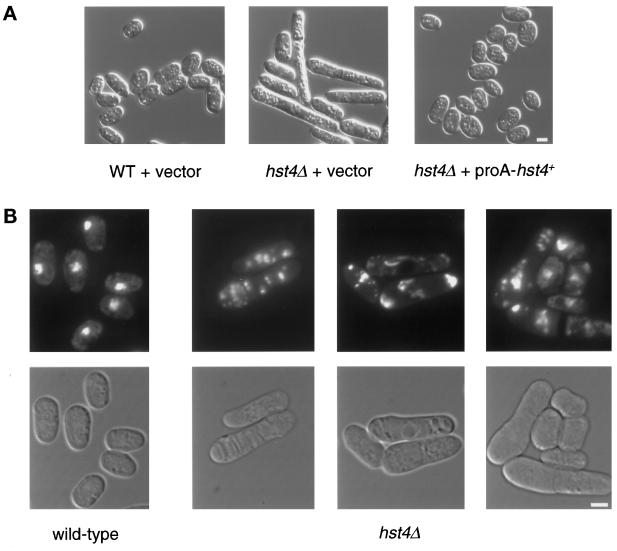
Morphological defects in hst4Δ cells were present in logarithmically growing populations. Ordinarily, S. pombe cells elongate throughout the cell cycle, doubling their size before cytokinesis. In hst4Δ mutant cultures, a subset of cells was significantly longer than normal, and this subpopulation became more prevalent as cultures reached saturation. In a saturated wild-type culture grown in rich medium, cells arrest with G2 DNA content but small cell size (Costello et al., 1986). In hst4Δ mutants, 33% (558 of 1670) of the cells were at least twice as long as saturated wild-type cells and reached lengths up to seven times that of wild-type cells. In contrast, only 5% (64 of 1412) of wild-type cells were twice as long as average wild-type length (Figure 3A). This phenotype was specifically attributable to the null allele because expression of a proA-tagged hst4+ plasmid construct restored wild-type morphology (Figure 3A).
Figure 3.
(A) S. pombe hst4Δ mutants have morphological defects. A wild-type strain containing the pSK248 vector (LPY4019) and an isogenic hst4Δ mutant strain containing either the same vector (LPY4011) or N-terminally proA-tagged hst4+ under the control of its endogenous promoter (LPY4012) were grown to saturation in liquid YES medium at 30°C. The cells were photographed with the use of Nomarski optics. Bar, 5 μm. (B) hst4Δ mutant cells have fragmented chromatin. A wild-type strain (LPY3280) and an isogenic hst4Δ strain (LPY3278) were grown to saturation at 30°C in liquid YES medium, fixed, and stained with DAPI to visualize their chromatin. The bottom panels show Nomarski images of the DAPI-stained cells seen in the top panels. Bar, 5 μm.
To determine if the long cells were viable, both wild-type and short and long cells from a saturated mutant culture were micromanipulated to follow their subsequent divisions individually. Of 78 normal wild-type cells micromanipulated, 60 (77%) grew to form colonies. The remainder failed to divide or divided at most twice after micromanipulation. In contrast, of 66 apparently normal short hst4Δ mutant cells, only 29 (44%) produced colonies. Of 66 long mutant cells, only 3 (5%) continued division to form a colony. Thus, even normal-appearing mutant cells have decreased viability, and the abnormally long cells are not viable in most instances. This decreased viability probably contributes to the modest sporulation and germination defects of hst4Δ null mutants that we have observed (our unpublished results).
Examination of chromatin in the long hst4Δ mutant cells with the use of DAPI staining revealed striking differences from wild-type cells (Figure 3B). At saturation, cells normally arrest with duplicated DNA that appears as a compact sphere in the center of the small cells (Costello et al., 1986). In contrast, the DNA of the long mutant cells appeared to be fragmented. There was often more than one focus of DAPI-stained material, and it was frequently dispersed in the cell (Figure 3B). The fragmented DNA was observed in 17% (225 of 1322) of the mutant cells. Fragmented chromatin may result from one of several situations. For example, the chromosomes could be intact in a fragmented nucleus, or the chromatin could be fragmented in an otherwise intact nucleus. To evaluate these possibilities, we followed the nuclear envelope in cells in which the nuclear pore component Cut11p was tagged with Green Fluorescent Protein (West et al., 1998). In cells in which the DNA had begun to fragment, the nuclear envelope remained intact and relatively normal. However, in some cells, the nuclear envelope became exaggerated in size as fragmentation became more severe. Thus, it is possible that changes in both nuclear and chromatin integrity contributed to the fragmentation visible in the DAPI-stained cells. The long cells also had aberrant septa, with many cells having multiple, wide, or off-center septa or deposits of septal material that did not appear to bisect the cell (our unpublished results).
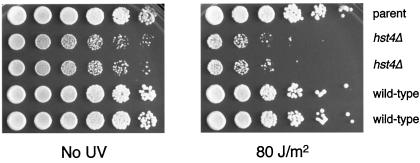
In S. cerevisiae, an hst3Δ hst4Δ rad9Δ triple mutant is hypersensitive to UV irradiation (Brachmann et al., 1995). To determine if the S. pombe mutant was UV sensitive, wild-type and mutant cells were treated with a range of UV irradiation. A series of fivefold dilutions of cells from four spore products of a parental ditype tetrad were plated on rich medium and treated with 80 J/m2 UV immediately after plating and again after 12 h (Figure 4). At this intermediate level of radiation, there is little to no sensitivity of the parent or wild-type spores compared with the untreated control. However, there was a modest but reproducible UV hypersensitivity seen in the two mutant spore products.
Figure 4.
The S. pombe hst4Δ mutant is sensitive to UV irradiation. Fivefold serial dilutions of a wild-type parent (FY1648) and four sister spore products from an hst4Δ heterozygous diploid (LPY3277 and LPY3278 are the hst4Δ strains, LPY3279 and LPY3280 are the isogenic wild-type strains) were grown to saturation in liquid YES medium at 30°C. Serial fivefold dilutions were plated onto YES plates and either left untreated or UV irradiated at 80 J/m2 with the use of a Stratalinker (Stratagene) immediately after plating and again after 12 h.
When wild-type cells are treated with UV radiation, they arrest with an elongated morphology as part of the DNA-damage checkpoint (Saka et al., 1997). We examined wild-type and mutant cells that had been treated with UV. The irradiated hst4Δ cells arrested comparably to wild-type cells. Therefore, the mutant’s UV sensitivity does not appear to be due to a defect in the checkpoint response but rather to some other mechanism.
hst4+ Is Required for Silencing at the Telomeres and Centromeres
Transcriptional silencing has been described in S. pombe at three loci: the telomeres (Nimmo et al., 1994), the silent mating loci (Thon and Klar, 1992; Thon et al., 1994), and the centromeres (Allshire et al., 1994, 1995). Genes placed within or adjacent to these loci are transcriptionally repressed. To determine whether hst4+ plays a role in transcriptional silencing, we crossed the hst4Δ mutants into strains containing reporter genes at each of the three loci.
Although a majority of known silencing genes in both S. pombe and S. cerevisiae were identified based on their phenotypes at the silent mating loci, this did not appear to be the primary target for hst4+ function. hst4Δ mutants had a very modest, if any, effect on silencing the mating loci. Furthermore, the mutant strains did not exhibit the haploid sporulation phenotype typical of other S. pombe silencing mutants (Lorentz et al., 1992; Thon and Klar, 1992; Thon et al., 1994; Grewal et al., 1998; our unpublished results).
Telomeric silencing was monitored in two ways: at a natural telomere and with the use of a minichromosome construct. In the natural context, expression of a his3+ reporter gene adjacent to the left telomere of chromosome I was evaluated. In wild-type strains, this reporter is completely silenced and cells are unable to grow on medium lacking histidine (Nimmo et al., 1998). To assess whether derepression of this reporter occurred in an hst4Δ mutant, we crossed the hst4Δ mutant into the marked strain. The null mutant was unable to restore growth on medium lacking histidine and thus did not derepress this reporter gene (Figure 5A). The second assay for telomeric silencing uses a minichromosome that is marked at the telomere with ura4+. In this construct, both the reporter gene and the telomeric context of the minichromosome are distinct from the his3+ marked natural telomere (Nimmo et al., 1994, 1998). We crossed the minichromosome into an hst4Δ strain and plated a series of fivefold dilutions onto various media: plates lacking adenine to select for the minichromosome and to control for the number of cells plated, plates lacking uracil to evaluate the low levels of ura4+ expressed from the telomeric locus, and 5-FOA plates to assess expression of the ura4+ gene from the telomere of the minichromosome (Figure 5B). Because 5-FOA is toxic to cells expressing the ura4+ gene product (Boeke et al., 1984; Allshire et al., 1994), cells expressing ura4+ are unable to grow on medium containing this compound. In a wild-type background, the ura4+ gene is epigenetically silenced. That is, the strains are able to form colonies on medium lacking uracil, but they are also able to form colonies on medium containing 5-FOA. Thus, in a wild-type population, some cells express the ura4+ reporter gene and some repress its transcription. In this assay, the hst4Δ mutant cells had a 25- to 625-fold growth defect on 5-FOA (Figure 5B), demonstrating increased expression of the normally silenced reporter gene.
Figure 5.
S. pombe hst4Δ mutants have telomeric silencing defects. (A) hst4Δ cells maintain normal silencing of an his3+ gene located at the left telomere of chromosome I. Fivefold serial dilutions of an his+ wild-type control (FY1180), the parent telomere-marked strain (FY1863), and wild-type (LPY3590) and hst4Δ (LPY3591) telomere-marked strains from the same tetrad were plated onto YES medium or EMM lacking histidine and grown at 32°C. The presence of the reporter gene was confirmed by molecular amplification (our unpublished results). (B) hst4Δ mutants derepress a ura4+ gene located at the telomere of the Ch16 minichromosome. Fivefold serial dilutions of the parent strain (LPY3537) and wild-type (LPY3555) and hst4Δ (LPY3556) strains from the same tetrad were plated onto EMM plates lacking adenine (ade−) to show the number of cells plated that contain the Ch16 minichromosome, onto EMM plates lacking uracil (ura−) to show the low levels of ura4+ expressed from the telomeric locus, and onto 5-FOA EMM plates lacking adenine (ade− FOA), which assay the expression levels of the ura4+ reporter gene. Note that all strains assayed are ura4− at the chromosomal locus (see Table 1).
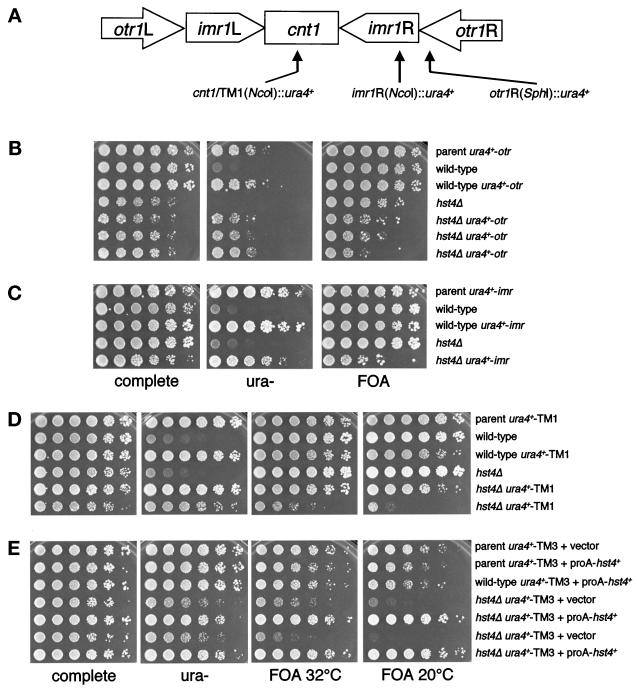
Like previously characterized S. pombe silencing mutants, an hst4Δ strain displayed centromeric silencing defects that varied depending on the location of the reporter gene within the centromeric repeats. S. pombe centromeres consist of a central domain that is flanked by large inverted repeats (Chikashige et al., 1989; Clarke and Baum, 1990; Hahnenberger et al., 1991; Murakami et al., 1991; Takahashi et al., 1992) (Figure 6A). We examined silencing of a ura4+ reporter gene placed within the central domain of centromere 1 (cen1) and centromere 3 (cen3), the imr repeat of cen1, and the otr repeat of cen1. In a wild-type background, ura4+ inserted at any of these locations is epigenetically silenced, so the strains are able to form colonies on both 5-FOA and medium lacking uracil (Allshire et al., 1994, 1995).
Figure 6.
S. pombe hst4+ is required for centromeric silencing. (A) Schematic representation of S. pombe centromere 1, which contains a central domain flanked by the imr and otr inverted repeats. (B) hst4Δ mutants derepress the otr locus. Fivefold dilutions were plated onto EMM plates as a growth control (complete), onto EMM plates lacking uracil (ura−), and onto 5-FOA plates (FOA) to assay silencing of the ura4+ reporter gene. Shown are the marked parent strain, four sister spore products plated at 32°C, and two additional isolates of marked hst4Δ strains. Strains in order of plating are: FY973, LPY3563, LPY3564, LPY3562, LPY3565, LPY3566, and LPY3570. The derepression seen was the same when plates were grown at 20°C (our unpublished results). (C) hst4+ is required for silencing within the imr repeat. As above, the parent strain, the marked and unmarked wild-type strains, and the marked and unmarked hst4Δ strains were plated onto EMM plates as a growth control (complete), onto EMM plates lacking uracil (ura−), and onto 5-FOA plates (FOA). Strains in order of plating are: FY498, LPY3993, LPY3994, LPY3992, and LPY3991. The derepression phenotype was the same when plates were grown at 20°C. (D) hst4+ is also required for silencing within the central domain of cen1 (TM1). The variable 5-FOA sensitivity seen at this locus is shown on the 5-FOA plate, where one isolate of the marked hst4Δ showed no sensitivity yet a second isolate had an ∼25-fold growth defect on 5-FOA at 32°C. When the plates were grown at 20°C rather than 32°C, the 5-FOA sensitivity increased to 625-fold in the marked hst4Δ strain. Strains in order of plating are: FY336, LPY3979, LPY3978, LPY3980, LPY3977, and LPY4387. (E) The hst4Δ mutant also shows temperature-dependent derepression within the central domain of cen3. The marked hst4Δ strain was ∼25-fold more 5-FOA sensitive than the unmarked strain at 32°C and ∼625-fold more sensitive at 20°C. This sensitivity at both temperatures was rescued by expression of N-terminally proA-tagged hst4+. Strains in order of plating are: LPY4379, LPY4380, LPY4381, LPY4384, and LPY4385. Note that, as in Figure 5B, all strains assayed in panels B–E are ura4− at the chromosomal locus (see Table 1).
An hst4Δ mutant strain exhibited variable growth defects of up to 625-fold on 5-FOA medium when a ura4+ gene was located within the otr repeat of cen1 (Figure 6B). Similar growth defects were seen when the reporter gene was located within the imr repeat or the central domain of cen1 (TM1) (Figure 6, C and D). The growth defect on 5-FOA seen at TM1 was temperature dependent; when plates were grown at 20°C, the defect increased to 625-fold (Figure 6D). However, the derepression was variable. Figure 6D shows an example of a strain that shows strong derepression and a separate isolate that has apparently wild-type levels of silencing. There was also dramatic and temperature-dependent 5-FOA sensitivity seen when the reporter was present in the central domain of cen3 (TM3). At 32°C, there was variable 5-FOA sensitivity similar to that seen at the other loci. At room temperature, however, the 5-FOA sensitivity was almost complete (Figure 6E). Wild-type cells grew well on 5-FOA at room temperature, but the mutant strains showed very little growth. This silencing defect was rescued by expression of the proA-tagged hst4+ plasmid construct (Figure 6E). Thus, the hst4Δ mutant exhibits silencing defects at telomeres and centromeres.
Increased Chromosome Loss in hst4Δ Mutants
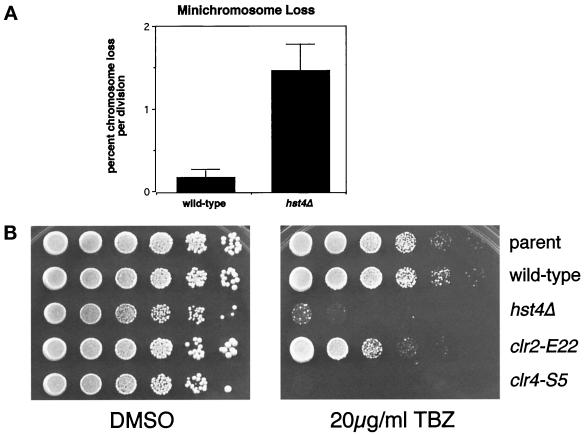
Because the hst4Δ mutants had decreased viability associated with fragmented DNA and centromeric silencing defects, it seemed possible that the hst4Δ phenotypes might result from aberrant chromatin structure, particularly at the centromeres. At least four previously characterized genes known to be important for centromeric silencing are also important for chromosome maintenance and thus centromeric function. clr4, rik1, swi6, and clr6 mutants are reported to have chromosome-loss rates from 9- to >100-fold greater than wild-type (Allshire et al., 1995; Ekwall et al., 1995, 1996; Grewal et al., 1998). To determine if the centromeric silencing defect was associated with a defect in centromeric function in the hst4Δ mutant, we assayed maintenance of the Ch16 minichromosome using colony color. This minichromosome construct contains the ade6-216 allele that complements the chromosomal ade6-210 allele present in the mutant and wild-type strains. Cells that maintain the minichromosome are ade+ and give rise to white colonies. Loss of the minichromosome results in red colony color. When the chromosome-loss event occurs in the first cell division, colonies arise that are half white and half red. To examine chromosome maintenance in the hst4Δ strain, the hst4Δ mutation was crossed into a strain containing the Ch16 minichromosome. The hst4Δ mutant lost the minichromosome in 1.5% of cell divisions, a rate that was eightfold higher than in the wild-type strain (Figure 7A). Thus, hst4Δ cells are defective in chromosome maintenance as well as silencing.
Figure 7.
(A) hst4Δ mutants have increased chromosome loss. Wild-type (LPY3552) and hst4Δ (LPY3551) strains containing the Ch16 minichromosome were grown to saturation in liquid YES at 30°C and plated onto YE plates supplemented with 12 mg/l adenine. The percent chromosome loss per division was calculated from the number of half-red colonies divided by the number of white colonies plus half-red colonies. For the hst4Δ strain, a total of 18,059 colonies were scored in four separate experiments. For the wild-type strain, a total of 14,529 colonies were scored in three experiments. (B) hst4Δ mutants are TBZ sensitive. Fivefold dilutions of a parent strain (LPY3102), a wild-type strain (LPY3279), and an isogenic hst4Δ mutant strain (LPY3277), as well as a TBZ-insensitive clr2 mutant (FY691) and a TBZ-sensitive clr4 mutant (FY695), were plated onto YES plates with DMSO as a growth control and YES plates with 20 μg/ml TBZ.
Genes that function in regulating centromeric chromatin structure might be predicted to affect the interaction of microtubules with the kinetochore during mitosis. In fact, at least three of the four previously identified S. pombe genes that are required for chromosome maintenance are sensitive to the microtubule-destabilizing drug thiabendazole (TBZ) and have genetic interactions with the gene encoding β-tubulin, nda3+ (Ekwall et al., 1996). To determine if hst4+ may function in a similar way, we assayed TBZ sensitivity in the hst4Δ mutant and compared it with the previously established sensitivity of a clr4 mutant and insensitivity of clr2 mutant and wild-type strains. The parent and wild-type strains and the clr2 mutant strain grew well, as expected. However, the hst4Δ mutant was sensitive to TBZ, similar to the clr4 mutant control strain (Figure 7B). When TBZ-treated cells were examined microscopically, the arrested hst4Δ and wild-type cells had the same morphology. Thus, TBZ sensitivity of the hst4Δ mutant may reflect either a potential role in mitotic spindle-chromosome interactions or a more general structural role in chromatin.
S. pombe Hst4 Is a Nuclear Protein Enriched in the Nucleolus
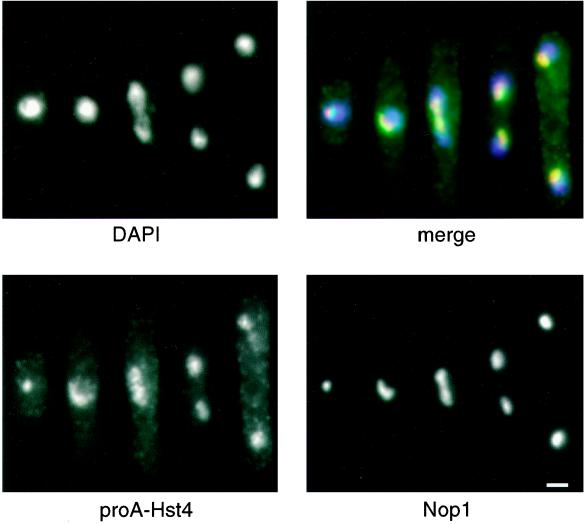
To determine its subcellular localization, we generated epitope-tagged constructs of Hst4p. A proA tag was inserted into the N terminus of Hst4, directly after the initiating methionine, and the proA-Hst4 protein was expressed from a plasmid under the control of the endogenous hst4+ promoter. This tagged construct appeared fully functional in that it was able to rescue the long-cell phenotype and the TM3 centromeric derepression of the hst4Δ mutant (Figures 3A and 6E). Indirect immunofluorescence of cells expressing this proA-Hst4 construct demonstrated that Hst4p was nuclear and was concentrated in a condensed spot within the nucleus. Colocalization of proA-Hst4 and Nop1, a nucleolar antigen (Aris and Blobel, 1988), identified this subnuclear staining as nucleolar (Figure 8). The nucleolar concentration was observed in cells from all stages of the cell cycle, and clear colocalization was seen in 90% (159 of 177) of cells examined.
Figure 8.
S. pombe Hst4p localizes to the nucleolus throughout the cell cycle. A wild-type strain containing N-terminally tagged proA-hst4+ under the control of its endogenous promoter (LPY4020) was fixed and stained with DAPI to visualize DNA (top left), with rabbit immunoglobulin G to visualize proA-hst4+ (bottom left), and with anti-Nop1p antibodies to stain the nucleolus (bottom right). A merged image of the five cells in different stages of the cell cycle is shown at top right. The DAPI staining is in blue, the proA-Hst4 staining is in green, and the Nop1p staining is in red. In the merged image, the overlap between the proA-Hst4p and Nop1p signals is yellow. The speckled cytoplasmic staining present in the proA-hst4+ panel is nonspecific in that it was also seen with secondary antibody alone in wild-type cells not expressing the proA-hst4+ construct (our unpublished results). Bar, 2 μm.
S. pombe hst4+ Is a Functional Homologue of S. cerevisiae HST3 and HST4
Because members of the SIR2 gene family share a high degree of sequence similarity, it was of interest to determine the extent of their functional conservation. As detailed above, hst4+ shares molecular and phenotypic similarities with HST3 and HST4 from S. cerevisiae. Therefore, we asked if hst4+ could rescue the phenotypes of a S. cerevisiae sir2Δ mutant or an hst3Δ hst4Δ double mutant. Overexpression of hst4+ was unable to rescue the silencing phenotypes of a S. cerevisiae sir2Δ mutant. This result was not necessarily surprising because a positive result demands both cross-species and cross-subfamily complementation, and it was previously shown that overexpressed HST3 cannot rescue sir2Δ mutant phenotypes (Brachmann et al., 1995). We next asked whether hst4+ could complement hst3Δ hst4Δ double mutant phenotypes, because these three genes are most closely related. Although hst3Δ and hst4Δ mutants do not independently have strong defects, the double mutant displays pleiotropic phenotypes, including temperature sensitivity for growth at 37°C, decreased chromosome stability, and hypersensitivity to UV irradiation in combination with a rad9Δ mutant (Brachmann et al., 1995). Furthermore, HST3 and HST4 are required for telomeric silencing, because reporter genes placed at the telomere are derepressed in a double mutant (Brachmann et al., 1995). To determine if hst4+ could complement these phenotypes, we transformed an hst3Δ hst4Δ double mutant with N-terminally epitope-tagged HA-hst4+ or proA-hst4+ overexpressed under the control of a galactose-inducible promoter.
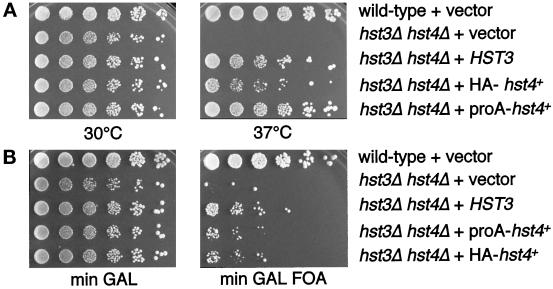
hst4+ complemented the temperature-sensitive growth phenotype of the hst3Δ hst4Δ double mutant. Figure 9A shows a wild-type strain and an hst3Δ hst4Δ double mutant transformed with vector, HST3, HA-hst4+, or proA-hst4+. The wild-type strain grew equally well at 30 and 37°C. The double mutant containing vector alone grew at 30°C but was unable to grow at 37°C. The HST3 control transformant complemented this temperature sensitivity, as expected. Both tagged versions of hst4+ were able to rescue the hst3Δ hst4Δ temperature-sensitive phenotype, demonstrating functional conservation between S. pombe and S. cerevisiae HST genes.
Figure 9.
S. pombe hst4+ is a functional homologue of S. cerevisiae HST3 and HST4. (A) S. pombe hst4+ complements the temperature-sensitive phenotype of an hst3Δ hst4Δ double mutant. Shown are serial fivefold dilutions plated at 30 or 37°C onto rich galactose plates to induce expression of the HA- or proA-tagged hst4+, which are under the control of the GAL10 promoter. Strains in order of plating are: LPY4377, LPY4378, LPY4361, LPY4364, and LPY4366. (B) S. pombe hst4+ is also able to partially rescue the telomeric silencing defect of the hst3Δ hst4Δ double mutant. Shown are fivefold dilutions plated onto minimal GAL plates and minimal GAL 5-FOA plates. Strains in order of plating are: LPY4377, LPY4378, LPY4361, LPY4367, and LPY4364.
hst4+ was also able to relieve the telomeric silencing defect of the double mutant (Figure 9B). A wild-type strain containing a telomeric URA3 reporter gene grew on medium containing 5-FOA, indicative of the transcriptional silencing of the URA3 gene. An hst3Δ hst4Δ double mutant with the same reporter was unable to grow on 5-FOA, and this derepression of URA3 was complemented by HST3. The telomeric derepression was also partially rescued by expression of hst4+ (Figure 9B). hst4+ could not, however, complement the UV hypersensitivity of an hst3Δ hst4Δ rad9Δ triple mutant (our unpublished results). Thus, because hst4+ rescued the temperature-sensitivity and telomeric silencing defects of the hst3Δ hst4Δ mutant, partial function of these genes has been conserved in the two yeasts.
DISCUSSION
The fission yeast S. pombe and the budding yeast S. cerevisiae are only distantly related, yet much has been learned through analysis of both their comparable and contrasting ways of accomplishing such essential processes as DNA replication and cell cycle control (for review, see Forsburg, 1999). Likewise, many parallels can be drawn between silencing in the two yeasts. In both, the silent mating-type loci and the telomeres are transcriptionally silenced (Gottschling et al., 1990; Aparicio et al., 1991; Thon and Klar, 1992; Nimmo et al., 1994; Thon et al., 1994). In contrast, S. pombe’s larger and more complex centromeres are also silenced (Chikashige et al., 1989; Clarke and Baum, 1990; Hahnenberger et al., 1991; Murakami et al., 1991; Takahashi et al., 1992; Steiner et al., 1993; Allshire et al., 1994, 1995). Even though the two organisms share at least two silenced loci, to date no homologues of the S. cerevisiae SIR silencing proteins have been reported in S. pombe.
Here we have described the identification of a S. pombe silencing gene homologue that is a member of the SIR2 silencing gene family. S. pombe hst4+ is most closely related to the S. cerevisiae genes HST3 and HST4. Like these homologues, it plays a role in chromatin structure. Indeed, hst4+ is required for transcriptional silencing, chromatin integrity, and genomic stability. However, it is distinguished from SIR2 and other HSTs by its apparent role in centromeric function. These studies thus functionally extend the SIR2 gene family to S. pombe and demonstrate that similar silencing mechanisms may exist in the two distantly related yeasts.
hst4+ Is Closely Related to the SIR2 Gene Family Both Molecularly and Functionally
S. pombe hst4Δ mutants are phenotypically similar to S. cerevisiae hst3Δ hst4Δ double mutants. S. pombe hst4Δ mutants accumulate large cells that are inviable. The abnormally long hst4Δ mutant cells are reminiscent of those seen in several cell-division-cycle (cdc) mutants in which cell growth continues after nuclear division arrests (see Nurse et al., 1976; Kelly et al., 1993; Maiorano et al., 1996; Gould et al., 1998). If the mutant cells experience irreparable alteration of their chromatin that triggers arrest of the nuclear division cycle without halting cell growth, long cells would arise, as observed in many cdc mutants. HST3 and HST4 in S. cerevisiae are redundant genes that are together required for cell cycle progression, telomeric silencing, and chromosome maintenance (Brachmann et al., 1995). Parallel to the S. pombe hst4Δ mutant cell cycle defect, S. cerevisiae hst3Δ hst4Δ mutants accumulate as large-budded cells that are larger than wild-type cells and have reduced viability (Brachmann et al., 1995). The growth and morphology phenotypes in both yeasts may be related to a role for these HST genes in the maintenance of chromatin structure and integrity.
Additional phenotypes of these family members in both yeasts support the idea that the genes are important in the maintenance of chromatin structure. The S. pombe hst4Δ mutant (Figure 4) and the S. cerevisiae hst3Δ hst4Δ double mutant (in combination with a rad9Δ mutation) (Brachmann et al., 1995) are sensitive to UV irradiation. In both yeasts, UV sensitivity has been correlated with mutations in genes thought to play a role in chromatin structure (Kaufman et al., 1997; Grewal et al., 1998). Alteration of chromatin structure in the hst mutants could make the DNA more accessible to UV radiation–induced damage. Additionally, the SIR2 family members in both yeasts, including hst4+, are required for transcriptional silencing. S. pombe hst4Δ mutants (Figures 5 and 6) and S. cerevisiae hst3Δ hst4Δ mutants (Brachmann et al., 1995) exhibit decreased silencing, indicating that these proteins may be involved either directly or indirectly in chromatin structure.
Direct evidence that these homologues are important for chromatin structure and integrity comes from chromosome-maintenance assays. hst4+ is important for chromosome maintenance, as demonstrated by the fact that a S. pombe hst4Δ strain is at least eight times more likely to missegregate the Ch16 minichromosome per cell division than a wild-type strain. This quantitative effect is almost certainly an underestimate of the chromosome-maintenance defect of the null mutant because, in S. pombe, each of the three chromosomes is essential for viability. Thus, for the assay to detect a chromosome loss, an event must occur that results in loss of the minichromosome and that leaves the remaining chromatin intact, allowing the cell to proceed through several rounds of cell division to form a colony. Because at least 30% of a population of hst4Δ cells is aberrantly long and not capable of giving rise to a colony, these clearly defective cells cannot be scored and are thus unrepresented in the chromosome-loss quantitation. Based on the dispersed DNA phenotype seen in many long cells (Figure 3B), it is likely that chromosome-loss events contribute to the loss of viability in the mutant cells. If it were possible to take into account the rate of chromosome loss in these cells, the total loss rate would be expected to be much higher.
We found that overexpression of hst4+ in S. cerevisiae rescues a subset of the hst3Δ hst4Δ double mutant phenotypes. hst4+ is able to rescue the temperature sensitivity for growth and to partially rescue the telomeric silencing defect of the S. cerevisiae mutants. However, hst4+ does not rescue the UV-hypersensitivity phenotype seen in the S. cerevisiae mutant. Thus, partial function has been conserved in these two yeasts. The S. cerevisiae genome duplicated ∼108 years ago, long after the S. cerevisiae and S. pombe lineages split (Wolfe and Shields, 1997). This duplication of the genome may have allowed similar genes to evolve different functions. Although HST3 and HST4 lie outside a genomic block that was duplicated between chromosome XV and chromosome IV (Wolfe and Shields, 1997), given their redundant functions and proximity to duplicated blocks it seems possible that they were part of the duplication event and that intermediate genes have undergone subsequent rearrangements. Knowledge of the functions of this subfamily is limited, in part, by the redundancy of the S. cerevisiae homologues. In S. pombe, hst4+ may fulfill the role of both S. cerevisiae genes, thus providing a unique opportunity to learn more about the subfamily.
hst4+ Is Similar to but Distinct from Other S. pombe Silencing Genes That Have a Role in Chromatin Structure
Several genes are already known to be important for transcriptional silencing in S. pombe. clr1+, clr2+, clr3+, clr4+, clr6+, rik1+, and swi6+, like hst4+, are required for wild-type levels of silencing (Egel et al., 1989; Lorentz et al., 1992, 1994; Thon and Klar, 1992; Ekwall and Ruusala, 1994; Thon et al., 1994; Allshire et al., 1995; Grewal and Klar, 1997; Grewal et al., 1998). These silencing genes fall into two classes based on additional phenotypes. Mutations in the first class include clr1, clr2, and clr3, which disrupt silencing but have no additional phenotypes. Mutations in the second class (clr4, clr6, rik1, and swi6) result in increased chromosome loss, linking their centromeric silencing defects with defects in centromeric function (Allshire et al., 1995; Ekwall et al., 1995, 1996; Grewal et al., 1998). It has been proposed that Clr4p, Rik1p, and Swi6p interact directly or indirectly with microtubules at the kinetochore (Ekwall et al., 1996). Mutants in all three genes are sensitive to the microtubule-destabilizing drug TBZ and show genetic interactions with the β-tubulin gene nda3 (Ekwall et al., 1996). Phenotypic analysis places hst4+ in the second class of silencing genes because it is important for chromosome maintenance and is also sensitive to TBZ. If these genes are required to maintain proper centromeric chromatin structure, minor alterations in chromatin context could result in silencing defects. More severe alterations could result in kinetochore defects and ultimately chromosome loss and reduced viability.
Several other phenotypes link hst4+ with the second class of silencing genes. Both clr6 and hst4Δ mutants are UV sensitive, a phenotype common in genes controlling the assembly of chromatin (Kaufman et al., 1997; Grewal et al., 1998). Furthermore, Hst4 protein localizes to the nucleolus, like overexpressed Clr4p (Sawin and Nurse, 1996; Ivanova et al., 1998) and S. cerevisiae Sir2p (Gotta et al., 1997). Because Hst4p also has diffuse nuclear localization outside the nucleolus, it is possible that it localizes to the silenced centromeres and telomeres as well. In S. cerevisiae, reporter genes placed within the rDNA repeats are silenced, and SIR2 is required for this transcriptional silencing (Bryk et al., 1997; Fritze et al., 1997; Smith and Boeke, 1997). Reporter genes have not been integrated into S. pombe rDNA. However, by analogy to S. cerevisiae, it seems likely that this region may be silenced. hst4+ and clr4+ could play a role in silencing the rDNA locus in S. pombe.
Although hst4+ shares many phenotypes with the second class of silencing genes in S. pombe, it also has distinct phenotypes. In particular, hst4Δ mutants have growth and morphology defects not seen in other silencing gene mutants. Furthermore, whereas clr4, rik1, and swi6 mutants have chromosomes that lag on the mitotic spindle, severely fragmented DNA like that seen in hst4Δ mutants is not seen in clr4, clr6, rik1, or swi6 mutants (Ekwall et al., 1995, 1996; Grewal et al., 1998). There are several possibilities to explain these similar but distinct phenotypes. Double mutants between hst4Δ and clr1, clr2, clr3, clr4, rik1, and swi6 do not have dramatically enhanced silencing defects (our unpublished results), supporting the hypothesis that the genes act in the same pathway. However, the different phenotypes seen in each individual mutant suggest that hst4+ function is not fully overlapping with that of the other genes but may converge on some of the same functions. This apparent contradiction may be explained if under certain circumstances or at some loci hst4+ works together with the other silencing genes to maintain proper chromatin structure, whereas in other cases it works in a different pathway or in a different complex. For example, Sir2p is a shared member of multiple complexes that function at distinct loci in S. cerevisiae (Gotta et al., 1997; Moazed et al., 1997; San-Segundo and Roeder, 1999; Shou et al., 1999; Straight et al., 1999).
Conserved HST Function from Bacteria to Humans
The SIR2 gene family is broadly conserved. Here we provide direct evidence that this gene family is functionally conserved as well, supporting the idea that SIR-like silencing mechanisms may be universally conserved. Our study further establishes a distinct role for members of this gene family in centromeric function hinted at by earlier chromosome-loss phenotypes in S. cerevisiae mutants (Brachmann et al., 1995). Silencing of centromeric or centromere-like loci occurs in S. pombe, D. melanogaster, and bacteria, and a link between centromeric silencing and centromeric function also exists (Allshire et al., 1994; Weiler and Wakimoto, 1995; Rodionov et al., 1999). In S. pombe, mutations in clr4, clr6, rik1, swi6, and hst4 disrupt centromeric silencing and result in elevated chromosome-loss rates, indicating that centromeric function is perturbed (Allshire et al., 1995; Ekwall et al., 1995, 1996; Grewal et al., 1998). In D. melanogaster, certain Su-var3(6) alleles that suppress position-effect variegation seen within centromeric heterochromatin also result in abnormal chromosome segregation (Baksa et al., 1993). Indeed, alleles of Su-var205, the gene that encodes the heterochromatin-associated protein HP1, suppress repression of markers within centromeric heterochromatin and are also defective in chromosome segregation (Kellum and Alberts, 1995). Furthermore, the mitotic transmission of an unstable marker chromosome is modified by genes known to affect position-effect-variegation (Wines and Henikoff, 1992). Finally, even bacteria show this link between transcriptional silencing and chromosomal segregation functions. Partition modules on bacterial plasmids have a centromere-like role. They are required for proper segregation of the plasmid into daughter cells after replication. Genes placed within the partitioning module are silenced, and in mutants with decreased silencing, plasmid partitioning is defective (Rodionov et al., 1999). Thus, the link between centromeric silencing and centromeric structure is preserved from prokaryotes to multicellular eukaryotes.
Our study links a member of the SIR2 gene family with centromeric silencing and centromeric function. hst4Δ mutants have growth and morphology defects in addition to phenotypes that suggest a role for the gene in chromatin structure. The mutants have fragmented DNA, silencing defects, and chromosome-maintenance defects. Like other recently identified S. pombe silencing genes, hst4+ appears to have a specialized function rather than a generic role at all silenced loci (see Nimmo et al., 1998). It is possible that other S. pombe SIR2 homologues will be found to function at other loci. Indeed, additional S. pombe homologues exist that may fulfill these roles (Freeman-Cook and Pillus, unpublished results; Derbyshire and Strathern, personal communication). The phenotypes of S. pombe hst4Δ mutants and the cognate S. cerevisiae mutants suggest a conserved chromosomal function for specialized SIR2 homologues in other organisms, including humans.
The extent of divergent functions of the other conserved SIR2/HST genes will be important to define. For example, it has been suggested that a Salmonella typhimurium homologue may have phosphoribosyltransferase activity (Tsang and Escalante-Semerena, 1998). Indeed, in recent studies, recombinant S. typhimurium and H. sapiens proteins have been shown to have weak ADP ribosyltransferase activity in vitro (Frye, 1999). In Leishmania major, a homologue is reported to encode a cytoplasmic and secreted protein that may contribute to antigenicity and ultimately influence the host immune response to this parasite (Zemzoumi et al., 1998). In C. albicans, a SIR2 homologue appears to influence switching between filamentous and colonial growth, an epigenetic program that is correlated with pathogenicity (Perez-Martin et al., 1999). Finally, a new role for SIR2 itself has been established with the observation that sir2 mutants are defective in the pachytene checkpoint of meiosis (San-Segundo and Roeder, 1999). Although upon initial consideration these observations may seem perplexingly distinct, we consider it likely that an underlying mechanistic similarity exists for SIR2/HST gene function. Determining what that function may be, whether it is an enzymatic activity, a structural role, or a more direct effect on transcriptional regulation, will be critical for ultimately understanding conserved mechanisms of silencing.
ACKNOWLEDGMENTS
We thank our colleagues M. Rose, F. LaCroute, J. Aris, R. West, H. Browning, C. Troxell, and M. Winey for providing reagents and advice and Y. Han for assistance with sequencing. Mapping of hst4+ was performed with the use of a S. pombe cosmid filter kindly provided by Dr. E. Maier (Hoheisel et al., 1993) and with the help of the Reference Library Database (Max Planck Institute, Berlin-Dahlem, Germany). We thank M. Derbyshire and J. Strathern for sharing unpublished results and A. Clarke, E. Stone, S. Garcia, R. West, and H. Browning for critical reading of the manuscript. This work was supported by a Howard Hughes Medical Institute predoctoral fellowship to L.L.F.-C., an American Cancer Society postdoctoral fellowship to J.M.S., and funding from the National Science Foundation and National Institutes of Health to L.P. Deconvolution microscopy in the Department of Molecular, Cellular, and Developmental Biology was made possible, in part, by a gift from Virginia and Mel Clark.
REFERENCES
- Aitchison JD, Blobel G, Rout MP. Nup120p: a yeast nucleoporin required for NPC distribution and mRNA transport. J Cell Biol. 1995;131:1659–1675. doi: 10.1083/jcb.131.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire RC, Javerzat JP, Redhead NJ, Cranston G. Position effect variegation at fission yeast centromeres. Cell. 1994;76:157–169. doi: 10.1016/0092-8674(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- Aris JP, Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J Cell Biol. 1988;107:17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksa K, et al. Mutations in the protein phosphatase 1 gene at 87B can differentially affect suppression of position-effect variegation and mitosis in Drosophila melanogaster. Genetics. 1993;135:117–125. doi: 10.1093/genetics/135.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Clark-Walker GD. sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol Cell Biol. 1994;14:4501–4508. doi: 10.1128/mcb.14.7.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, Niwa O, Yanagida M. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell. 1989;57:739–751. doi: 10.1016/0092-8674(89)90789-7. [DOI] [PubMed] [Google Scholar]
- Clarke L, Baum MP. Functional analysis of a centromere from fission yeast: a role for centromere-specific repeated DNA sequences. Mol Cell Biol. 1990;10:1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello G, Rodgers L, Beach D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- Derbyshire MK, Weinstock KG, Strathern JN. HST1, a new member of the SIR2 family of genes. Yeast. 1996;12:631–640. doi: 10.1002/(SICI)1097-0061(19960615)12:7%3C631::AID-YEA960%3E3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Egel R, Willer M, Nielsen O. Unblocking of meiotic crossing-over between the silent mating-type cassettes of fission yeast, conditioned by the recessive, pleiotropic mutant rik1. Curr Genet. 1989;15:407–410. [Google Scholar]
- Ekwall K, Javerzat JP, Lorentz A, Schmidt H, Cranston G, Allshire R. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science. 1995;269:1429–1431. doi: 10.1126/science.7660126. [DOI] [PubMed] [Google Scholar]
- Ekwall K, Nimmo ER, Javerzat JP, Borgstrom B, Egel R, Cranston G, Allshire R. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localization of the chromo domain protein Swi6p and impair centromere function. J Cell Sci. 1996;109:2637–2648. doi: 10.1242/jcs.109.11.2637. [DOI] [PubMed] [Google Scholar]
- Ekwall K, Ruusala T. Mutations in rik1, clr2, clr3 and clr4 genes asymmetrically derepress the silent mating-type loci in fission yeast. Genetics. 1994;136:53–64. doi: 10.1093/genetics/136.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. The best yeast? Trends Genet. 1999;15:340–344. doi: 10.1016/s0168-9525(99)01798-9. [DOI] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (Sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy BK, Grunstein M, Gasser SM. Localization of Sir2p: the nucleolus as a compartment for silent information regulators. EMBO J. 1997;16:3243–3255. doi: 10.1093/emboj/16.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE. Telomere-proximal DNA in Saccharomyces cerevisiae is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci USA. 1992;89:4062–4065. doi: 10.1073/pnas.89.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Gould K, Burns CG, Feoktistova A, Hu C, Pasion S, Forsburg SL. Fission yeast cdc24+ encodes a novel replication factor required for chromosomal integrity. Genetics. 1998;149:1221–1233. doi: 10.1093/genetics/149.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Bonaduce MJ, Klar AJ. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics. 1998;150:563–576. doi: 10.1093/genetics/150.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Klar AJ. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics. 1997;146:1221–1238. doi: 10.1093/genetics/146.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnenberger KM, Carbon J, Clarke L. Identification of DNA regions required for mitotic and meiotic functions within the centromere of Schizosaccharomyces pombe chromosome I. Mol Cell Biol. 1991;11:2206–2215. doi: 10.1128/mcb.11.4.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel JD, Maier E, Mott R, McCarthy L, Grigoriev AV, Schalkwyk LC, Nizetic D, Francis F, Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993;73:109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Ivanova AV, Bonaduce MJ, Ivanov SV, Klar AJ. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat Genet. 1998;19:192–195. doi: 10.1038/566. [DOI] [PubMed] [Google Scholar]
- Ivy JM, Klar AJ, Hicks JB. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:688–702. doi: 10.1128/mcb.6.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Kobayashi R, Stillman B. UV radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- Kellum R, Alberts BM. Heterochromatin protein 1 is required for correct chromosome segregation in Drosophila embryos. J Cell Sci. 1995;108:1419–1431. doi: 10.1242/jcs.108.4.1419. [DOI] [PubMed] [Google Scholar]
- Kelly TJ, Martin GS, Forsburg SL, Stephen RJ, Russo A, Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Bonaduce MJ. swi6, a gene required for mating-type switching, prohibits meiotic recombination in the mat2-mat3 “cold spot” of fission yeast. Genetics. 1991;129:1033–1042. doi: 10.1093/genetics/129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G, Liu K, Liu C, Lustig AJ. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- Loo S, Rine J. Silencers and domains of generalized repression. Science. 1994;264:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- Loo S, Rine J. Silencing and heritable domains of gene expression. Annu Rev Cell Dev Biol. 1995;11:519–548. doi: 10.1146/annurev.cb.11.110195.002511. [DOI] [PubMed] [Google Scholar]
- Lorentz A, Heim L, Schmidt H. The switching gene swi6 affects recombination and gene expression in the mating-type region of Schizosaccharomyces pombe. Mol Gen Genet. 1992;233:436–442. doi: 10.1007/BF00265441. [DOI] [PubMed] [Google Scholar]
- Lorentz A, Ostermann K, Fleck O, Schmidt H. Switching gene swi6, involved in repression of silent mating-type loci in fission yeast, encodes a homologue of chromatin-associated proteins from Drosophila and mammals. Gene. 1994;143:139–143. doi: 10.1016/0378-1119(94)90619-x. [DOI] [PubMed] [Google Scholar]
- Lowell JE, Pillus L. Telomere tales: chromatin, telomerase and telomere function in Saccharomyces cerevisiae. Cell Mol Life Sci. 1998;54:32–49. doi: 10.1007/s000180050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano D, von Assendelft GB, Kearsey SE. Fission yeast cdc21, a member of the MCM protein family, is required for onset of S phase and is located in the nucleus throughout the cell cycle. EMBO J. 1996;15:861–872. [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Kistler A, Axelrod A, Rine J, Johnson AD. Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci USA. 1997;94:2186–2191. doi: 10.1073/pnas.94.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Murakami S, Matsumoto T, Niwa O, Yanagida M. Structure of the fission yeast centromere cen3: direct analysis of the reiterated inverted region. Chromosoma. 1991;101:214–221. doi: 10.1007/BF00365153. [DOI] [PubMed] [Google Scholar]
- Nasmyth KA. The regulation of yeast mating-type chromatin structure by SIR: an action at a distance affecting both transcription and transposition. Cell. 1982;30:567–578. doi: 10.1016/0092-8674(82)90253-7. [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Cranston G, Allshire RC. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 1994;13:3801–3811. doi: 10.1002/j.1460-2075.1994.tb06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo ER, Pidoux AL, Perry PE, Allshire RC. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature. 1998;392:825–828. doi: 10.1038/33941. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Ohi R, Feoktistova A, Gould KL. Construction of vectors and a genomic library for use with his3− deficient strains of Schizosaccharomyces pombe. Gene. 1996;174:315–318. doi: 10.1016/0378-1119(96)00085-6. [DOI] [PubMed] [Google Scholar]
- Perez-Martin J, Uria JA, Johnson AD. Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J. 1999;18:2580–2592. doi: 10.1093/emboj/18.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov O, Lobocka M, Yarmolinsky M. Silencing of genes flanking the P1 plasmid centromere. Science. 1999;283:546–549. doi: 10.1126/science.283.5401.546. [DOI] [PubMed] [Google Scholar]
- Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes Dev. 1997;11:3387–3400. doi: 10.1101/gad.11.24.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Segundo P, Roeder GS. Pch2 links chromatin silencing to meiotic checkpoint control. Cell. 1999;97:313–324. doi: 10.1016/s0092-8674(00)80741-2. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Nurse P. Identification of fission yeast nuclear markers using random polypeptide fusions with green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:15146–15151. doi: 10.1073/pnas.93.26.15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sherman J, Pillus L. An uncertain silence. Trends Genet. 1997;13:308–313. doi: 10.1016/s0168-9525(97)01198-0. [DOI] [PubMed] [Google Scholar]
- Sherman JM, Stone EM, Freeman-Cook LL, Brachmann CB, Boeke JD, Pillus L. The conserved core of a human SIR2 homologue functions in yeast silencing. Mol Biol Cell. 1999;10:3045–3059. doi: 10.1091/mbc.10.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D, Squire M, Nasmyth KA. Characterization of two genes required for the position-effect control of yeast mating-type genes. EMBO J. 1984;3:2817–2823. doi: 10.1002/j.1460-2075.1984.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZWS, Jang J, Shevchenko A, Charbonneau H, Deshaies RJ. Exit from mitosis is triggered by Tem-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Goel V, Klar AJS. A novel function of the DNA repair gene rhp6 in mating-type silencing by chromatin remodeling in fission yeast. Mol Cell Biol. 1998;18:5511–5522. doi: 10.1128/mcb.18.9.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Klar AJ. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: a novel in vivo probe for chromatin structure of yeast. Genes Dev. 1992;6:186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Steiner NC, Hahnenberger KM, Clarke L. Centromeres of the fission yeast Schizosaccharomyces pombe are highly variable genetic loci. Mol Cell Biol. 1993;13:4578–4587. doi: 10.1128/mcb.13.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Yamano H, Kinoshita N, Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993;3:13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Straight AF, Shou W, Dowd GJ, Turck CW, Deshaies RJ, Johnson AD, Moazed D. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell. 1999;97:245–256. doi: 10.1016/s0092-8674(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Murakami S, Chikashige Y, Funabiki H, Niwa O, Yanagida M. A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol Biol Cell. 1992;3:819–835. doi: 10.1091/mbc.3.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Cohen A, Klar AJ. Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics. 1994;138:29–38. doi: 10.1093/genetics/138.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Klar AJ. The clr1 locus regulates the expression of the cryptic mating-type loci of fission yeast. Genetics. 1992;131:287–296. doi: 10.1093/genetics/131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang AW, Escalante-Semerena JC. CobB, a new member of the SIR2 family of eucaryotic regulatory proteins, is required to compensate for the lack of nicotinate mononucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase activity in cobT mutants during cobalamin biosynthesis in Salmonella typhimurium LT2. J Biol Chem. 1998;273:31788–31794. doi: 10.1074/jbc.273.48.31788. [DOI] [PubMed] [Google Scholar]
- Van Holde KE. Chromatin. New York: Springer-Verlag; 1989. [Google Scholar]
- Weaver DC, Shpakovski GV, Caputo E, Levin HL, Boeke JD. Sequence analysis of closely related retrotransposon families from fission yeast. Gene. 1993;131:135–139. doi: 10.1016/0378-1119(93)90682-s. [DOI] [PubMed] [Google Scholar]
- Weiler KS, Wakimoto BT. Heterochromatin and gene expression in Drosophila. Annu Rev Genet. 1995;29:577–605. doi: 10.1146/annurev.ge.29.120195.003045. [DOI] [PubMed] [Google Scholar]
- West RR, Vaisberg EV, Ding R, Nurse P, McIntosh JR. cut11+: a gene required for cell cycle–dependent spindle pole body anchoring in the nuclear envelope and bipolar spindle formation in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:2839–2855. doi: 10.1091/mbc.9.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wines DR, Henikoff S. Somatic instability of a Drosophila chromosome. Genetics. 1992;131:683–691. doi: 10.1093/genetics/131.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KH, Shields DC. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]
- Wolffe A. Chromatin Structure and Function. New York: Academic Press; 1992. [Google Scholar]
- Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisiae. Gene. 1996;169:115–118. doi: 10.1016/0378-1119(95)00785-7. [DOI] [PubMed] [Google Scholar]
- Zemzoumi K, Sereno D, Francois C, Guilvard E, Lemesre JL, Ouaissi A. Leishmania major: cell type dependent distribution of a 43 kDa antigen related to silent information regulatory-2 protein family. Biol Cell. 1998;90:239–245. doi: 10.1016/s0248-4900(98)80020-8. [DOI] [PubMed] [Google Scholar]