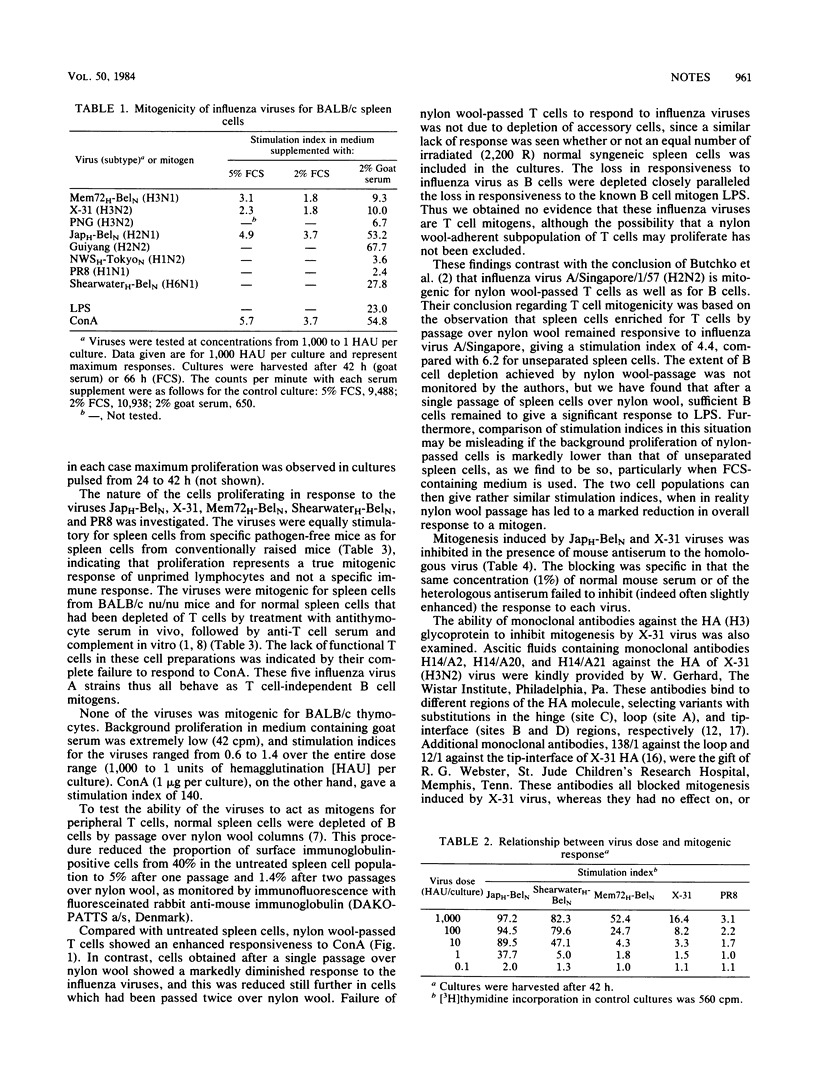
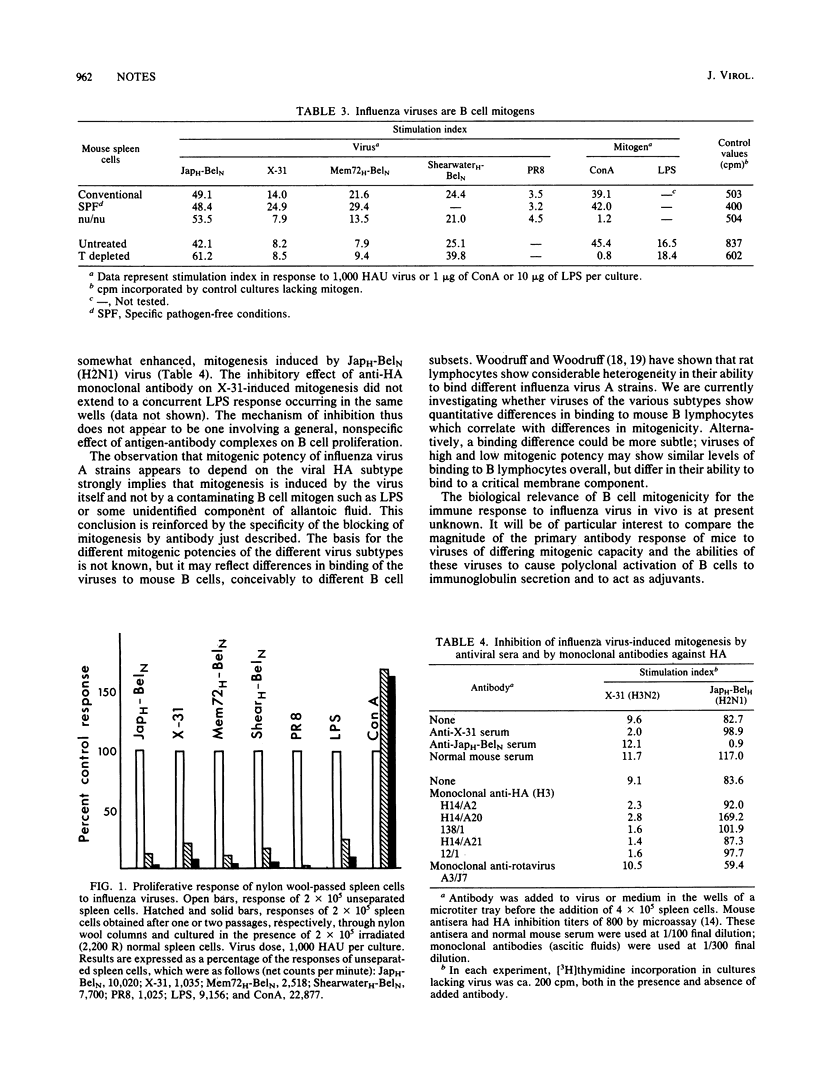
Abstract
UV-inactivated influenza virus A strains of subtypes H1, H2, H3, and H6 were shown to be mitogenic for unprimed splenic lymphocytes from BALB/c mice. Representative viruses of these four subtypes all behaved as T cell-independent B cell mitogens. The magnitude of the proliferative response was determined by the subtype of the hemagglutinin molecule: H2 and H6 viruses were the most potent mitogens, and H3 viruses were moderately mitogenic, whereas H1 viruses induced only low, but significant, levels of proliferation. Mitogenesis was inhibited by antiviral sera and by monoclonal antibodies directed against hemagglutinin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Peppard P. M., Burns W. H., White D. O. In vitro antibody response to influenza virus. I. T cell dependence of secondary response to hemagglutinin. J Immunol. 1979 Sep;123(3):1356–1361. [PubMed] [Google Scholar]
- Butchko G. M., Armstrong R. B., Martin W. J., Ennis F. A. Influenza A viruses of the H2N2 subtype are lymphocyte mitogens. Nature. 1978 Jan 5;271(5640):66–67. doi: 10.1038/271066a0. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Snitkoff G. W., McSharry J. J. Activation of mouse lymphocytes by vesicular stomatitis virus. J Virol. 1980 Sep;35(3):757–765. doi: 10.1128/jvi.35.3.757-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Snitkoff G., McSharry J. J. Mitogenic activity of Sindbis virus and its isolated glycoproteins. Infect Immun. 1982 Dec;38(3):1242–1248. doi: 10.1128/iai.38.3.1242-1248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. C. Functional heterogeneity among the T-derived lymphocytes of the mouse. III. Helper and suppressor T-cells activated by concanavalin A. Cell Immunol. 1975 Jul;18(1):9–20. doi: 10.1016/0008-8749(75)90031-3. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Darai G., Hirt H. M., Keyssner K., Munk K. In vitro mitogenic stimulation of murine spleen cells by herpes simplex virus. J Immunol. 1978 Feb;120(2):641–645. [PubMed] [Google Scholar]
- Kizaka S., Goodman-Snitkoff G., McSharry J. J. Sendai virus glycoproteins are T cell-dependent B cell mitogens. Infect Immun. 1983 May;40(2):592–600. doi: 10.1128/iai.40.2.592-600.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Gerhard W., Ward C. W., Dopheide T. A. Antigenic drift in type A influenza virus: sequence differences in the hemagglutinin of Hong Kong (H3N2) variants selected with monoclonal hybridoma antibodies. Virology. 1979 Oct 15;98(1):226–237. doi: 10.1016/0042-6822(79)90540-3. [DOI] [PubMed] [Google Scholar]
- Mochizuki D., Hedrick S., Watson J., Kingsbury D. T. The interaction of Herpes Simplex Virus with murine lymphocytes. I. Mitogenic properties of herpes simplex virus. J Exp Med. 1977 Dec 1;146(6):1500–1510. doi: 10.1084/jem.146.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov B. F. Viruses as nonspecific modulators of immunological reactivity. Acta Virol. 1981 Mar;25(2):122–128. [PubMed] [Google Scholar]
- Webster R. G., Brown L. E., Jackson D. C. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983 Apr 30;126(2):587–599. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Influenza A virus interaction with murine lymphocytes. III. Recirculating rat T and B cells differ on the basis of receptors for Cam (H1N1) virus. Cell Immunol. 1981 Jan 15;57(2):486–494. doi: 10.1016/0008-8749(81)90106-4. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Lymphocyte receptors for myxoviruses and paramyxoviruses. J Immunol. 1974 Jun;112(6):2176–2183. [PubMed] [Google Scholar]


