Abstract
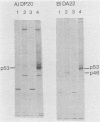
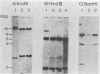
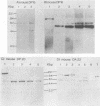
The erythroleukemia induced by Friend virus complex in adult mice is a multistage malignancy characterized by the emergence, late in the disease, of tumorigenic cell clones. We have previously shown that a significant proportion of these clones have unique rearrangements in their cellular p53 oncogene. The clonal relationships among Friend tumor cells isolated in the late stages of Friend erythroleukemia were analyzed by examining the unique integration site of Friend murine leukemia virus and the unique rearrangement in their cellular p53 oncogene. The majority of clones isolated from individual mice infected with Friend virus were clonally related as judged by the site of Friend murine leukemia virus integration. However, Southern gel analysis of DNA from individual Friend cell clones indicated that all of the clones with a normal p53 gene from the same mice were clonally related, but were unrelated to the Friend cell lines with a rearranged p53 gene. These results suggest that Friend tumor cells with rearrangements in their p53 gene arise as the result of a unique transformation event, rather than by progression from already existing tumor cells with a normal p53 gene. They also suggest that such rearrangements in the p53 gene confer a strong selective advantage to these cells in vivo.
Full text
PDF

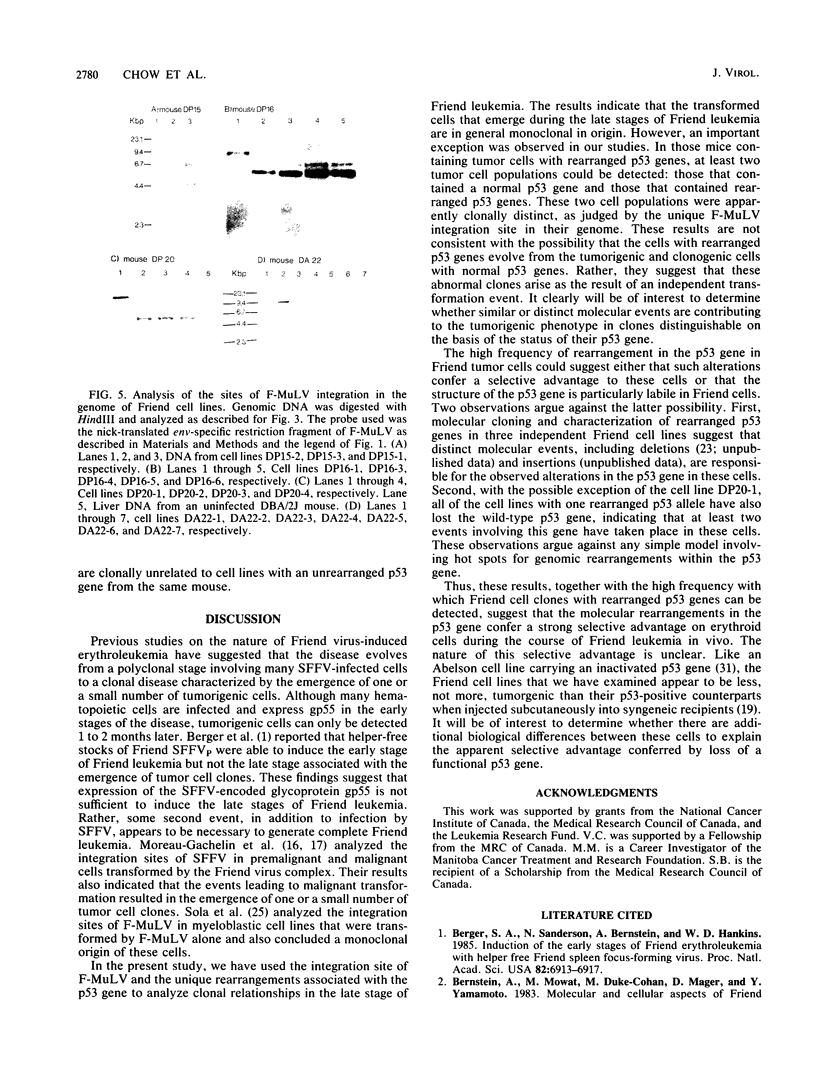

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. A., Sanderson N., Bernstein A., Hankins W. D. Induction of the early stages of Friend erythroleukemia with helper-free Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6913–6917. doi: 10.1073/pnas.82.20.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C., HADDAD J. R. Tumor formation with transplants of spleen or liver from mice with virus-induced leukemia. J Natl Cancer Inst. 1960 Dec;25:1279–1285. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Redmond S., Wade-Evans A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucleic Acids Res. 1984 Jul 25;12(14):5609–5626. doi: 10.1093/nar/12.14.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRAND E. A., PRENTICE T., HOFFMAN J. G., GRACE J. T., Jr Effect of Friend virus in Swiss and DBA/1 mice on Fe59 uptake. Proc Soc Exp Biol Med. 1961 Feb;106:423–426. doi: 10.3181/00379727-106-26358. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Bernstein A. Induction of clonogenic and erythroleukemic cells by different helper virus pseudotypes of Friend spleen focus-forming virus. Virology. 1985 Mar;141(2):337–341. doi: 10.1016/0042-6822(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Mak T. W., Bernstein A. Quantitative colony method for tumorigenic cells transformed by two distinct strains of Friend leukemia virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1703–1707. doi: 10.1073/pnas.78.3.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager D., Mak T. W., Bernstein A. Friend leukaemia virus-transformed cells, unlike normal stem cells, form spleen colonies in Sl/sld mice. Nature. 1980 Dec 11;288(5791):592–594. doi: 10.1038/288592a0. [DOI] [PubMed] [Google Scholar]
- Moreau-Gachelin F., D'Auriol L., Robert-Lezenes J., Galibert F., Tambourin P., Tavitian A. Analysis of integrated proviral DNA sequences with an octadecanucleotide probe designed for specific identification of spleen focus-forming virus in the mouse genome. J Virol. 1986 Jan;57(1):349–352. doi: 10.1128/jvi.57.1.349-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Robert-Lezenes J., Wendling F., Tavitian A., Tambourin P. Integration of spleen focus-forming virus proviruses in Friend tumor cells. J Virol. 1985 Jan;53(1):292–295. doi: 10.1128/jvi.53.1.292-295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat M., Bernstein A. Linkage of the Fv-2 gene to a newly reinserted ecotropic retrovirus in Fv-2 congenic mice. J Virol. 1983 Sep;47(3):471–477. doi: 10.1128/jvi.47.3.471-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S. A 2.4-kilobase-pair fragment of the Friend murine leukemia virus genome contains the sequences responsible for friend murine leukemia virus-induced erythroleukemia. J Virol. 1983 Jun;46(3):718–725. doi: 10.1128/jvi.46.3.718-725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rovinski B., Munroe D., Peacock J., Mowat M., Bernstein A., Benchimol S. Deletion of 5'-coding sequences of the cellular p53 gene in mouse erythroleukemia: a novel mechanism of oncogene regulation. Mol Cell Biol. 1987 Feb;7(2):847–853. doi: 10.1128/mcb.7.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Oliff A. The pathophysiology of murine retrovirus-induced leukemias. Crit Rev Oncol Hematol. 1986;5(3):257–323. doi: 10.1016/s1040-8428(86)80041-5. [DOI] [PubMed] [Google Scholar]
- Sola B., Heard J. M., Fichelson S., Martial M. A., Pozo F., Bordereaux D., Gisselbrecht S. Monoclonal proliferation of Friend murine leukemia virus-transformed myeloblastic cells occurs early in the leukemogenic process. Mol Cell Biol. 1985 May;5(5):1009–1014. doi: 10.1128/mcb.5.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tambourin P. E., Wendling F., Jasmin C., Smadja-Joffe F. The physiopathology of Friend leukemia. Leuk Res. 1979;3(3):117–129. doi: 10.1016/0145-2126(79)90009-2. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Harris N., Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984 Aug;38(1):119–126. doi: 10.1016/0092-8674(84)90532-4. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Oren M., Bienz B., Lavie V., Hazum S., Givol D. A single gene and a pseudogene for the cellular tumour antigen p53. Nature. 1983 Dec 8;306(5943):594–597. doi: 10.1038/306594a0. [DOI] [PubMed] [Google Scholar]