Abstract
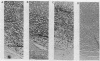
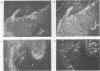
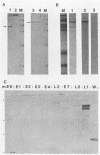
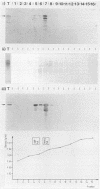
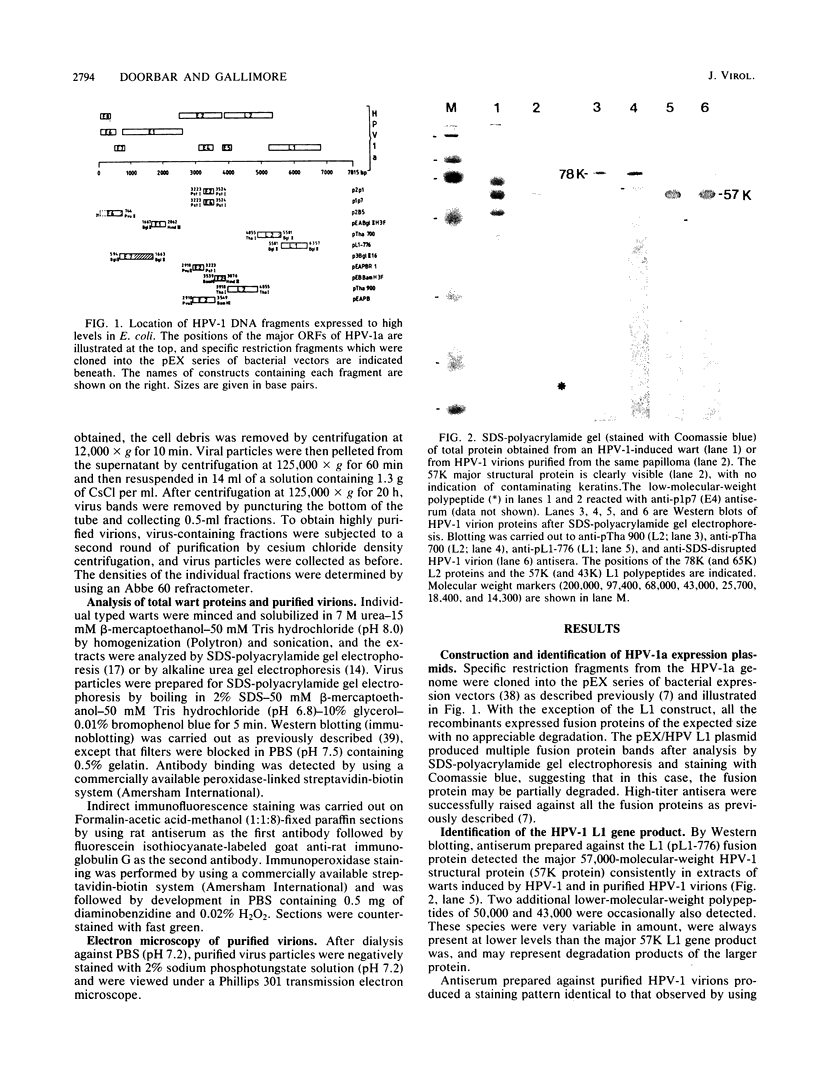
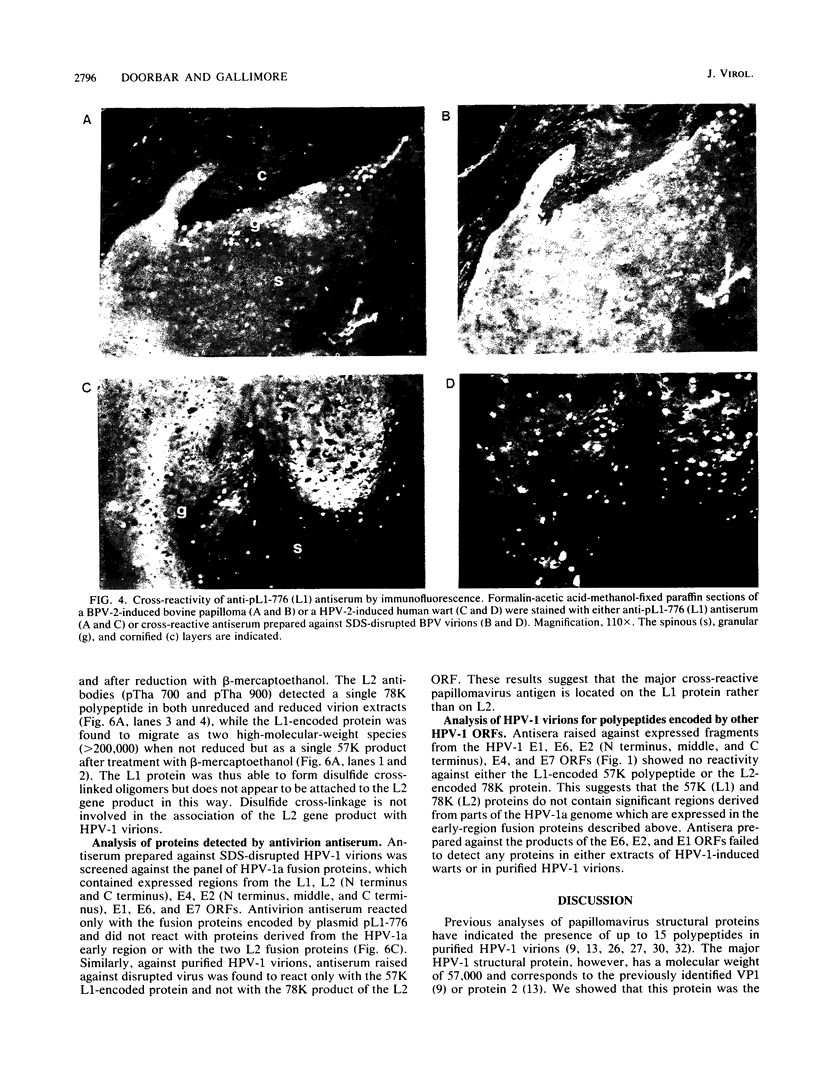
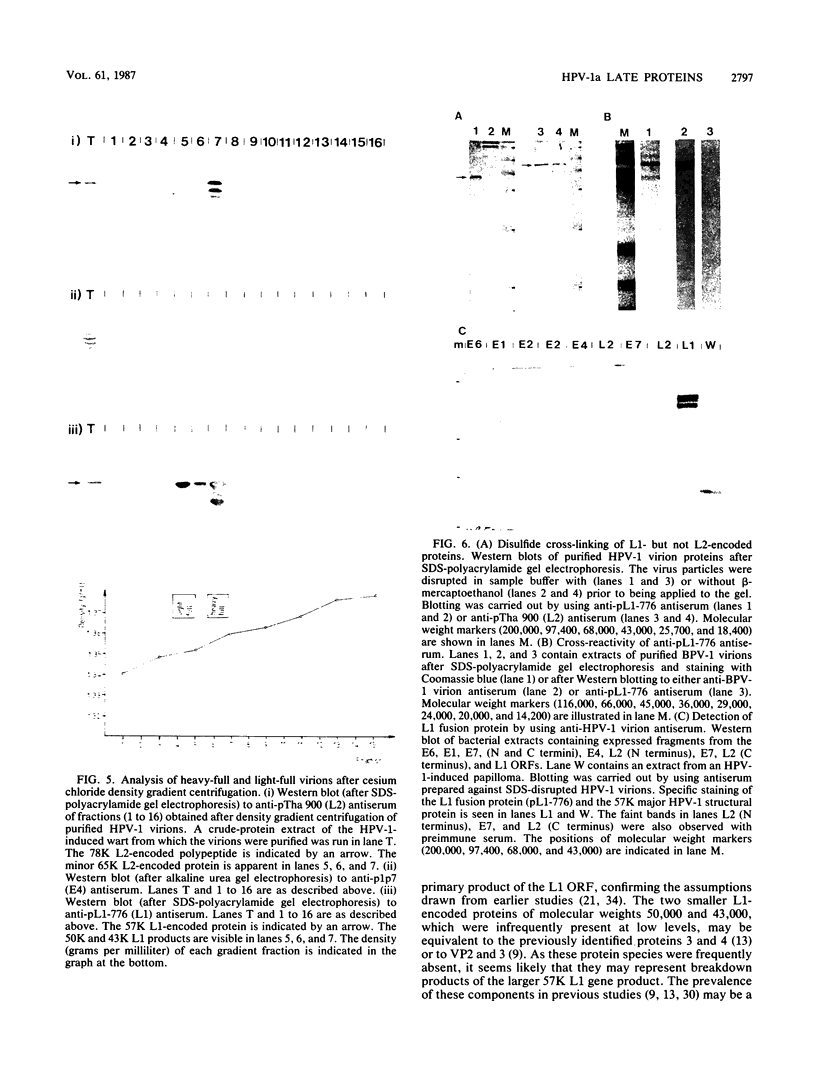
The human papillomavirus 1 (HPV-1) virion is composed of two virally encoded proteins: a 57,000-molecular-weight polypeptide (57K polypeptide), which is the product of the L1 open reading frame (ORF), and a 78K polypeptide, which is derived from the L2 ORF. The 57K (L1) product, which represents the major structural component, appears to be disulfide cross-linked in virus particles. The 78K (L2) protein is a minor component of the virion and does not appear to be disulfide linked either to the L1 gene product or to itself. Analysis of virus particles banding at different buoyant densities revealed differences in the L2 content of heavy-full and light-full virions. Antiserum prepared against a bacterially expressed fragment of the L1 ORF was found by immunofluorescence to cross-react with HPV-2 and bovine papillomavirus 1 virions in wart sections. No cross-reactivity was observed with antisera prepared against either the N- or C-terminal halves of the L2-encoded protein. Similarly, antisera prepared against purified virus particles (disrupted and nondisrupted) reacted only with an expressed fragment of the L1 ORF and not with either L2-encoded polypeptides or proteins derived from the E1, E2, E4, E6, or E7 ORFs. This indicates that the L1 protein contains the papillomavirus common antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMEIDA J. D., HOWATSON A. F., WILLIAMS M. G. Electron microscope study of human warts; sites of virus production and nature of the inclusion bodies. J Invest Dermatol. 1962 Jun;38:337–345. [PubMed] [Google Scholar]
- BARRERA-ORO J. G., SMITH K. O., MELNICK J. L. Quantitation of papova virus particles in human warts. J Natl Cancer Inst. 1962 Sep;29:583–595. [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Croissant O., Breitburd F., Orth G. Specificity of cytopathic effect of cutaneous human papillomaviruses. Clin Dermatol. 1985 Oct-Dec;3(4):43–55. doi: 10.1016/0738-081x(85)90048-3. [DOI] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J., Campbell D., Grand R. J., Gallimore P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 1986 Feb;5(2):355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Heilman C. A., Howley P. M. Transcriptional organization of bovine papillomavirus type 1. J Virol. 1983 Sep;47(3):516–528. doi: 10.1128/jvi.47.3.516-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 May;15(5):1239–1247. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Pfister H., Zur Hausen H. Human papilloma viruses (HPV): characterization of four different isolates. Virology. 1977 Feb;76(2):569–580. doi: 10.1016/0042-6822(77)90239-2. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson A. B., Rosenthal J. D., Olson C., Pass F., Lancaster W. D., Shah K. Immunologic relatedness of papillomaviruses from different species. J Natl Cancer Inst. 1980 Mar;64(3):495–500. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Jenson A. B. Evidence for papillomavirus genus-specific antigens and DNA in laryngeal papilloma. Intervirology. 1981;15(4):204–212. doi: 10.1159/000149233. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L. Papova virus group. Science. 1962 Mar 30;135(3509):1128–1130. doi: 10.1126/science.135.3509.1128. [DOI] [PubMed] [Google Scholar]
- Meinke W., Meinke G. C. Isolation and characterization of the major capsid protein of bovine papilloma virus type 1. J Gen Virol. 1981 Jan;52(Pt 1):15–24. doi: 10.1099/0022-1317-52-1-15. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Allison A. C., Butel J. S., Eckhart W., Eddy B. E., Kit S., Levine A. J., Miles J. A., Pagano J. S., Sachs L. Papovaviridae. Intervirology. 1974;3(1-2):106–120. doi: 10.1159/000149746. [DOI] [PubMed] [Google Scholar]
- NOYES W. F., MELLORS R. C. Fluorescent antibody detection of the antigens of the Shope papilloma virus in papillomas of the wild and domestic rabbit. J Exp Med. 1957 Oct 1;106(4):555–562. doi: 10.1084/jem.106.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. Differences exist between viral transcripts in cottontail rabbit papillomavirus-induced benign and malignant tumors as well as non-virus-producing and virus-producing tumors. J Virol. 1984 Sep;51(3):706–712. doi: 10.1128/jvi.51.3.706-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Favre M., Croissant O. Characterization of a new type of human papillomavirus that causes skin warts. J Virol. 1977 Oct;24(1):108–120. doi: 10.1128/jvi.24.1.108-120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Pfister H., Gissmann L., zur Hausen H. Partial characterization of the proteins of human papilloma viruses (HPV) 1-3. Virology. 1977 Nov;83(1):131–137. doi: 10.1016/0042-6822(77)90216-1. [DOI] [PubMed] [Google Scholar]
- Pfister H., Meszaros J. Partial characterization of a canine oral papillomavirus. Virology. 1980 Jul 15;104(1):243–246. doi: 10.1016/0042-6822(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Pfister H., zur Hausen H. Characterization of proteins of human papilloma viruses (HPV) and antibody response to HPV 1. Med Microbiol Immunol. 1978 Nov 17;166(1-4):13–19. doi: 10.1007/BF02121129. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Leary S. L., Faras A. J. Shope papillomavirus transcription in benign and malignant rabbit tumors. Virology. 1985 Oct 15;146(1):120–129. doi: 10.1016/0042-6822(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Ranki A., Kianto U., Kanerva L., Tolvanen E., Johansson E. Immunohistochemical and electron microscopic characterization of the cellular infiltrate in alopecia (areata, totalis, and universalis). J Invest Dermatol. 1984 Jul;83(1):7–11. doi: 10.1111/1523-1747.ep12261627. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]