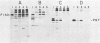Abstract
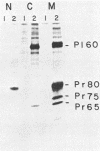
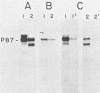
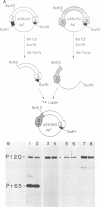
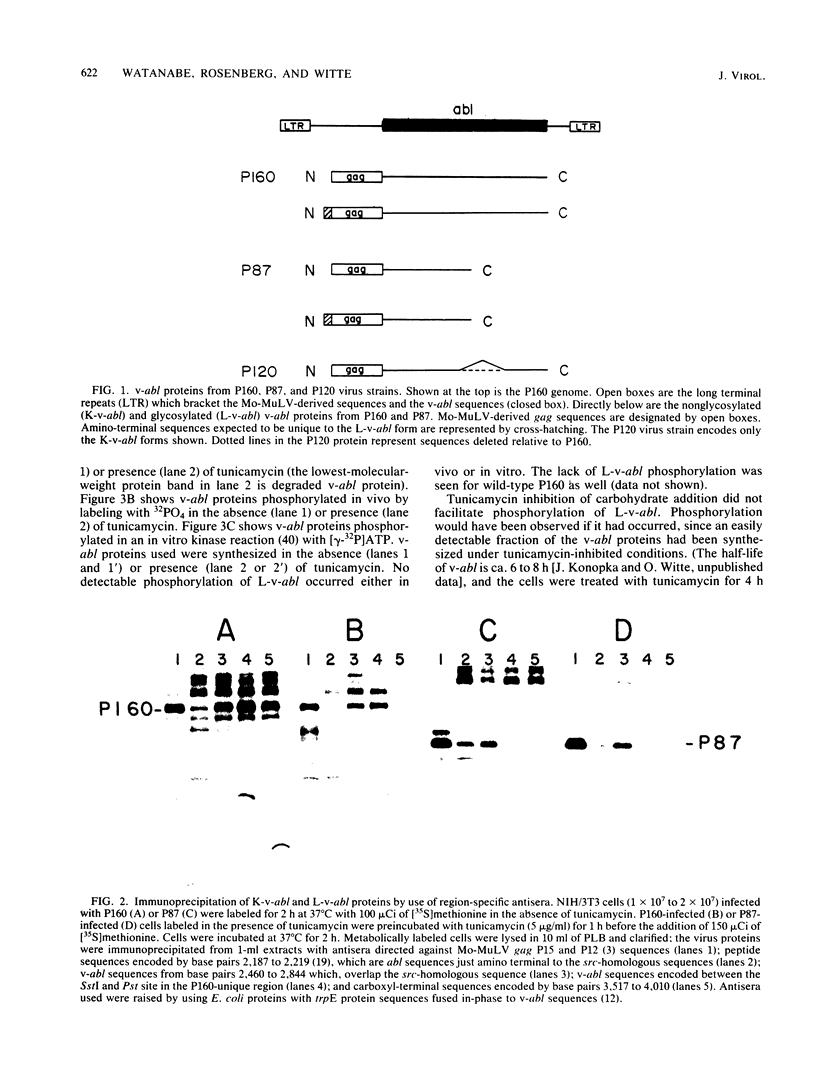
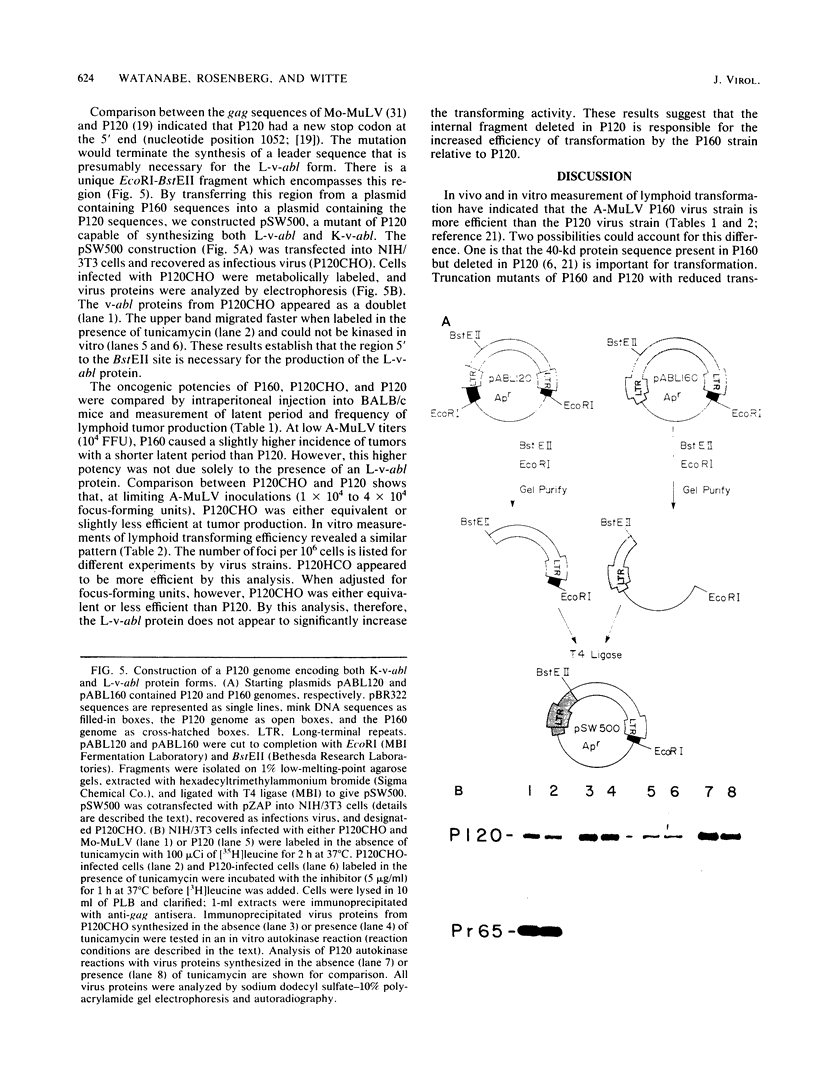
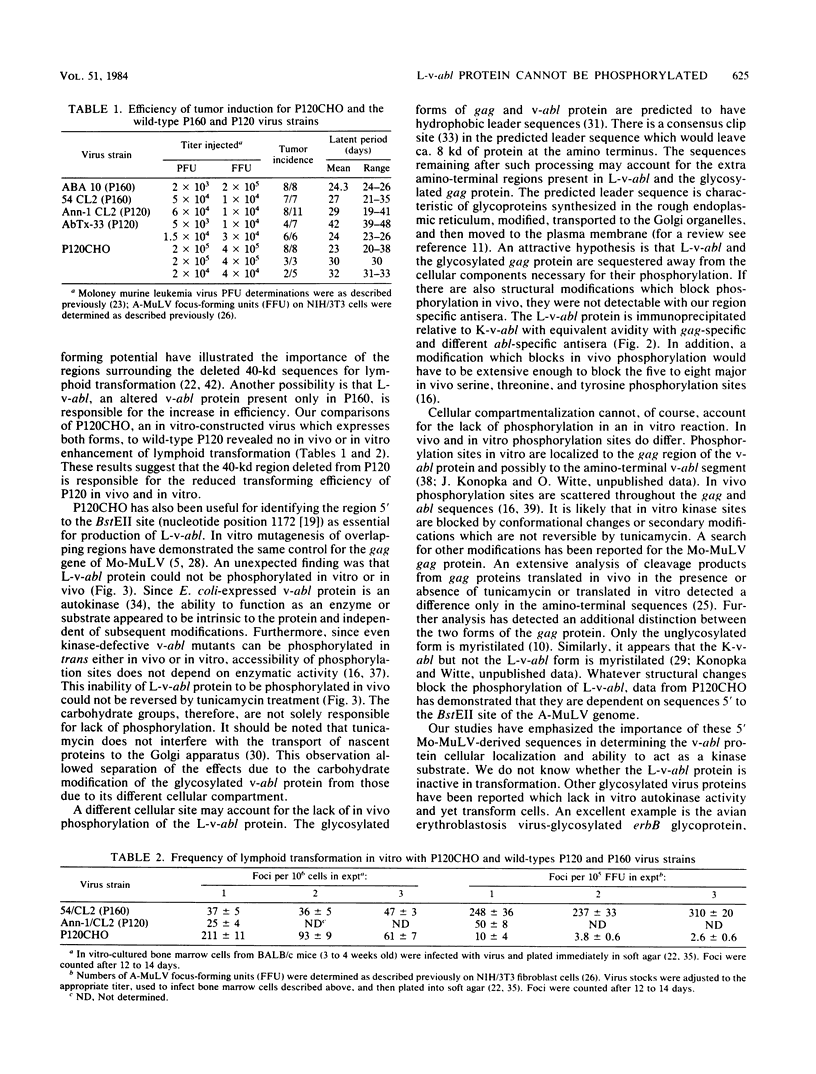
Abelson murine leukemia virus encodes a transforming protein which contains tyrosine kinase activity and is phosphorylated in vivo and in vitro. We found that P160 and P160-derived virus strains expressed an additional, altered v-abl protein which could not be phosphorylated. The altered v-abl protein (L-v-abl) differed from the phosphorylated form (K-v-abl) in that it was glycosylated and localized exclusively to the membrane fraction. Tunicamycin inhibition of N-linked carbohydrate addition did not restore phosphorylation. It did, however, reveal that L-v-abl had additional sequences relative to K-v-abl. The coding sequences required for this region and for the expression of L-v-abl were identified by replacing sequences in the P120 virus genome, which did not express L-v-abl, with sequences from the P160 virus genome. The necessary sequences were localized to the Moloney murine leukemia virus-derived gag gene. Comparison between the in vitro altered P120 and wild-type P120 virus strains indicated that expression of L-v-abl did not increase the efficiency of lymphoid transformation. Although the biological role of L-v-abl is not clear, our analyses have revealed that a specific amino terminal gag sequence can prevent v-abl from acting as a kinase substrate and can alter the cellular localization and modification of v-abl. These properties distinguish L-v-abl from previously reported v-abl proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Furth M., Wolff L., Ruscetti S. K., Sherr C. J. Monoclonal antibodies to the transformation-specific glycoprotein encoded by the feline retroviral oncogene v-fms. J Virol. 1982 Nov;44(2):696–702. doi: 10.1128/jvi.44.2.696-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Rosenberg N., Witte O. N. Transformation of immature lymphoid cells by Abelson murine leukemia virus. Immunol Rev. 1979;48:3–22. doi: 10.1111/j.1600-065x.1979.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Chute H., Chao E., Feuerman M. Construction and characterization of Moloney murine leukemia virus mutants unable to synthesize glycosylated gag polyprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5965–5969. doi: 10.1073/pnas.80.19.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Witte O. N., Gilboa E., Rosenberg N., Baltimore D. Genome structure of Abelson murine leukemia virus variants: proviruses in fibroblasts and lymphoid cells. J Virol. 1981 May;38(2):460–468. doi: 10.1128/jvi.38.2.460-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Goff S. P., Tabin C. J., Paskind M., Wang J. Y., Baltimore D. Cloning and analysis of reverse transcript P160 genomes of Abelson murine leukemia virus. J Virol. 1983 Mar;45(3):1195–1199. doi: 10.1128/jvi.45.3.1195-1199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Nowinski R. C., Eisenman R. N. Biosynthesis and metabolism of viral proteins expressed on the surface of murine leukemia virus-infected cells. Virology. 1978 Nov;91(1):116–129. doi: 10.1016/0042-6822(78)90360-4. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Ponticelli A. S., Whitlock C. A., Rosenberg N., Witte O. N. In vivo tyrosine phosphorylations of the Abelson virus transforming protein are absent in its normal cellular homolog. Cell. 1982 Jul;29(3):953–960. doi: 10.1016/0092-8674(82)90458-5. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Sealy L., Bishop J. M., McGrath J. P., Levinson A. D. The product of the avian erythroblastosis virus erbB locus is a glycoprotein. Cell. 1983 Apr;32(4):1257–1267. doi: 10.1016/0092-8674(83)90307-0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. Abelson murine leukemia virus mutants with alterations in the virus-specific P120 molecule. J Virol. 1980 Jan;33(1):340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris C. J., van Eenbergen J., Liskamp R. M., Bloemers H. P. Structure of glycosylated and unglycosylated gag and gag-pol precursor proteins of Moloney murine leukemia virus. J Virol. 1983 Jun;46(3):841–859. doi: 10.1128/jvi.46.3.841-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol. 1983 May;46(2):538–546. doi: 10.1128/jvi.46.2.538-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shida H., Matsumoto S. Analysis of the hemagglutinin glycoprotein from mutants of vaccinia virus that accumulates on the nuclear envelope. Cell. 1983 Jun;33(2):423–434. doi: 10.1016/0092-8674(83)90424-5. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Queen C., Baltimore D. Expression of an Abelson murine leukemia virus-encoded protein in Escherichia coli causes extensive phosphorylation of tyrosine residues. J Biol Chem. 1982 Nov 25;257(22):13181–13184. [PubMed] [Google Scholar]
- Watanabe S. M., Witte O. N. Site-directed deletions of Abelson murine leukemia virus define 3' sequences essential for transformation and lethality. J Virol. 1983 Mar;45(3):1028–1036. doi: 10.1128/jvi.45.3.1028-1036.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Ponticelli A., Gifford A., Baltimore D., Rosenberg N., Elder J. Phosphorylation of the Abelson murine leukemia virus transforming protein. J Virol. 1981 Sep;39(3):870–878. doi: 10.1128/jvi.39.3.870-878.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]