Abstract
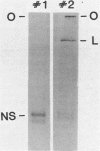
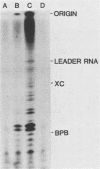
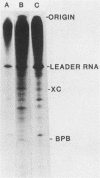
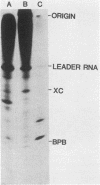
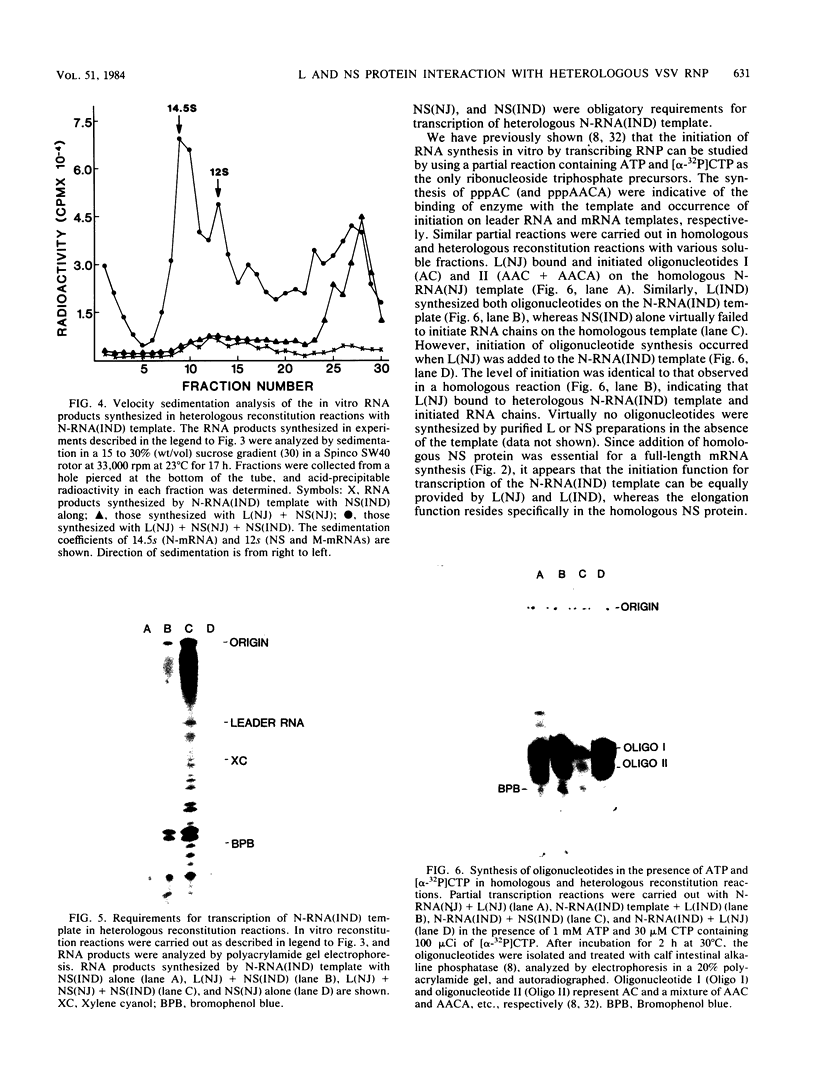
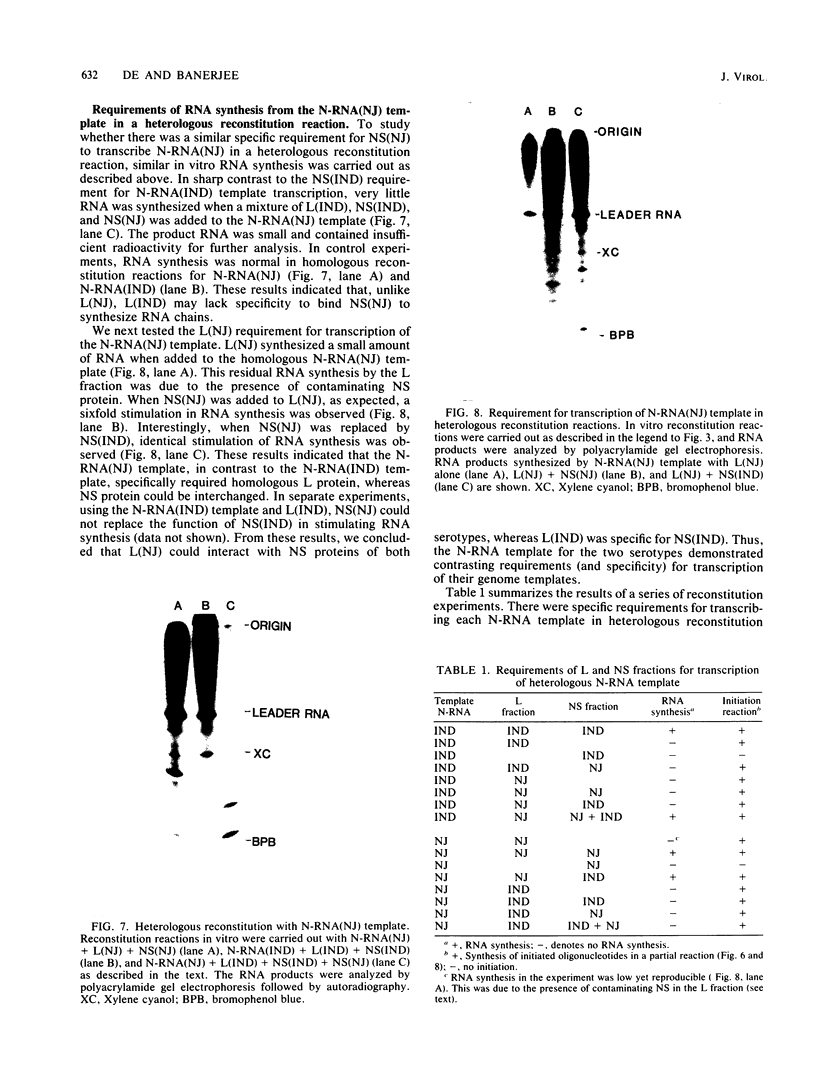
Two dissociable proteins, L and NS, and N-RNA template were purified from two serologically distinct vesicular stomatitis viruses, Indiana [VSV(IND)] and New Jersey [VSV(NJ)]. Requirements for RNA synthesis in heterologous reconstitution reactions in vitro were studied. The L and NS proteins of VSV(NJ) failed to synthesize full-length leader RNA and mRNAs in vitro when reconstituted with N-RNA(IND) template. However, when purified homologous NS(IND) was added to the reaction mixture, mRNA synthesis ensued. The requirements for transcription of N-RNA(NJ) template were different from those for N-RNA(IND). For RNA synthesis, transcription specifically required L(NJ), but the NS(NJ) and NS(IND) proteins were interchangeable. This suggests that there are specific domains on the L(NJ) protein, at which NS proteins of both serotypes may interact to form an active RNA polymerase complex, whereas L(IND) lacked such domains for interaction with NS(NJ). The function of the L protein appeared primarily to initiate RNA chains, and the NS protein was required for chain elongation. The results of these in vitro complementation experiments are discussed in light of previous in vivo complementation studies.
Full text
PDF

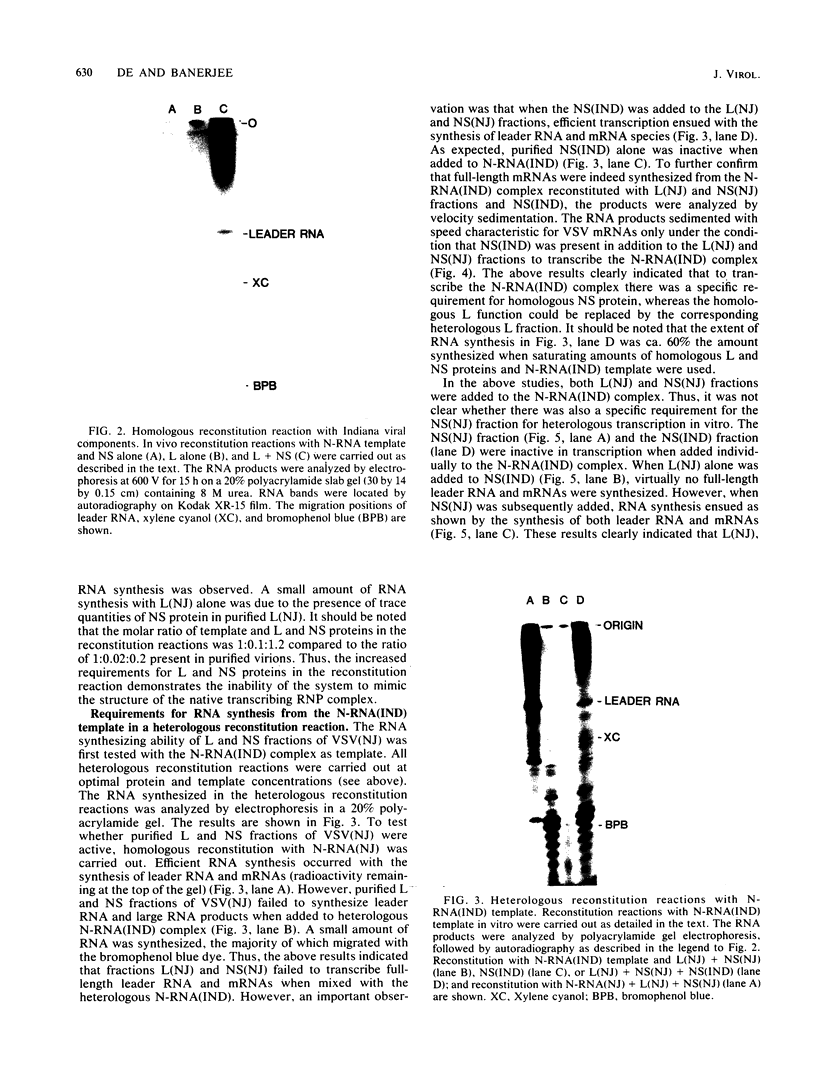


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Lazzarini R. A. Elementary aspects of autointerference and the replication of defective interfering virus particles. Virology. 1978 Jun 1;87(1):152–163. doi: 10.1016/0042-6822(78)90167-8. [DOI] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Emerson S. U., Flamand A. Reconstitution of infectivity and transcriptase activity of homologous and heterologous viruses: vesicular stomatitis (Indiana serotype), Chandipura, vesicular stomatitis (New Jersey serotype), and Cocal viruses. J Virol. 1974 Jul;14(1):139–144. doi: 10.1128/jvi.14.1.139-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Identification of promoter-proximal oligonucleotides and a unique dinucleotide, pppGpC, from in vitro transcription products of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):93–103. doi: 10.1128/jvi.39.1.93-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. M., Schnitzlein W. M., Reichmann M. E. Expression of genetic information contained in the RNA of a defective interfering particle of vesicular stomatitis virus. Virology. 1977 Apr;77(2):579–588. doi: 10.1016/0042-6822(77)90483-4. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Nucleotide sequence of the leader RNA of the New Jersey serotype of vesicular stomatitis virus. Nucleic Acids Res. 1978 Nov;5(11):4165–4176. doi: 10.1093/nar/5.11.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Tahara S. M., Banerjee A. K. Production and characterization of a monoclonal antibody to the N protein of vesicular stomatitis virus (Indiana serotype). Virology. 1982 Oct 30;122(2):510–514. doi: 10.1016/0042-6822(82)90255-0. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze-Fernandez M. T., Banerjee A. K. In vitro RNA transcription by the New Jersey serotype of vesicular stomatitis virus. I. Characterization of the mRNA species. J Virol. 1978 Apr;26(1):179–187. doi: 10.1128/jvi.26.1.179-187.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Summers D. F. Characterization of monospecific antisera against all five vesicular stomatitis virus-specific proteins: anti-L and anti-NS inhibit transcription in vitro. Virology. 1982 Jul 15;120(1):194–204. doi: 10.1016/0042-6822(82)90017-4. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Inhibition of viral transcriptase by immunoglobulin directed against the nucleocapsid NS protein of vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1357–1366. doi: 10.1128/jvi.15.6.1357-1366.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Thornton B. J., Emerson S. U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault D., Takayesu D., Prevec L. Heterotypic exclusion between vesicular stomatitis viruses of the New Jersey and Indiana serotypes. J Gen Virol. 1977 Apr;35(1):53–65. doi: 10.1099/0022-1317-35-1-53. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Emerson S. U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J Virol. 1978 Sep;27(3):560–567. doi: 10.1128/jvi.27.3.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Prevec L., Kang C. Y. Homotypic and heterotypic interference by defective particles of vesicular stomatitis virus. Nature. 1970 Oct 3;228(5266):25–27. doi: 10.1038/228025a0. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B., Stevenson M. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. J Virol. 1971 Dec;8(6):836–841. doi: 10.1128/jvi.8.6.836-841.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Synthesis of RNA by mutants of vesicular stomatitis virus (Indiana serotype) and the ability of wild-type VSV New Jersey to complement the VSV Indiana ts G I-114 transcription defect. J Virol. 1976 Oct;20(1):157–169. doi: 10.1128/jvi.20.1.157-169.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Banerjee A. K. Poly(A)-adjacent sequence of the 14.5 S mRNA of vesicular stomatitis virus (New Jersey serotype). Virology. 1980 Aug;105(1):297–300. doi: 10.1016/0042-6822(80)90184-1. [DOI] [PubMed] [Google Scholar]
- Rhodes D. P., Moyer S. A., Banerjee A. K. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1974 Dec;3(4):327–333. doi: 10.1016/0092-8674(74)90046-4. [DOI] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. The size and the cistronic origin of defective vesicular stomatitis virus particle RNAs in relation to homotypic and heterotypic interference. J Mol Biol. 1976 Mar 5;101(3):307–325. doi: 10.1016/0022-2836(76)90150-9. [DOI] [PubMed] [Google Scholar]
- Thornton G. B., De B. P., Banerjee A. K. Interaction of L and NS proteins of vesicular stomatitis virus with its template ribonucleoprotein during RNA synthesis in vitro. J Gen Virol. 1984 Mar;65(Pt 3):663–668. doi: 10.1099/0022-1317-65-3-663. [DOI] [PubMed] [Google Scholar]
- Thornton G. B., Kopchick J. J., Stacey D. W., Banerjee A. K. Microinjection of vesicular stomatitis virus ribonucleoprotein into animal cells yields infectious virus. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1160–1167. doi: 10.1016/s0006-291x(83)80264-2. [DOI] [PubMed] [Google Scholar]