Abstract
Surprisingly, although highly temperature-sensitive, the bimA1APC3 anaphase-promoting complex/cyclosome (APC/C) mutation does not cause arrest of mitotic exit. Instead, rapid inactivation of bimA1APC3 is shown to promote repeating oscillations of chromosome condensation and decondensation, activation and inactivation of NIMA and p34cdc2 kinases, and accumulation and degradation of NIMA, which all coordinately cycle multiple times without causing nuclear division. These bimA1APC3-induced cell cycle oscillations require active NIMA, because a nimA5 + bimA1APC3 double mutant arrests in a mitotic state with very high p34cdc2 H1 kinase activity. NIMA protein instability during S phase and G2 was also found to be controlled by the APC/C. The bimA1APC3 mutation therefore first inactivates the APC/C but then allows its activation in a cyclic manner; these cycles depend on NIMA. We hypothesize that bimAAPC3 could be part of a cell cycle clock mechanism that is reset after inactivation of bimA1APC3. The bimA1APC3 mutation may also make the APC/C resistant to activation by mitotic substrates of the APC/C, such as cyclin B, Polo, and NIMA, causing mitotic delay. Once these regulators accumulate, they activate the APC/C, and cells exit from mitosis, which then allows this cycle to repeat. The data indicate that bimAAPC3 regulates the APC/C in a NIMA-dependent manner.
INTRODUCTION
The bimE and bimA genes were originally defined by temperature-sensitive mutations in functions required for normal progression through mitosis in Aspergillus nidulans (Morris, 1976; Osmani et al., 1988; Engle et al., 1990; O’Donnell et al., 1991) and were subsequently found to encode highly conserved proteins whose homologues have been isolated from many organisms ranging from fungi to humans (Hirano et al., 1990, 1994; Mirabito and Morris, 1993; Lamb et al., 1994; Starborg et al., 1994; King et al., 1995; Tugendreich et al., 1995). Most significantly, they were recently identified as components of the anaphase-promoting complex (APC) (King et al., 1995; Peters et al., 1996; Zachariae et al., 1996). The APC is also known as the cyclosome (Sudakin et al., 1995) and is therefore abbreviated here to APC/C (see Townsley and Ruderman, 1998, for review).
bimE encodes the largest subunit of the APC/C and has recently been termed APC1 (Peters et al., 1996). We will use the designation bimEAPC1 for the gene and BIMEAPC1 for the protein. bimA encodes a member of the tetratricopeptide repeat family of proteins, being most similar to Schizosaccharomyces pombe nuc2 (Hirano et al., 1990, 1994) and Saccharomyces cerevisiae CDC27 (Sikorski et al., 1990; Lamb et al., 1994), which was recently termed APC3 (Peters et al., 1996). The APC/C functions as an E3 ubiquitin ligase, which specifically targets proteins (such as mitotic cyclins) for degradation in a destruction box (D box)-dependent manner (Glotzer et al., 1991; King et al., 1996b) through the ubiquitin–proteasome pathway, to promote initiation of anaphase and exit from mitosis into G1 (for reviews see Murray, 1995; King et al., 1996a; Hershko, 1997; Townsley and Ruderman, 1998).
APC/C activity is cell cycle regulated (Hershko et al., 1994; Zachariae and Nasmyth, 1996) potentially by reversible phosphorylation (Lahav-Baratz et al., 1995) of certain subunits (Peters et al., 1996). Recent data indicate that Polo-related kinases play a role in the activation of the APC/C during mitosis (Charles et al., 1998; Descombes and Nigg, 1998; Shirayama et al., 1998). In S. cerevisiae, the APC/C is activated during the metaphase to anaphase transition and remains active during G1 (Amon et al., 1994), as also occurs in mammalian cells (Brandeis and Hunt, 1996). As cells progress into S phase from G1, mitosis-specific APC/C activity is turned off to allow accumulation of mitotic cyclins in preparation for the next mitosis.
It has been shown that the APC/C subunit BIMEAPC1 also has an interphase function to negatively regulate mitosis, as loss of BIMEAPC1 overrides the nimA5 G2 arrest and, in combination with non–tyrosinephosphorylated p34cdc2, promotes premature mitosis from S phase (Osmani et al., 1988; James et al., 1995; Ye et al., 1996). It has been proposed that some checkpoint controls over mitosis are switched from the APC/C during G1 to include tyrosine phosphorylation of p34cdc2 upon initiation of DNA replication (Ye et al., 1997a). BIMEAPC1 may therefore normally play a role to prevent activation of NIMA kinase, and other mitotic regulators, during interphase to prevent premature mitosis.
The levels of NIMEcyclinB and NIMA proteins oscillate once each cell cycle in a strikingly similar manner (Evans et al., 1983; Alfa et al., 1990; Draetta et al., 1990; Ghiara et al., 1991; Hunt et al., 1992; Richardson et al., 1992; Grandin and Reed, 1993; Pu and Osmani, 1995; Ye et al., 1995; Yamano et al., 1996). Both proteins accumulate during late S to G2, peak at early M, and are rapidly degraded as cells progress out of mitosis with slightly different kinetics (Pu and Osmani, 1995; Ye et al., 1995). If cells are arrested at mitosis, for instance by the bimE7APC1 mutation or by addition of nocodazole, then both NIMEcyclinB and NIMA proteins remain stable (Ye et al., 1995). Additionally, degradation-deficient forms of either cyclin B (Murray et al., 1989; Luca et al., 1991; Gallant and Nigg, 1992; Surana et al., 1993) or NIMA (O’Connell et al., 1994; Pu and Osmani, 1995) prevent normal exit from mitosis. This raises the possibility that the degradation of NIMEcyclinB and NIMA proteins may be mediated, at least partly, by the same proteolytic pathway. Although the mitosis-specific proteolysis of cyclin B through D box-directed ubiquitination has been well characterized in several systems (see Murray, 1995, for review), how NIMA kinase is specifically degraded during M phase progression is not understood. The determinants for mitotic instability of NIMA reside in its C-terminal domain, which contains PEST sequences and p34cdc2 phosphorylation sites (O’Connell et al., 1994; Pu and Osmani, 1995).
In the present work, experiments using APC/C mutations and biochemical analysis of NIMA stability uncover a regulatory role for BIMAAPC3 involving APC/C-dependent instability of NIMA.
MATERIALS AND METHODS
Aspergillus Strains and General Techniques
Strains of A. nidulans used in this study were R153 (pyroA4; wA3); GR5 (pyrG89; pyroA4; wA3); SO1 (bimE7; pyrG89; pyroA4; wA2); SO4 (bimE7; pabaA1; wA2); SO15 (nimA5; bimE7; pabaA1; riboB2; wA2); SO54 (nimA5; wA2); 5C (alcA::nimA, pyr4+; pyrG89; benA22; pabaA1; fwA1); RP5 (alcA::nimA; pyrG89, pyr4+; nicB8; riboA1; yA2); MAT68 (alcA::nimE at argB; pabaA1); AT62 (bimA1; alcA::nimA, pyr4+; pyrG89; choA1; pabaA1); AT92 (alcA::nimE at argB; choA1; yA2); AT114 (bimE7; alcA::nimE at argB; yA2); AT120 (bimA1; alcA::nimE at argB; choA1; yA2); AT128 (bimE7; alcA::nimA, pyr4+ pyrG89; wA2); and PM156 (bimA1; pabaA1; wA2). Media and general techniques for culture, transformation, light microscopy, protein extraction, immunoprecipitation, protein kinase assays, and Western blotting were as previously described (Ye et al., 1995). NIMA and p34cdc2 H1 kinase activities were quantified, based on incorporation of 32P into their in vitro substrates β-casein and histone H1, using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The relative amount of NIMA and NIMEcyclinB was measured after Western blotting with the use of a densitometer and ImageQuant software (Molecular Dynamics).
nimA Deletion
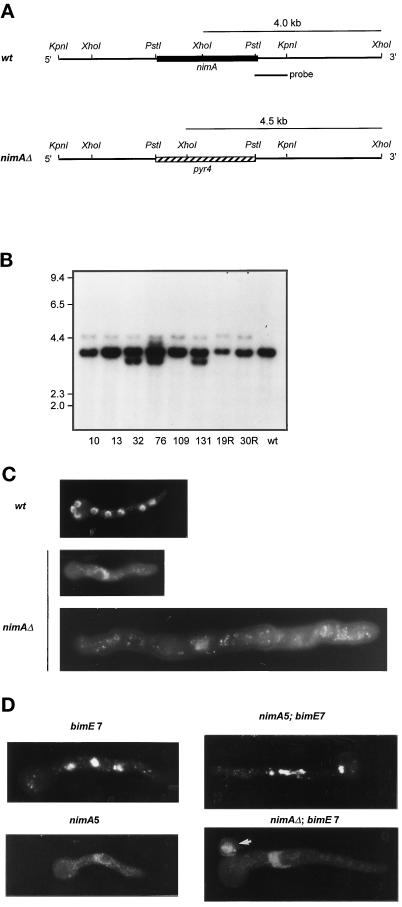
A 5.0-kb KpnI fragment containing the nimA open reading frame was cloned into pGEM giving rise to plasmid pWC1. A PstI fragment, 129 bp upstream of the start codon and 83 bp before the stop codon of nimA, was replaced with the PstI fragment of the pyr4 gene from Neurospora crassa, which complements the pyrG89 mutation of A. nidulans. Two plasmids containing opposite orientations of the pyr4+ gene were obtained called pnimAΔA and pnimAΔB. A linear KpnI fragment from pnimAΔB, as shown in Figure 7A, was used to delete nimA by transformation of GR5 and SO1.
Figure 7.
Targeted nimA deletion. (A) Restriction maps of the nimA locus and nimAΔ locus after targeted deletion. The PstI fragment of nimA was replaced with a PstI fragment containing the pyr4 gene. The linear genomic KpnI fragment containing the pyr4 insert was used for transformation in a wild-type (wt = GR5) and a bimE7APC1 strain. Diagnostic XhoI fragments of 4.0 kb (wt) and 4.5 kb (nimAΔ) when probed with the PstI–KpnI fragment are indicated. (B) Autoradiogram of a Southern blot of XhoI-digested genomic DNA isolated from putative heterokaryons after deletion of nimA probed with the radiolabled PstI–KpnI fragment indicated in A. 10, 13, 32, 76, 109, and 131 are deletions in GR5, and 19R and 30R indicate deletions in a bimE7APC1 strain. Heterokaryons 10, 13, 109, 19R, and 30R all contain both a specific deletion and the wild-type allele. (C) Micrographs of DAPI-stained germlings. The wild-type germling is from an 8-h sample and contains eight interphase nuclei. The nimAΔ germlings are derived from a heterokaryon germinated for 8 and 15 h, respectively, at 32°C. (D) Representative micrographs of DAPI-stained germlings from strains with the indicated pertinent genotypes. Spores were first allowed to germinate at 32°C for 7 h before shifting to 42°C for 4 h. The nimA5 mutant germling was grown at 42°C directly for 7 h. It is to be noted that the single nucleus of the large nimAΔ + bimE7APC1 germling remained at interphase upon shift to 42°C for 4 h, whereas nuclei in the bimE7APC1 or nimA5 + bimE7APC1 mutant germlings all became condensed. The arrow indicates an ungerminated spore carrying the parental genotype (GR5 = pyrg89) derived from the nimAΔ + bimE7APC1 heterokaryon.
Transformants were screened for heterokaryons (Osmani et al., 1988) by streaking conidiospores (conidia) onto selective YAG without uridine and uracil. If a transformant is maintained as a heterokaryon conidia derived from such a transformant, it will not be able to grow in the absence of uridine and uracil. This is because conidia containing the parental nuclei (they are uninucleate) will not be able to germinate in the absence of uridine and uracil because of the pryG89 mutation. On the other hand, conidia containing deleted nimA, which can germinate in the absence of uridine and uracil because of the pyr4+ gene, will not be able to undergo nuclear division and will cease growth as a single nucleate germling (Osmani et al., 1988). Suspected heterokaryons were maintained as mycelial colonies on selective medium.
Southern blot analysis of selected heterokaryons was carried out to confirm specific nimA deletion. Isolated DNA was digested to completion with XhoI, separated by electrophoresis, and blotted onto nylon membranes. A 0.8-kb KpnI–PstI fragment was isolated from pWC1 and was used as probe for random primer labeling. Hybridization conditions were as previously described (Ye et al., 1996).
Temperature Shifts
We usually do temperature upshift to 42°C by vigorously shaking the culture in a 60°C water bath. In this study, we also harvested Aspergillus cells by vacuum filtration through a layer of Miracloth (Calbiochem-Novabiochem, La Jolla, CA) and then transferred the cells directly to prewarmed medium at 42°C to cause instant temperature change of the culture.
Induction and Repression of NIMEcyclinB and NIMA Expression from the alcA Promoter
Induction and repression of NIMA and NIMEcyclinB expression from the alcA promoter were as previously described (Pu and Osmani, 1995). In this study, we also incorporated hydroxyurea (100 mM) into the medium to cause S phase arrest before ethanol induction.
RESULTS
Unsuccessful Cell Cycle Oscillations Are Induced by Rapid Inactivation of bimAAPC3
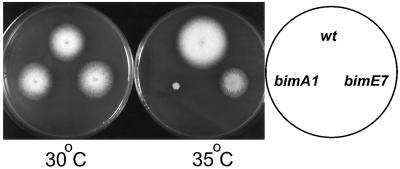
Temperature-sensitive mutations in two genes, bimAAPC3 and bimEAPC1, which both encode components of the APC/C, have been previously identified in A. nidulans (Morris, 1976; Engle et al., 1990; O’Donnell et al., 1991; King et al., 1995; Tugendreich et al., 1995; Peters et al., 1996; Zachariae et al., 1996). When shifted to restrictive temperature, the bimE7APC1 mutation causes a rapid cell cycle arrest in mitosis (Morris, 1976; Osmani et al., 1988; James et al., 1995), consistent with this mutation causing inactivation of APC/C function and preventing mitotic exit. However, the bimA1APC3 mutation takes much longer to cause a mitotic arrest (O’Donnell et al., 1991), even though this allele is more temperature sensitive than bimE7APC1 (Figure 1). The extreme temperature sensitivity caused by bimA1APC3, and the lack of first cell cycle arrest in mitosis caused by this mutation, indicate that irreversible loss of APC/C function is not caused by the bimA1APC3 allele at restrictive temperature.
Figure 1.
Temperature sensitivity of bimA1APC3 and bimE7APC1 strains. Strains containing no temperature-sensitive mutation (R153) or bimA1APC3 (PM156) or bimE7APC1 (SO4) were replica plated and incubated at permissive temperature (30°C) or semirestrictive temperature (35°C) for 3 d. Greater temperature sensitivity caused by bimA1APC3 when compared with bimE7APC1 is apparent.
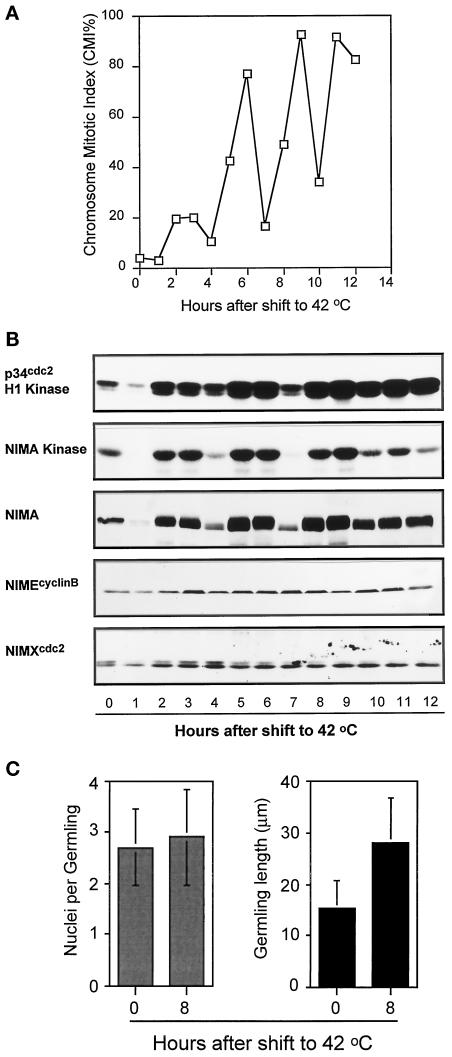
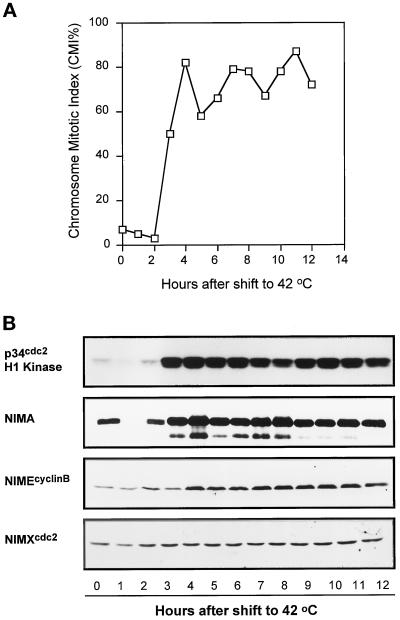
In an attempt to generate a more effective mitotic block with bimA1APC3, we transferred cells, after filtration from 32°C medium, directly into medium prewarmed to 42°C with the expectation that this may more immediately inactivate APC/C function. This shortened the period to attain restrictive temperature from several minutes, using our standard technique, to a matter of a second or so. To our surprise, the instant temperature shift induced multiple synchronized mitotic oscillations, of increasing magnitude, with four peaks of chromosome mitotic index (CMI) occurring over 12 h (Figure 2A). This was specific for the bimA1APC3 mutation, as such synchronized mitotic oscillations were absent in wild-type and bimE7APC1 mutant cells when treated identically (our unpublished results). When bimA1APC3 mutant cultures were shifted to 42°C by shaking in a 60°C water bath (our standard temperature shift regimen), no cell cycle synchrony was apparent, but instead the CMI increased slowly to ∼70% by 8 h after temperature shift.
Figure 2.
Mitotic oscillations induced by bimA1APC3 upon rapid temperature shift. (A) CMI of a bimA1APC3 mutant strain after rapid temperature shift. (B) Autoradiograms of p34cdc2 H1 and NIMA kinase activities and Western blot analysis of NIMA, NIMEcyclinB, and p34cdc2. (C) Nuclear division and germling growth of the nimA5 + bimA1APC3 mutant cells after rapid temperature upshift. Spores were first germinated at 32°C for 7.5 h, and, after harvesting by centrifugation, the germlings were rapidly transferred to new medium prewarmed to 42°C and incubated at 42°C for 8 h. The number of nuclei and germling length were measured before and 8 h after temperature shift.
As rapid temperature-shifted bimA1APC3 cultures underwent repeated rounds of chromosome condensation, we followed biochemically the abundance and activities of several mitotic regulators during these apparent cell cycle oscillations (Figure 2B). Both the activities of p34cdc2 and NIMA mitotic-promoting kinases oscillated in the same manner as the CMI values. When the CMI was low, the mitotic kinase activities were low, and when the CMI was high, so were the kinase activities, most noticeably for NIMA kinase activity (Figure 2B). The chromosome mitotic oscillations caused by rapid inactivation of bimA1APC3 are therefore clearly cell cycle oscillations driven by activation and inactivation of mitosis-promoting protein kinases. This conclusion was further ratified by the dramatic periodic accumulation and hyperphosphorylation, followed by degradation, of the NIMA protein revealed by Western blot analysis (Figures 2B and 4B, NIMA). No such oscillation in p34cdc2 abundance was detected, and only minor oscillations of NIMEcyclinB protein could be detected (Figure 2B).
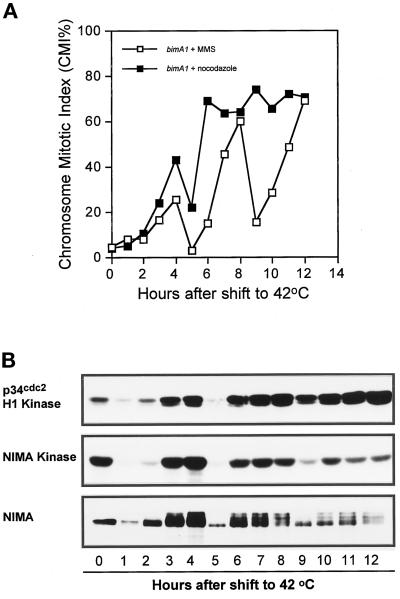
Figure 4.
Effects of addition of nocodazole and MMS on the bimA1APC3-induced mitotic oscillations. Nocodazole or MMS was added to bimA1APC3 mutant cells 4 h after temperature shift and remained in the culture during the duration of the experiment. (A) CMI. (B) Autoradiograms of p34cdc2 H1 and NIMA kinase activities and NIMA Western blot in the MMS-treated bimA1APC3 cells.
The oscillations in cell cycle parameters caused by rapid inactivation of bimA1APC3 did not lead to successful nuclear division. If relatively young cultures, which contained germlings having two to four nuclei per cell, were shifted rapidly to restrictive temperature, virtually no increase in nuclear number was observed (Figure 2C). The germlings did continue to grow, but they were unable to complete successful mitotic division over a period in which at least two divisions would be expected to have taken place. In addition, it should be noted that no obvious increase in DNA ploidy occurred, based on DAPI staining, during the bimA1-induced cell cycle oscillations. During incubation at 42°C for 13 h (e.g., Figure 2) the normal 1n ploidy of A. nidulans should have increased to 16n if DNA synthesis was occurring without division. However, no such large nuclei were observed during these experiments (our unpublished results).
To determine the role of NIMA in the cell cycle oscillations caused by inactivation of bimAAPC3, a double bimA1APC3 + nimA5 mutant strain was constructed and subjected to the rapid temperature shift protocol. In this double mutant no significant oscillation of p34cdc2 kinase activity was apparent, but instead p34cdc2 kinase activity dramatically increased 10-fold, and the chromosome mitotic index also increased to a high sustained level with only minor variations (Figure 3A). Additionally, no oscillations were observed in the level or phosphorylation state of NIMA or NIMEcyclinB; rather, these two proteins accumulated after the temperature shift (Figure 3B). These data indicate that active NIMA is required to generate the cell cycle oscillations observed after rapid inactivation of bimAAPC3. The data also indicate that rapid inactivation of bimAAPC3 can apparently override the G2 arrest caused by nimA5 in a manner reminiscent of how bimE7APC1 also overrides the nimA5 G2 arrest (Osmani et al., 1988, 1991b; James et al., 1995) (see below).
Figure 3.
nimA dependence of the mitotic oscillations induced by bimA1APC3. (A) CMI of a nimA5 + bimA1 double mutant strain after rapid temperature shift. (B) Autoradiogram of p34cdc2 H1 kinase activity and Western blots of NIMA, NIMEcyclinB, and p34cdc2 after rapid temperature shift.
The mitotic oscillations promoted by rapid temperature shift of bimA1APC3-containing cells were also tested for sensitivity to checkpoint control mechanisms. Mitotic oscillations were instigated by rapid temperature shift to 42°C. After 4 h incubation, cells were tested for the DNA damage checkpoint by using methyl methanesulfonate (MMS) and were also treated with the microtubule poison nocodazole, which is known to generate a pseudomitotic arrest. No effect was observed on the oscillations of either the CMI or NIMA or p34cdc2 kinase activities after treatment with MMS (Figure 4, A and B), whereas, as noted previously, the wild-type delays the cell cycle after sustaining DNA damage by inactivating p34cdc2 (Ye et al., 1997b). Again the oscillations observed after MMS treatment were particularly apparent for the abundance and phosphorylation state of NIMA. In contrast, addition of nocodazole largely suppressed the cell cycle oscillations, and these cells arrested with high CMI values (Figure 4A) and also high levels of NIMA, NIMEcyclinB, and NIMA and p34cdc2 kinase activities (our unpublished results). These results suggest that, after rapid temperature shift, the bimA1APC3 mutant cells have lost the DNA damage checkpoint but still have an intact spindle assembly checkpoint. The ability of nocodazole to trap bimA1APC3 cells in mitosis also confirms that this mutation causes a reversible delay to mitotic progression and not an arrest in mitosis.
NIMA and NIMEcyclinB Are Stabilized in Cells with Inactive bimEAPC1 and bimAAPC3
Previous work has demonstrated that NIMA protein abundance is regulated in a cell cycle-dependent manner, being low during S phase and maximal at mitosis, similar to cyclin B (Ye et al., 1995). Additionally, degradation of NIMA, also like cyclin B, is required for exit from mitosis (O’Connell et al., 1994; Pu and Osmani, 1995), and the APC/C is known to regulate the stability of other mitotically unstable proteins (for review see Cohen-Fix and Koshland, 1997; Townsley and Ruderman, 1998). These observations, and the dramatic oscillations in NIMA abundance caused by rapid inactivation of the APC/C subunit bimAAPC3 (Figures 2B and 4B), prompted us to investigate the potential role of the APC/C in the regulation of NIMA stability.
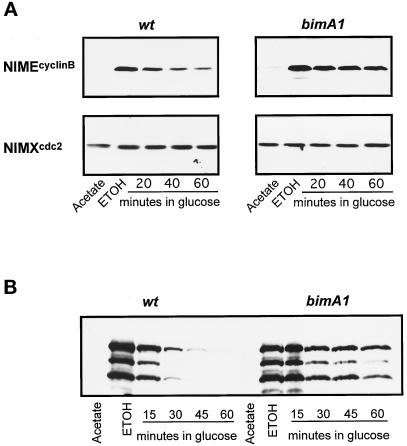
To directly assess the role of the APC/C in NIMA degradation, we measured the stability of NIMA expressed from the heterologous alcA promoter in S phase-arrested cells, as we have shown previously that NIMA is very unstable during S phase arrest (Ye et al., 1995; Ye et al., 1996). The stability of NIMEcyclinB when expressed from the alcA promoter was similarly determined. Three conditions were used: stability with normal APC/C function, stability with bimAAPC3 inactivated, and stability with bimEAPC1 inactivated. As shown in Figure 5, A and B, in the presence of APC/C function both NIMEcyclinB and NIMA are unstable in the hydroxyurea (HU)-arrested S phase cells with a half-life of <20 min. In contrast, both NIMEcyclinB and NIMA are greatly stabilized when the APC/C subunit BIMAAPC3 was inactive, and their half lives were increased to >60 min. Similar results were obtained when the BIMEAPC1 APC/C component was inactivated and both NIMEcyclinB and NIMA proteins were both highly stabilized (our unpublished results). These results provide strong biochemical evidence for a role of the APC/C in NIMA degradation at a point in the cell cycle where NIMA is known to be unstable.
Figure 5.
APC/C-dependent stabilization of NIMEcyclinB and NIMA. (A) Cells of the wild-type (wt = R153) and bimA1APC3 cells containing a copy of alcA-driven nimEcyclinB were grown at 32°C to early log phase in acetate media (repressing for alcA). HU (100 mM) was added to the cells for 2 h to cause S phase arrest before rapid transfer to inducing medium (ethanol) also containing 100 mM HU but at 42°C. One hour after transfer to inducing medium at 42°C, glucose was added to repress expression from the alcA promoter. The abundance of NIMEcyclinB was determined by Western blotting. The same blots were also subsequently detected for p34cdc2 as loading control. (B) The experimental procedures for NIMA induction and repression in HU-arrested cells were as described in A by using a strain with alcA inducible nimA. NIMA was detected by Western blot after immunoprecipitation. No general proteolysis was observed in the samples from which NIMA was isolated, and the level of p34cdc2 was found by Western blotting to be constant. However, in addition to full-length NIMA (top band) numerous smaller NIMA degradation products were observed.
Mutations of the APC/C Override the nimA5 G2 Arrest by Elevating p34cdc2 H1 Kinase Activity and Making nimA5 Leaky
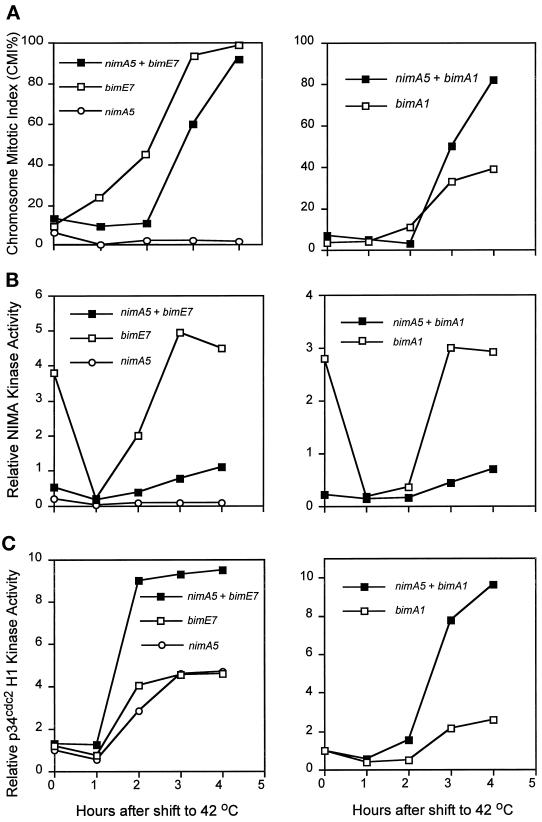
The nimA5 mutation arrests cells in G2 at restrictive temperature even though p34cdc2 kinase activity increases to mitotic levels (Osmani et al., 1991a; Ye et al., 1995). Our current data (Figure 3A) and previous studies (Osmani et al., 1991b; James et al., 1995) indicate that mutation of the APC/C allows cells to bypass the nimA5 G2 arrest and enter mitosis, albeit a defective mitosis (Osmani et al., 1991b). To further understand this phenomenon we have carried out two types of experiment. First, biochemical analysis was completed to see whether mitosis was occurring without NIMA kinase activity in APC− + nimA5 double mutant strains. Second, nimA was deleted from bimE7APC1 cells to see whether an APC/C mutation could override complete lack of NIMA function.
Strains containing single nimA5, bimA1APC3, or bimE7APC1 mutations, and those containing nimA5 + bimA1APC3 and nimA5 + bimE7APC1 were subjected to temperature shift and analyzed for CMI, NIMA, and p34cdc2 H1 kinase activities (Figure 6). Two features of the analysis are particularly interesting. First, it is clear that both the bimA1APC3 and bimE7APC1 mutations allow some activation of the mutant NIMA kinase to a level higher than that observed in the single nimA5 mutant strain (Figure 6B). This partial activation could contribute to the defective mitosis seen in bimE7APC1 + nimA5 mutant strains. Second, dramatic activation of the p34cdc2 H1 kinase was promoted in both bimE7APC1 + nimA5 and bimA1APC3 + nimA5 strains to a level much higher than that observed in normal mitosis and greater than that recorded in cells blocked in mitosis by the bimE7APC1 mutation or at the restrictive temperature for bimA1APC3 or nimA5 (Figure 6C). Such high activation of p34cdc2 H1 kinase is likely to play a role in the ability of bimE7APC1 and bimA1APC3 mutations to override the nimA5 G2 arrest (Figure 6A).
Figure 6.
APC/C mutations override the nimA5 G2 arrest with very high p34cdc2 H1 kinase activity. (A) Mutant cells, as indicated, were grown to early log phase at 32°C and shifted to 42°C at time 0. At the times indicated the CMI was determined after DAPI staining and fluorescent microscopy. p34cdc2 H1 (B) and NIMA kinase (C) activities were assayed after immunoprecipitation with specific antibodies.
Deletion of nimA Prevents an APC/C Mutant from Entering Mitosis
To see whether some NIMA activity is required to allow mitotic events when bimEAPC1 is inactivated, we removed nimA by targeted deletion in both a wild-type strain and a bimE7APC1 strain. A linear DNA deletion strategy was used (Figure 7A) in which nimA was replaced with the pyr4 nutritional marker from Neurospora crasser, which encodes orotidine-5′-phosphate decarboxylase and complements the pyrG89 mutation in A. nidulans. The deleted allele in the homokaryotic state is lethal (see below) and was therefore maintained in heterokaryons as previously described (Osmani et al., 1988; Oakley and Osmani, 1993).
After transformation with the linear deletion construct and preliminary screening of transformants for heterokaryons (see MATERIALS AND METHODS), Southern blot analysis identified several heterokaryons containing both a wild-type allele and a clean deleted allele (Figure 7, A and B) termed nimAΔ. It was noticeable that all deleted heterokaryons contained much lower levels of the deleted allele. This is most likely due to selection for the wild-type allele, as NIMA is expressed at a low level, is cell cycle regulated, and is very unstable. It is therefore likely that a 50:50 ratio of nimA to nimAΔ nuclei in a heterokaryon would not supply enough NIMA to enable normal cell cycle progression within that heterokaryon. This therefore leads to the selection for high ratios of the wild-type allele.
The ability to maintain nuclei containing a deletion of an essential gene in the heterokaryotic state in A. nidulans allows the analysis of the null phenotype because the heterokaryotic state is broken when conidia are formed, as they are uninucleate (Osmani et al., 1988). Phenotypic analysis of germinated conidia derived from heterokaryons having a clean deletion of nimA identified a low percentage of conidia (∼3.0%) that germinated to produce polarized germlings with a single interphase nucleus (Figure 7C), which is the classic nim (never in mitosis) phenotype of A. nidulans. These cells clearly contain the deleted allele, as they are pyr4+. They also showed the expected phenotype for deletion of an essential cell cycle gene. All other conidia from the heterokaryon are parental pyrG89-containing cells because they did not form germlings on media lacking uridine and uracil. The low recovery of the nimAΔ allele in conidia from the heterokaryons confirms the Southern blot analysis, indicating that there is selection for the wild-type allele in these heterokaryons. We conclude that the nimA5 phenotype is very similar to the nimAΔ null allele and that nimA is an essential gene.
Phenotypic analysis of conidia derived from a heterokaryon containing a clean deletion of nimA in a bimE7APC1 background was used to determine whether inactivation of bimEAPC1 could cause mitosis in the absence of nimA. Conidia were plated on media lacking uridine and uracil and were germinated at 32°C for a period to allow identification of those containing the nimAΔ allele. After temperature shift to 42°C to inactivate BIMEAPC1, DAPI staining revealed that all the nimAΔ + bimE7APC1 cells (>150) remained arrested in interphase (Figure 7D). The experiment was repeated by germinating directly at 42°C, but the nimAΔ allele still prevented bimE7APC1 from promoting cells into mitosis. It is therefore necessary to have some NIMA activity or cells remain arrested before mitosis when the APC/C is inactivated by bimE7APC1.
DISCUSSION
The abundance of the NIMA kinase is regulated through the cell cycle, and its degradation is required for exit from mitosis (Osmani and Ye, 1996). Mitotic degradation of other regulatory proteins (Cohen-Fix et al., 1996; Funabiki et al., 1996; Juang et al., 1997; Michaelis et al., 1997; Charles et al., 1998; Shirayama et al., 1998) is also required for normal exit from mitosis, and these proteins are targeted by the APC/C for degradation. In the current work we provide evidence that BIMAAPC3 plays a regulatory role in APC/C function and show that NIMA kinase is likely a target of the APC/C.
Although the bimA1APC3 allele causes tight temperature sensitivity, it does not cause an irreversible arrest in mitosis, which would be expected for a mutation causing inactivation of the APC/C at restrictive temperature. Remarkably, we demonstrate that inactivation of bimA1APC3 instead induces repeating rounds of mitotic events characterized by chromosome condensation and decondensation, activation and inactivation of the p34cdc2 and NIMA protein kinases, accumulation and degradation of NIMA, and cyclic rounds of phosphorylation of NIMA. The tight correlation observed between these events strongly indicates that rapid inactivation of bimA1APC3 promotes cyclic activation and inactivation of the mitosis-promoting kinases, which then drive nuclei in and out of a mitotic state.
How does inactivation of an APC/C subunit cause cell cycle oscillations of the mitosis-promoting kinases? Some insight into this phenomenon is provided by data indicating that NIMA could be a target of the APC/C.
It is known that inhibition of DNA synthesis by addition of hydroxyurea to dividing A. nidulans cells causes S phase arrest and disappearance of NIMA protein (Ye et al., 1996). By expression of nimA from an inducible promoter after S phase arrest, we have shown that, like NIMEcyclinB, NIMA is rendered unstable in an APC/C-dependent manner. We have no direct evidence that this involves the E3 ubiquitin ligase activity of the APC/C, but this is an obvious possibility.
We have also found that the APC/C plays a role in NIMA stability at G2 because the previously reported ability of the bimE7APC1 mutation to override the nimA5 G2 arrest (Osmani et al., 1988) was found to be shared with the bimA1APC3 mutation. Thus mutations of subunits of the APC/C allow entry into mitosis when NIMA is defective. Biochemical analysis was used to reveal the molecular basis for this effect. First, the kinase activity of p34cdc2 is greatly increased in nimA5 + bimE7APC1 and nimA5 + bimA1APC3 double mutant strains to a level much higher than that found during normal mitosis. This increased level of p34cdc2 activity is at least in part because NIMEcyclinB accumulates to higher levels in these double mutants. Increases in NIMEcyclinB at the G2 arrest point of nimA5 can cause immediate elevation of p34cdc2 H1 kinase activity, as p34cdc2 is not subjected to inhibitory tyrosine phosphorylation at this point in the cell cycle (Osmani et al., 1991a). Second, inactivation of bimAAPC3 or bimEAPC1 renders the nimA5 mutation slightly leaky, giving rise to marginally elevated levels of NIMA kinase activity. This is, at least in part, due to an accumulation of NIMA protein to higher levels but could also be caused by the marked elevation of p34cdc2 H1 kinase activity observed in these strains. The data indicate that at G2 NIMA and NIMEcyclinB proteins are held in check via an APC/C-dependent mechanism. Therefore, APC/C mutations make the nimA5 mutation leaky and also superinduce p34cdc2 H1 kinase activity to promote defective mitotic events. Mutation of the APC/C cannot, however, override complete loss of NIMA function, as deletion of nimA arrests cells in G2 even after inactivation of bimE7APC1.
While this work was in preparation, it has been reported that promoter rundown of nimA, which causes a G2 arrest, can be overridden by inactivation of bimA1APC3 in cells containing “insignificant levels” of NIMA kinase. This experiment has been interpreted to mean that APC/C mutations can promote mitotic events in the complete absence of NIMA function (Lies et al., 1998). This apparent discrepancy with our data may be due to the different experimental approaches used. However, deletion of nimA from the genome generates a true null phenotype. Additionally, the insignificant levels of NIMA kinase reported (Lies et al., 1998) may be significant, because when NIMA and the APC/C are inhibited, p34cdc2 H1 kinase levels are induced to higher than mitotic levels (Figure 6). Additionally, as NIMA is likely a substrate of the APC/C (see below), promoter rundown of NIMA is probably less effective when the turnover rate of NIMA is reduced by inactivation of the APC/C.
The fact that mutation of APC/C subunits causes elevation of NIMA at the nimA5 arrest point and also renders NIMA more stable during S-phase arrest suggests that NIMA requires the APC/C for instability during interphase and indicates that NIMA is a substrate of the APC/C. In further support of this contention, we note that NIMA is degraded during mitosis and that it is stabilized when the spindle assembly checkpoint is engaged, which is thought to be imposed by inhibition of the APC/C (Osmani and Ye, 1996). Additionally, we have previously shown that forced expression of NIMA enhances the bimEAPC1 mutation, which is also consistent with NIMA being a substrate of the APC/C (Ye et al., 1996). We now show that the bimA1APC3 mutation causes amazing oscillations in the abundance of NIMA protein, indicating that this APC/C mutation promotes repeated rounds of inactivation and then activation of the APC/C toward NIMA. With these observations in mind we propose the following to help explain the oscillatory effects caused by bimA1APC3.
The bimA1APC3 mutation may uncouple an oscillatory clock mechanism that controls the APC/C and thus cell cycle progression. By rapid inactivation of bimA1APC3, perhaps this regulatory clock is reset to a base level. By restarting the clock from the same regulatory point in the cell cycle, all cells would then proceed through the cell cycle in a synchronous manner, resulting in the observed cell cycle-specific changes we documented regarding chromosome condensation, kinase activities, and protein abundance.
Second, to help explain why multiple oscillations occur, we suggest that the bimA1APC3 mutation makes the APC/C partially resistant to activation by its substrates, such as cyclin B, Polo, and NIMA, during mitosis. This would cause cells to delay in mitosis and accumulate APC/C substrates until a critical threshold level could accumulate and eventually activate the partially defective APC/C. After APC/C activation, cyclin B, Polo, and NIMA would be degraded and allow exit from mitosis. In this manner an extended mitotic delay would be imposed followed by progression through interphase into another mitotic delay until the defective APC/C could be activated again after accumulation of substrates, and so on. In this manner multiple cell cycle oscillations could be generated between interphase and mitotic states.
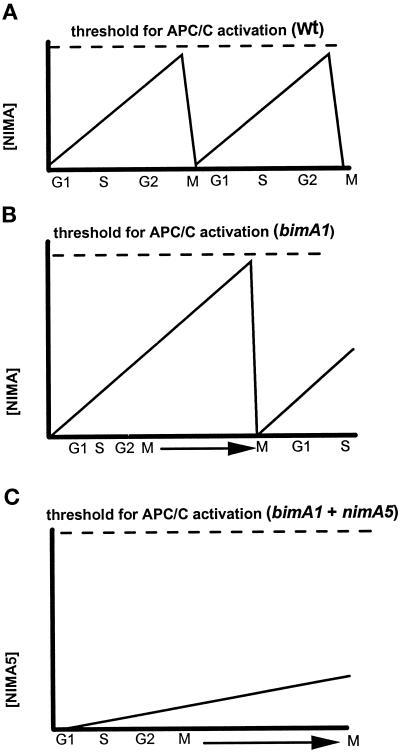
We have shown that NIMA is likely a substrate of the APC/C and that its level and activity oscillate widely after rapid inactivation of bimA1APC3. For this reason it is possible that NIMA accumulation plays a key role in the activation of the APC/C seen after rapid inactivation of bimA1APC3 (Figure 8). This possibility is further supported by the fact that in a nimA5 + bimA1APC3 double mutant the APC/C is not activated, and cells remain in an extended mitotic state with elevated levels of both NIMA and NIMEcyclinB. Thus, nimA is required for the activation of the APC/C after inhibition of bimAAPC3. This then explains why the nimA5 + bimA1APC3 double mutant arrests in mitosis but the single bimA1APC3 mutant oscillates in and out of mitosis. In bimA1APC3 strains, NIMA protein could accumulate with normal activity and help activate the APC/C to allow degradation of mitotic substrates and promote exit from mitosis (Figure 8B). By contrast, in nimA5 + bimA1APC3 strains, although NIMA5 protein accumulates and has enough activity to allow cells into mitosis, it does not properly activate the APC/C, thus causing the extended mitotic arrest observed (Figure 8C).
Figure 8.
Relationship between NIMA and BIMA1APC3. To help explain why the nimA5 mutation in combination with bimA1APC3 causes an effective mitotic arrest phenotype, whereas the bimA1APC3 mutation generates repeating rounds of mitotic oscillation, we propose the following. (A) Substrates of the APC/C, such as cyclin B, Polo-like kinases, and NIMA, accumulate to a threshold during mitosis, which activates the APC/C to trigger their demise and also to exit from mitosis. (B) The bimA1APC3 mutation increases the threshold of activation of the APC/C. This results in a mitotic delay during which NIMA protein and activity accumulate. When NIMA activity reaches the higher threshold level of activation, the APC/C is activated, and cells can exit mitosis. (C) In the bimA1 + nimA5 double mutant, although p34cdc2 H1 kinase activity and NIMA5 activity are increased to allow entry into mitosis, the level of NIMA5 activity stays below that required to activate the APC/C. This then causes an extended mitotic arrest, because cells cannot exit mitosis.
Of course it is possible that both p34cdc2/cyclin B and NIMA are involved in the activation of the APC/C at mitosis, along with other regulators such as Polo-like kinases (Charles et al., 1998; Descombes and Nigg, 1998; Shirayama et al., 1998). The important point is that bimAAPC3 apparently plays a regulatory role in APC/C function. We propose that bimAAPC3 could be part of a cell cycle clock mechanism and, additionally, that it is required for normal activation of the APC/C at mitosis in response to accumulation and activation of APC/C substrates during mitosis.
Finally, it should be noted that the cyclic oscillations of cell cycle events caused by inactivation of bimA1APC3 are unusual in several respects. Most noticeably they are not successful in dividing nuclei, despite repeated rounds of activation and inactivation of p34cdc2 and NIMA. Perhaps some APC/C substrates, such as Pds1 (Cohen-Fix et al., 1996), Cut2 (Funabiki et al., 1996), Scc1 (Michaelis et al., 1997), and Ase1 (Juang et al., 1997), that need to be removed to allow normal progression out of mitosis are not destroyed when bimAAPC3 is inhibited. The cell cycle oscillations are also not responsive to the DNA damage checkpoint. They are, however, responsive to the spindle assembly checkpoint activated by microtubule depolymerization. Because the bimA1APC3-induced cyclic oscillations are arrested at mitosis after microtubule depolymerization, it is indicated that the APC/C is still inhibited by Mad2p, which mediates this checkpoint arrest (He et al., 1997; Li et al., 1997).
Our data therefore indicate that bimAAPC3 inactivation prevents degradation of anaphase substrates of the APC/C while allowing normal regulation of the APC/C during the spindle checkpoint. This suggests that the regulation of the APC/C is disturbed in a relatively specific manner by the bimA1APC3 mutation. Perhaps the cyclic rounds of chromosome condensation and decondensation promoted by bimA1APC3 inactivation are driven primarily by cycles of NIMA activity, with most other cell cycle-specific events remaining unchanged. This could occur if the bimA1APC3 mutation interferes with the activation of the APC/C toward NIMA but not toward substrates required for anaphase progression and passage through other phases of the cell cycle. Future experiments will be aimed at validating our proposed regulatory role for bimAAPC3, which should further our understanding of the regulation of the APC/C, particularly with regard to its substrate specificity during cell cycle progression and checkpoint control.
ACKNOWLEDGMENTS
We thank the members of our laboratory for constructive suggestions on this work and Drs. Peter Mirabito and Matthew O’Connell for A. nidulans strains. This work was supported in part by a grant from the National Institutes of Health (GM-42564) to S.A.O.
REFERENCES
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body populations of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Amon A, Irniger S, Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO. The polo-related kinase cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Koshland D. The metaphase-to-anaphase transition: avoiding a mid-life crisis. Curr Opin Cell Biol. 1997;9:800–806. doi: 10.1016/s0955-0674(97)80080-4. [DOI] [PubMed] [Google Scholar]
- Cohen-Fix O, Peters J, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes & Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1990;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Engle DB, Osmani SA, Osmani AH, Rosborough S, Xiang X, Morris NR. A negative regulator of mitosis in Aspergillus is a putative membrane-spanning protein. J Biol Chem. 1990;265:16132–16137. [PubMed] [Google Scholar]
- Evans TE, Rosenthal J, Youngbloom K, Distel K, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Gallant P, Nigg EA. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992;117:213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara JB, Richardson HE, Sugimoto K, Henze M, Lew DJ, Wittenberg C, Reed SI. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991;65:163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Grandin N, Reed SI. Differential function and expression of Saccharomyces cerevisiae B-type cyclins in mitosis and meiosis. Mol Cell Biol. 1993;13:2113–2125. doi: 10.1128/mcb.13.4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Patterson TE, Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc Natl Acad Sci USA. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A. Roles of ubiquitin-medicated proteolysis in cell cycle control. Curr Opin Cell Biol. 1997;9:788–800. doi: 10.1016/s0955-0674(97)80079-8. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ganoth D, Sudakin V, Dahan A, Cohen LH, Luca FC, Ruderman J. Components of a system that ligates cyclin to ubiquitin and their regulation by protein kinase cdc2. J Biol Chem. 1994;269:4940–4946. [PubMed] [Google Scholar]
- Hirano T, Hiraoka Y, Yanagida M. A temperature sensitive mutation of the S. pombe gene nuc2+ that encodes a nuclear-scaffold protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1990;106:307–317. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Kinoshita N, Morikawa K, Yanagida M. Snap helix with a knob and hole essential repeats on S. pombe nuclear protein nuc2+ Cell. 1994;60:319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116:707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Mirabito P, Scacheri P, Morris N. The Aspergillus nidulans bimE (blocked-in-mitosis) gene encodes multiple cell cycle functions involved in mitotic checkpoint control and mitosis. J Cell Sci. 1995;108:3485–3499. doi: 10.1242/jcs.108.11.3485. [DOI] [PubMed] [Google Scholar]
- Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996a;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- King RW, Glotzer M, Kirschner MW. Mutagenic analysis of the destruction signal of mitotic cyclins and the structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996b;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Lahav-Baratz S, Sudakin V, Ruderman JV, Hershko A. Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc Natl Acad Sci USA. 1995;92:9303–9307. doi: 10.1073/pnas.92.20.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JR, Michaud WA, Sikorski RS, Hieter PA. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994;13:4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorbea C, Mahaffey D, Rechsteiner M, Benezra R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc Natl Acad Sci USA. 1997;94:12431–12436. doi: 10.1073/pnas.94.23.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies CC, Cheng J, James SW, Morris NR, O’Connell MJ, Mirabito PM. BIMAAPC3, a component of the Aspergillus anaphase promoting complex/cyclosome, is required for a G2 checkpoint blocking entry into mitosis in the absence of NIMA function. J Cell Sci. 1998;111:1453–1465. doi: 10.1242/jcs.111.10.1453. [DOI] [PubMed] [Google Scholar]
- Luca FC, Shibuya EK, Dohrmann CE, Ruderman JV. Both cyclin A Δ60 and B Δ97 are stable and arrest cells in M-phase, but only cyclin B Δ97 turns on cyclin destruction. EMBO J. 1991;10:4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K. Cohesins:chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Mirabito PM, Morris NR. BIMA, a TPR-containing protein required for mitosis, localizes to the spindle pole body in Aspergillus nidulans. J Cell Biol. 1993;120:959–968. doi: 10.1083/jcb.120.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NR. Mitotic mutants of Aspergillus nidulans. Genet Res. 1976;26:237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Murray A. Cyclin ubiquitination: the destructive and of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Norbury C, Nurse P. Premature chromatin condensation upon accumulation of NIMA. EMBO J. 1994;13:4926–4937. doi: 10.1002/j.1460-2075.1994.tb06820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KL, Osmani AH, Osmani SA, Morris NR. bimA encodes a member of the tetratricopeptide repeat family of proteins and is required for the completion of mitosis in Aspergillus nidulans. J Cell Sci. 1991;99:711–719. doi: 10.1242/jcs.99.4.711. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Osmani SA. Cell-cycle analysis using the filamentous fungus Aspergillus nidulans. In: Fantes P, Brooks R, editors. The Cell Cycle, a Practical Approach. Oxford, United Kingdom: IRL Press; 1993. pp. 127–142. [Google Scholar]
- Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell. 1991a;67:283–291. doi: 10.1016/0092-8674(91)90180-7. [DOI] [PubMed] [Google Scholar]
- Osmani AH, O’Donnell K, Pu RT, Osmani SA. Activation of the nimA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the bimE checkpoint. EMBO J. 1991b;10:2669–2679. doi: 10.1002/j.1460-2075.1991.tb07810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani SA, Engle DB, Doonan JH, Morris NR. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988;52:241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Osmani SA, Ye XS. Cell cycle regulation in Aspergillus by two protein kinases. Biochem J. 1996;317:633–641. doi: 10.1042/bj3170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, King RW, Hoog C, Kirschner MW. Identification of BIME as a subunit of the anaphase-promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- Pu RT, Osmani SA. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit. EMBO J. 1995;14:995–1003. doi: 10.1002/j.1460-2075.1995.tb07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Lew DJ, Henze M, Sugimoto K, Reed SI. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes & Dev. 1992;6:2021–2034. doi: 10.1101/gad.6.11.2021. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Starborg M, Brundell E, Gell K, Hoog C. A novel murine gene encoding a 216-kDa protein is related to a mitotic checkpoint regulator previously identified in Aspergillus nidulans. J Biol Chem. 1994;269:24133–24137. [PubMed] [Google Scholar]
- Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–198. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Ruderman JV. Proteolytic ratchets that control progression through mitosis. Trends Cell Biol. 1998;8:238–244. doi: 10.1016/s0962-8924(98)01268-9. [DOI] [PubMed] [Google Scholar]
- Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Ye XS, Fincher RR, Tang A, McNeal KK, Gygax SE, Wexler AN, Ryan KB, James S, Osmani SA. Proteolysis and tyrosine phosphorylation of p34cdc2/cyclin B: the role of MCM2 and initiation of DNA replication to allow tyrosine phosphorylation of p34cdc2. J Biol Chem. 1997a;272:33384–33393. doi: 10.1074/jbc.272.52.33384. [DOI] [PubMed] [Google Scholar]
- Ye XS, Fincher RR, Tang A, O’Donnell K, Osmani SA. Two S-phase checkpoint systems, one involving the function of both BIME and Tyr15 phosphorylation of p34cdc2, inhibit NIMA and prevent premature mitosis. EMBO J. 1996;15:3599–3610. [PMC free article] [PubMed] [Google Scholar]
- Ye XS, Fincher RR, Tang A, Osmani SA. The G2/M DNA damage checkpoint inhibits mitosis through Tyr15 phosphorylation of p34cdc2 in Aspergillus nidulans. EMBO J. 1997b;15:101–112. doi: 10.1093/emboj/16.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XS, Xu G, Pu PT, Fincher RR, McGuire SL, Osmani AH, Osmani SA. The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: coordination of two mitosis promoting kinases. EMBO J. 1995;14:986–994. doi: 10.1002/j.1460-2075.1995.tb07079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K. TPR proteins required for anaphase progression mediate ubiquitination of mitotic B-type cyclins in yeast. Mol Biol Cell. 1996;7:791–801. doi: 10.1091/mbc.7.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Shin TH, Galova M, Obermaier B, Nasmyth K. Identification of subunits of the anaphase-promoting complex of Saccharomyces cerevisiae. Science. 1996;274:1201–1204. doi: 10.1126/science.274.5290.1201. [DOI] [PubMed] [Google Scholar]