Abstract
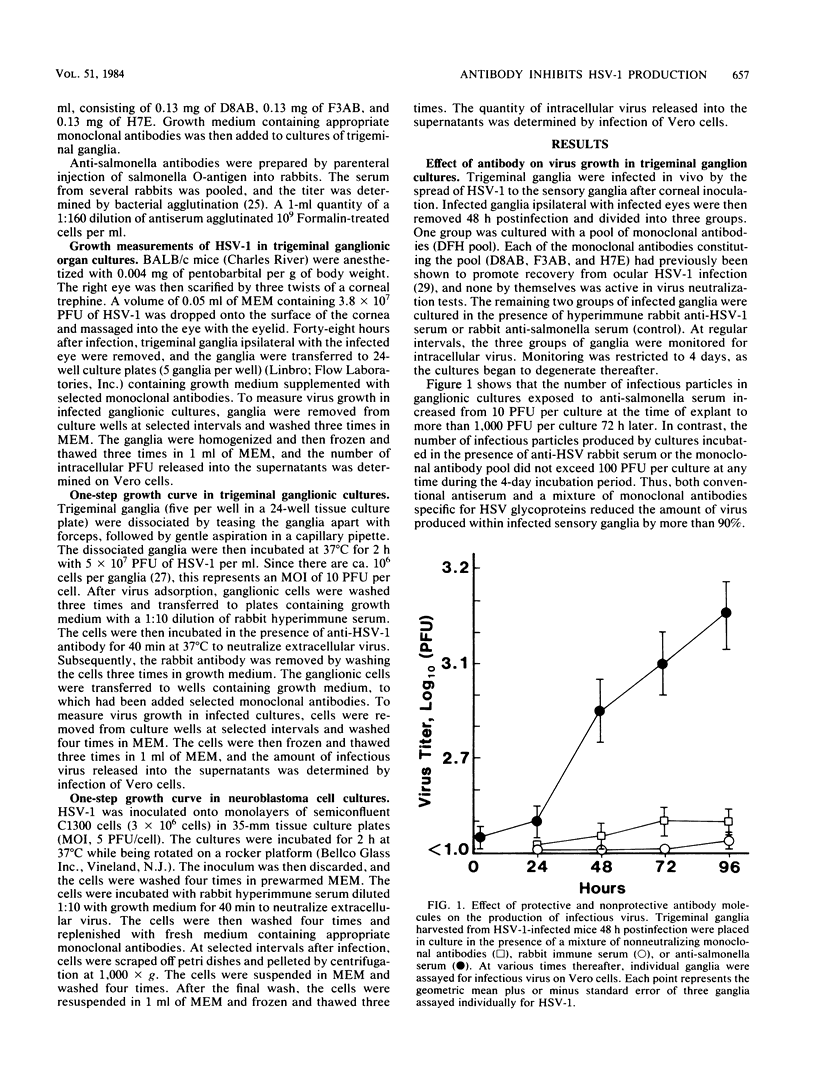
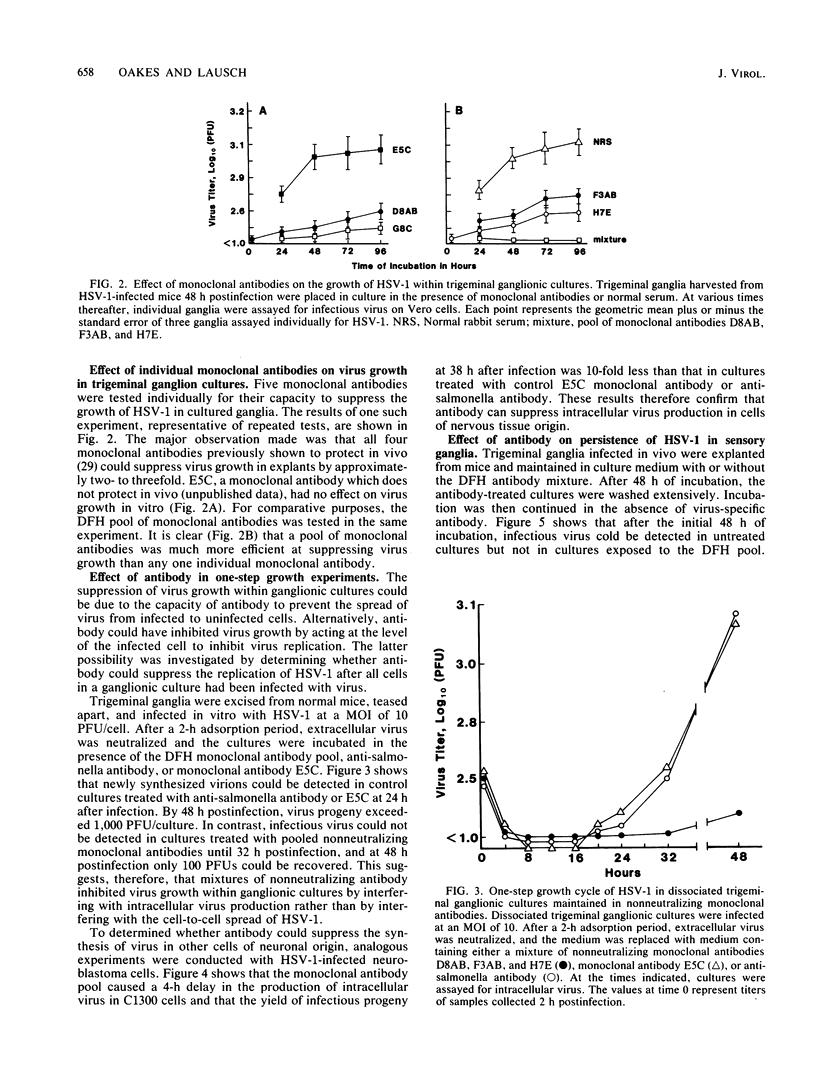
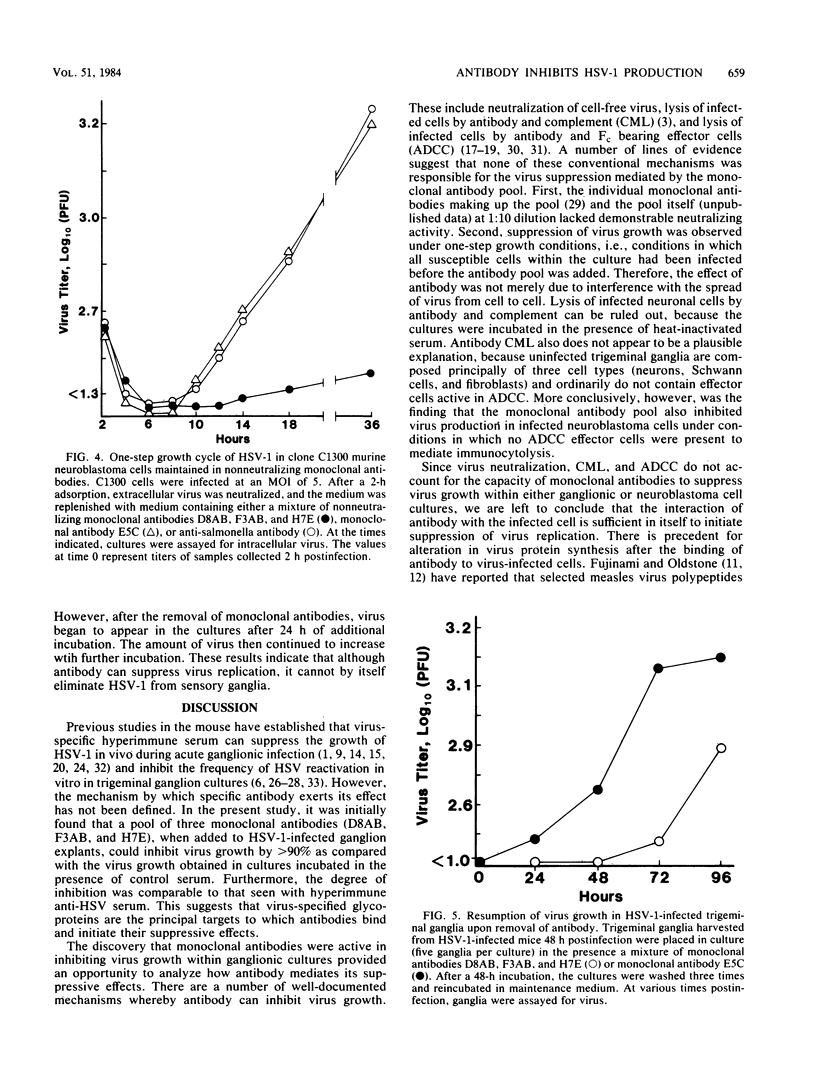
The effect of monoclonal antibodies on the growth of herpes simplex virus type 1 in trigeminal ganglia was investigated. Four-week-old mice were infected on an abrased cornea with herpes simplex virus type 1. Forty-eight hours after infection, trigeminal ganglia ipsilateral with infected eyes were removed and placed in culture. Incubation of infected ganglia in the presence of a pool of nonneutralizing monoclonal antibodies specific for glycoproteins of gB and gE suppressed virus growth by greater than 90%. This was comparable to the amount of suppression observed when infected ganglia were incubated in hyperimmune serum. Individual monoclonal antibodies were less efficient, being able to inhibit virus growth by only two- to threefold. The mechanism of suppression was examined. Reduction in virus growth was observed under conditions in which all susceptible ganglion cells were infected in vitro before nonneutralizing monoclonal antibody was added. Similar results were obtained in tests with virus-infected neuroblastoma cells. Furthermore, suppression of infectious progeny was seen in the absence of complement and immunologically reactive cells. Thus, neither virus neutralization nor immunocytolysis could account for the effects of antibody on virus growth. Rather, the data suggest that antibody can bind to herpes simplex virus type 1-infected neuronal cells and suppress intracellular virus replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal E., Puricelli L., de la Peña N. C., de Lustig E. S. Influence of human leukocyte interferon on IgG on the replication of herpes simplex virus in nervous tissue in vitro. Acta Virol. 1979 Nov;23(6):461–467. [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Costa J., Rabson A. S., Yee C., Tralka T. S. Immunoglobulin binding to herpes virus-induced Fc receptors inhibits virus growth. Nature. 1977 Sep 15;269(5625):251–252. doi: 10.1038/269251a0. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecob-Johnston M. S., Whetsell W. O., Jr Host-cell response to herpes virus infection in central and peripheral nervous tissue in vitro. J Gen Virol. 1979 Sep;44(3):747–757. doi: 10.1099/0022-1317-44-3-747. [DOI] [PubMed] [Google Scholar]
- Engler H., Zawatzky R., Goldbach A., Schröder C. H., Weyand C., Hämmerling G. J., Kirchner H. Experimental infection of inbred mice with herpes simplex virus. II. Interferon production and activation of natural killer cells in the peritoneal exudate. J Gen Virol. 1981 Jul;55(Pt 1):25–30. doi: 10.1099/0022-1317-55-1-25. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 Jul;125(1):78–85. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Kino Y., Hayashi Y., Hayashida I., Mori R. Dissemination of herpes simplex virus in nude mice after intracutaneous inoculation and effect of antibody on the course of infection. J Gen Virol. 1982 Dec;63(2):475–479. doi: 10.1099/0022-1317-63-2-475. [DOI] [PubMed] [Google Scholar]
- Klein R. J. Effect of immune serum on the establishment of herpes simplex virus infection in trigeminal ganglia of hairless mice. J Gen Virol. 1980 Aug;49(2):401–405. doi: 10.1099/0022-1317-49-2-401. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Kohl S., Cahall D. L., Walters D. L., Schaffner V. E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J Immunol. 1979 Jul;123(1):25–30. [PubMed] [Google Scholar]
- Kohl S., Loo L. S. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J Immunol. 1982 Jul;129(1):370–376. [PubMed] [Google Scholar]
- Kristensson K., Vahlne A., Persson L. A., Lycke E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J Neurol Sci. 1978 Feb;35(2-3):331–340. doi: 10.1016/0022-510x(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Larsen H. S., Russell R. G., Rouse B. T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983 Jul;41(1):197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Davis W. B., Taylor J. A., Weppner W. A. Lymphocyte reactivity contributes to protection conferred by specific antibody passively transferred to herpes simplex virus-infected mice. Infect Immun. 1980 Aug;29(2):642–649. doi: 10.1128/iai.29.2.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw H., Asher L. V., Wohlenberg C., Sekizawa T., Notkins A. L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979 Jul;44(1):205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Openshaw H., Asher L. V., Wohlenberg C., Sekizawa T., Notkins A. L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979 Jul;44(1):205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Openshaw H., Puga A., Notkins A. L. Herpes simplex virus infection in sensory ganglia: immune control, latency, and reactivation. Fed Proc. 1979 Dec;38(13):2660–2664. [PubMed] [Google Scholar]
- Rajcáni J., Ciampor F., Sabó A., Líbiková H., Rosenbergová M. Activation of latent herpesvirus hominis in explants of rabbit trigeminal ganglia: the influence of immune serum. Arch Virol. 1977;53(1-2):55–69. doi: 10.1007/BF01314847. [DOI] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Walz M. A., Yamamoto H., Notkins A. L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976 Dec 9;264(5586):554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]


