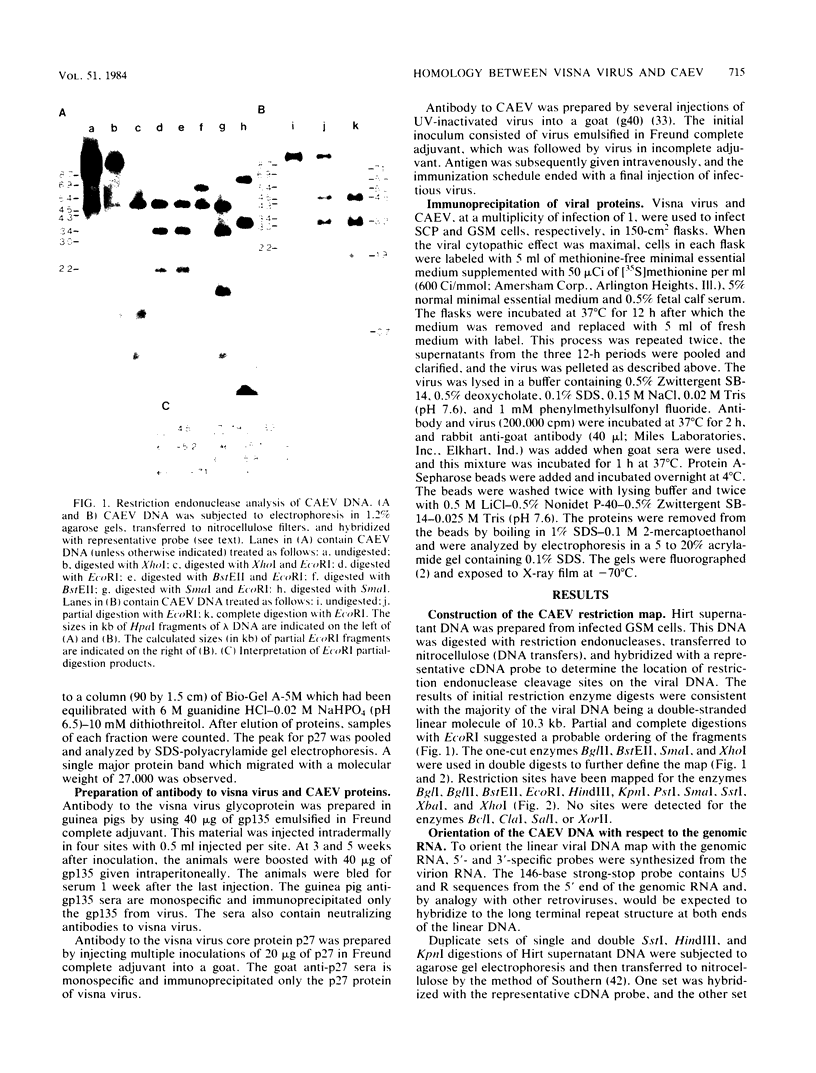
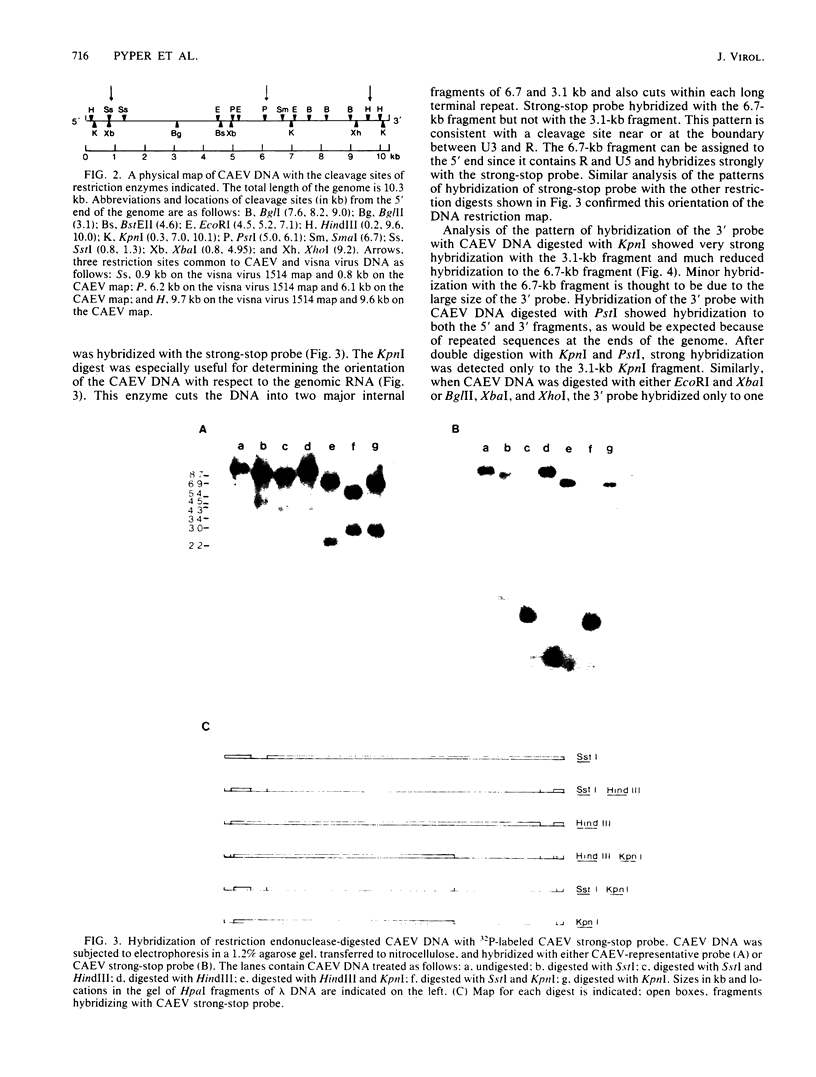
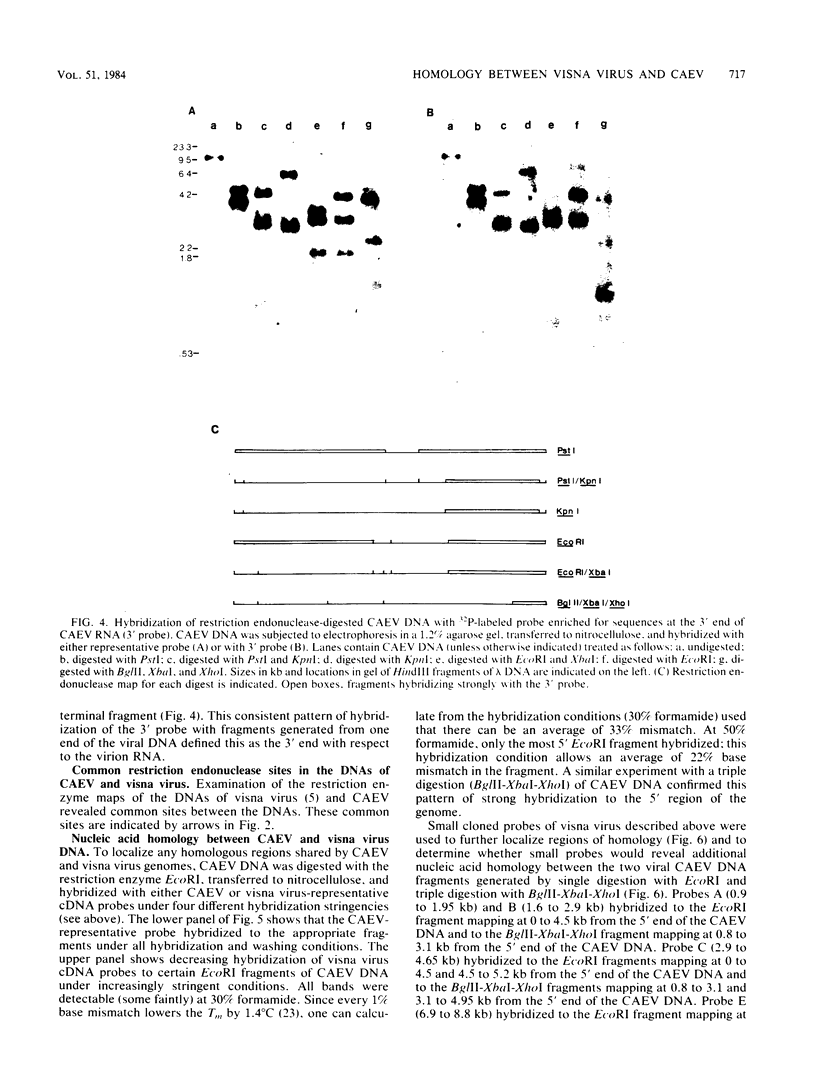
Abstract
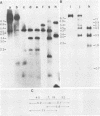
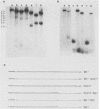
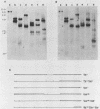
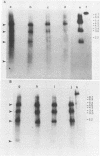
Visna virus of sheep and arthritis-encephalitis virus of goats are serologically related but genetically distinct retroviruses which cause slowly progressive diseases in their natural hosts. To localize homologous regions of the DNAs of these two viruses, we constructed a physical map of caprine arthritis-encephalitis virus DNA and aligned it with the viral RNA. Cloned probes of visna virus DNA were then used to localize regions of homology with the caprine arthritis-encephalitis virus DNA. These studies showed homology in the 5' region of the genome encompassing U5 and the gag and pol genes and also in a small region in the env gene. These findings correlate with biological data suggesting that the regions of the DNA which are homologous may be responsible for virus group characteristics such as the closely related virus core antigens. Regions which did not show homology such as large sections in the env gene may represent unique sequences which control highly strain-specific characteristics such as the neutralization antigen and specific cell tropisms.
Full text
PDF



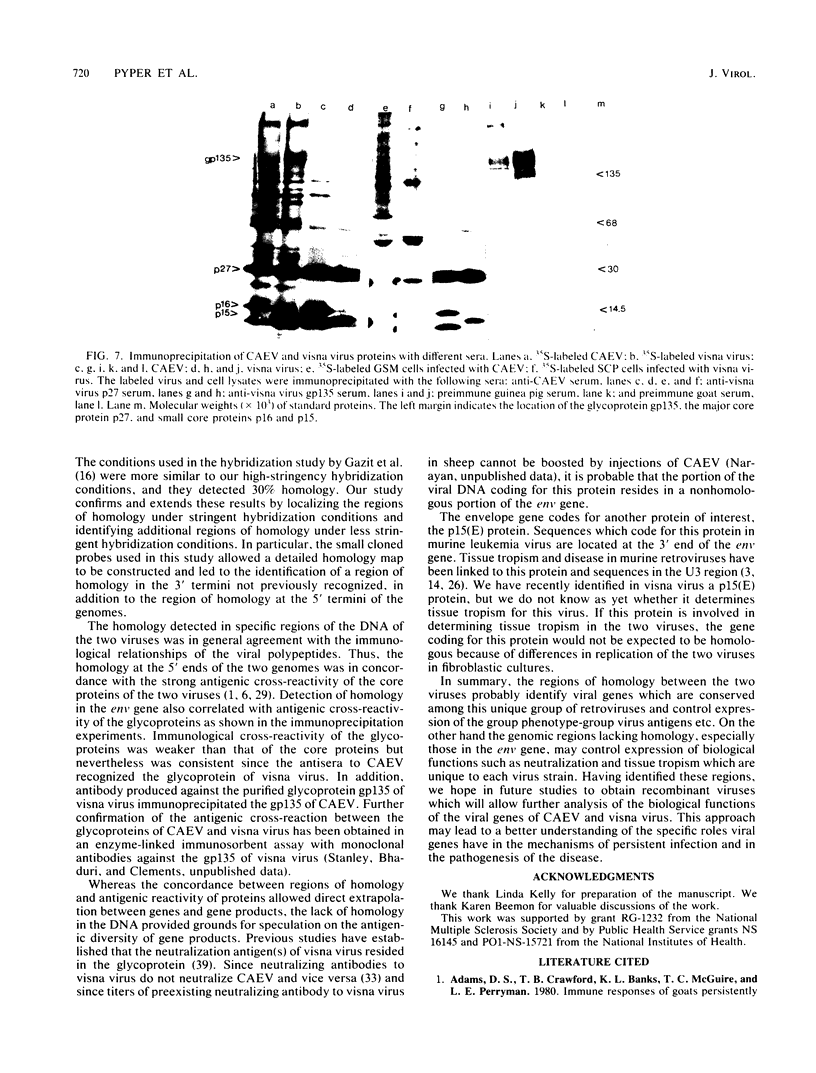

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. S., Crawford T. B., Banks K. L., McGuire T. C., Perryman L. E. Immune responses of goats persistently infected with caprine arthritis-encephalitis virus. Infect Immun. 1980 May;28(2):421–427. doi: 10.1128/iai.28.2.421-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. E., D'Antonio N., Narayan O. Genomic changes associated with antigenic variation of visna virus. II. Common nucleotide sequence changes detected in variants from independent isolations. J Mol Biol. 1982 Jul 5;158(3):415–434. doi: 10.1016/0022-2836(82)90207-8. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O. A physical map of the linear unintegrated DNA of Visna virus. Virology. 1981 Aug;113(1):412–415. doi: 10.1016/0042-6822(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Cork L. C. Biochemical characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Oct;50(2):423–427. doi: 10.1099/0022-1317-50-2-423. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Griffin D. E., Johnson R. T. The synthesis and structure of visna virus DNA. Virology. 1979 Mar;93(2):377–386. doi: 10.1016/0042-6822(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Invest. 1980 Jun;42(6):596–602. [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. J Am Vet Med Assoc. 1981 Apr 1;178(7):713–719. [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- De Boer G. F., Terpstra C., Houwers D. J., Hendriks J. Studies in epidemiology of maedi/visna in sheep. Res Vet Sci. 1979 Mar;26(2):202–208. [PubMed] [Google Scholar]
- De Boer G. F. Zwoegerziekte virus, the causative agent for progressive interstitial pneumonia (maedi) and meningo-leucoencephalitis (visna) in sheep. Res Vet Sci. 1975 Jan;18(1):15–25. [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaniv A., Dvir M., Perk K., Irving S. G., Dahlberg J. E. The caprine arthritis-encephalitis virus is a distinct virus within the Lentivirus group. Virology. 1983 Jan 15;124(1):192–195. doi: 10.1016/0042-6822(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Gudnadóttir M. Visna-maedi in sheep. Prog Med Virol. 1974;18(0):336–349. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Hu S. F., Lai M. M., Vogt P. K. Characterization of the env gene in avian oncoviruses by heteroduplex mapping. J Virol. 1978 Sep;27(3):667–676. doi: 10.1128/jvi.27.3.667-676.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R. W., Brunovskis I., Summers W. C. DNA base sequence homology between coliphages T7 and phiII and between T3 and phiII as determined by heteroduplex mapping in the electron microscope. J Mol Biol. 1973 Jun 25;77(2):189–196. doi: 10.1016/0022-2836(73)90330-6. [DOI] [PubMed] [Google Scholar]
- Klevjer-Anderson P., McGuire T. C. Neutralizing antibody response of rabbits and goats to caprine arthritis-encephalitis virus. Infect Immun. 1982 Nov;38(2):455–461. doi: 10.1128/iai.38.2.455-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Characterization of ribonucleic acid from visna virus. J Virol. 1971 May;7(5):582–587. doi: 10.1128/jvi.7.5.582-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung M. L., Hartley J. W., Rowe W. P., Hopkins N. H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983 Jan;45(1):275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux S., Clements J. E. Molecular cloning of unintegrated visna viral DNA and characterization of frequent deletions in the 3' terminus. Gene. 1983 Aug;23(2):137–148. doi: 10.1016/0378-1119(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Narayan O., Sheffer D., Griffin D. E., Clements J., Hess J. Lack of neutralizing antibodies to caprine arthritis-encephalitis lentivirus in persistently infected goats can be overcome by immunization with inactivated Mycobacterium tuberculosis. J Virol. 1984 Feb;49(2):349–355. doi: 10.1128/jvi.49.2.349-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Pétursson G., Nathanson N., Georgsson G., Panitch H., Pálsson P. A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Invest. 1976 Oct;35(4):402–412. [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M., Kalinyak J. E. Simple rapid method for the synthesis of radioactively labeled cDNA hybridization probes utilizing bacteriophage M13mp7. Proc Natl Acad Sci U S A. 1982 Feb;79(3):724–728. doi: 10.1073/pnas.79.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberson S. M., McGuire T. C., Klevjer-Anderson P., Gorham J. R., Cheevers W. P. Caprine arthritis-encephalitis virus is distinct from visna and progressive pneumonia viruses as measured by genome sequence homology. J Virol. 1982 Nov;44(2):755–758. doi: 10.1128/jvi.44.2.755-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A. Visna of sheep; a slow, demyelinating infection. Br J Exp Pathol. 1958 Oct;39(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Scott J. V., Stowring L., Haase A. T., Narayan O., Vigne R. Antigenic variation in visna virus. Cell. 1979 Oct;18(2):321–327. doi: 10.1016/0092-8674(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stowring L., Haase A. T., Charman H. P. Serological definition of the lentivirus group of retroviruses. J Virol. 1979 Feb;29(2):523–528. doi: 10.1128/jvi.29.2.523-528.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Traul K., Larson D., Stephens R., Wolff J., Munch K., Mayyasi S. New method for the removal of extraneous proteins from purified oncornaviruses. J Clin Microbiol. 1975 Sep;2(3):253–260. doi: 10.1128/jcm.2.3.253-260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]