Abstract
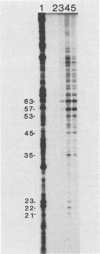
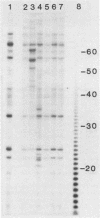
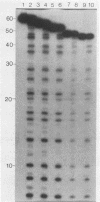
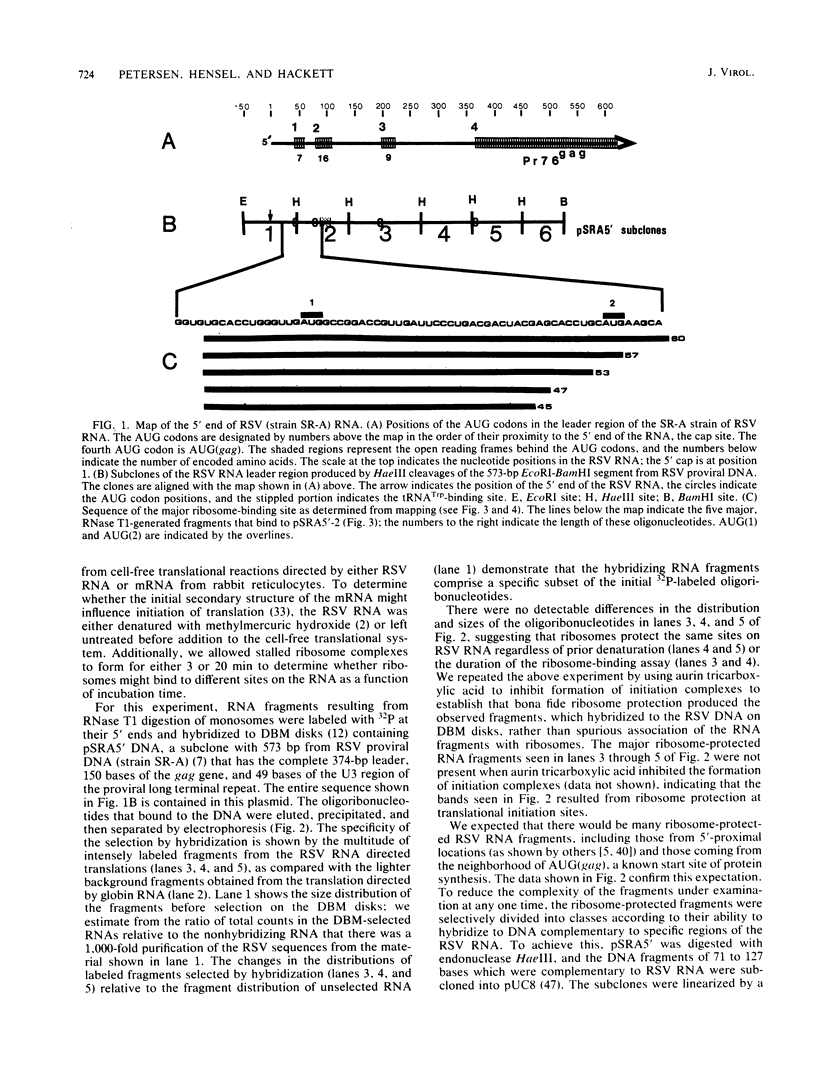
A new method for identifying ribosome-binding sites was developed to determine whether AUG codons in the 5'-terminal RNA sequence of Rous sarcoma virus were used to initiate protein synthesis. We found that when translation is inhibited, the major ribosome-binding site on Rous sarcoma virus RNA is at the 5'-proximal AUG codon, even though the primary translational product from this RNA, Pr76gag, is encoded behind the fourth AUG codon 331 bases downstream from the observed initiation site. These results suggest that ribosomes can initiate translation on Rous sarcoma virus RNA at more than one site, thereby producing a seven-amino-acid peptide, as well as the gag gene polyprotein precursor of Mr 76,000.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Shih D. S., Zimmern D., Kaesberg P. Two-step binding of eukaryotic ribosomes to brome mosaic virus RNA3. Nature. 1979 Sep 27;281(5729):277–282. doi: 10.1038/281277a0. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A., Jaton J. C. In vitro, the major ribosome binding site on Rous sarcoma virus RNA does not contain the nucleotide sequence coding for the N-terminal amino acids of the gag gene product. J Virol. 1979 Feb;29(2):597–611. doi: 10.1128/jvi.29.2.597-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Zuker M., Spahr P. F. Structure-function relationship of Rous sarcoma virus leader RNA. Nucleic Acids Res. 1982 Sep 11;10(17):5183–5196. doi: 10.1093/nar/10.17.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Dorner L. F., Larsen G. R., Wimmer E., Anderson C. W. Identification of the initiation site of poliovirus polyprotein synthesis. J Virol. 1982 Jun;42(3):1017–1028. doi: 10.1128/jvi.42.3.1017-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Haenni A. L. Binding of ribosomes to 5'-terminal leader sequences of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3111–3115. doi: 10.1073/pnas.76.7.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Egberts E., Traub P. Translation of ascites and mengovirus RNA in fractionated cell-free systems from uninfected and mengovirus-infected Ehrlich-ascites-tumor cells. Eur J Biochem. 1978 Feb;83(2):341–352. doi: 10.1111/j.1432-1033.1978.tb12100.x. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Swanstrom R., Varmus H. E., Bishop J. M. The leader sequence of the subgenomic mRNA's of Rous sarcoma virus is approximately 390 nucleotides. J Virol. 1982 Feb;41(2):527–534. doi: 10.1128/jvi.41.2.527-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Edbrooke M. R., Campbell P. N. Comparison of the nucleotide sequence of cloned human and guinea-pig pre-alpha-lactalbumin cDNA with that of chick pre-lysozyme cDNA suggests evolution from a common ancestral gene. Nucleic Acids Res. 1982 Jun 11;10(11):3503–3515. doi: 10.1093/nar/10.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendy G. N., Kronenberg H. M., Potts J. T., Jr, Rich A. Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7365–7369. doi: 10.1073/pnas.78.12.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Analysis of ribosome binding sites from the s1 message of reovirus. Initiation at the first and second AUG codons. J Mol Biol. 1982 Apr 25;156(4):807–820. doi: 10.1016/0022-2836(82)90143-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Mechanism of mRNA recognition by eukaryotic ribosomes during initiation of protein synthesis. Curr Top Microbiol Immunol. 1981;93:81–123. doi: 10.1007/978-3-642-68123-3_5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legon S. The binding of ribosomes to polyoma virus RNA. Possible role of the leader region in initiation site recognition. J Mol Biol. 1979 Oct 25;134(2):219–240. doi: 10.1016/0022-2836(79)90033-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lomedico P. T., McAndrew S. J. Eukaryotic ribosomes can recognize preproinsulin initiation codons irrespective of their position relative to the 5' end of mRNA. Nature. 1982 Sep 16;299(5880):221–226. doi: 10.1038/299221a0. [DOI] [PubMed] [Google Scholar]
- Mardon G., Varmus H. E. Frameshift and intragenic suppressor mutations in a Rous sarcoma provirus suggest src encodes two proteins. Cell. 1983 Mar;32(3):871–879. doi: 10.1016/0092-8674(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Lockard R. E., Vamvakopoulos N., Rieser L., RajBhandary U. L., Vournakis J. N. Secondary structure of mouse and rabbit alpha- and beta-globin mRNAs: differential accessibility of alpha and beta initiator AUG codons towards nucleases. Cell. 1980 Jan;19(1):91–102. doi: 10.1016/0092-8674(80)90391-8. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ravelonandro M., Godefroy-Colburn T., Pinck L. Structure of the 5'-terminal untranslated region of the genomic RNAs from two strains of alfalfa mosaic virus. Nucleic Acids Res. 1983 May 11;11(9):2815–2826. doi: 10.1093/nar/11.9.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete sequences of the ribosome recognition sites in vesicular stomatitis virus mRNAs: recognition by the 40S and 80S complexes. Cell. 1978 Jun;14(2):345–353. doi: 10.1016/0092-8674(78)90120-4. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Liarakos C. D., Gupta R. C., Randerath K., O'Malley B. W. Ribosome binding site analysis of ovalbumin messenger ribonucleic acid. Biochemistry. 1979 Dec 25;18(26):5798–5808. doi: 10.1021/bi00593a009. [DOI] [PubMed] [Google Scholar]
- Sherman F., McKnight G., Stewart J. W. AUG is the only initiation codon in eukaryotes. Biochim Biophys Acta. 1980 Sep 19;609(2):343–346. doi: 10.1016/0005-2787(80)90246-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of non-initiator regions in RNA bacteriophage messengers by ribosomes of Bacillus stearothermophilus. J Mol Biol. 1973 Jan;73(1):1–16. doi: 10.1016/0022-2836(73)90155-1. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vogeli G., Ohkubo H., Sobel M. E., Yamada Y., Pastan I., de Crombrugghe B. Structure of the promoter for chicken alpha 2 type I collagen gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5334–5338. doi: 10.1073/pnas.78.9.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]