Abstract
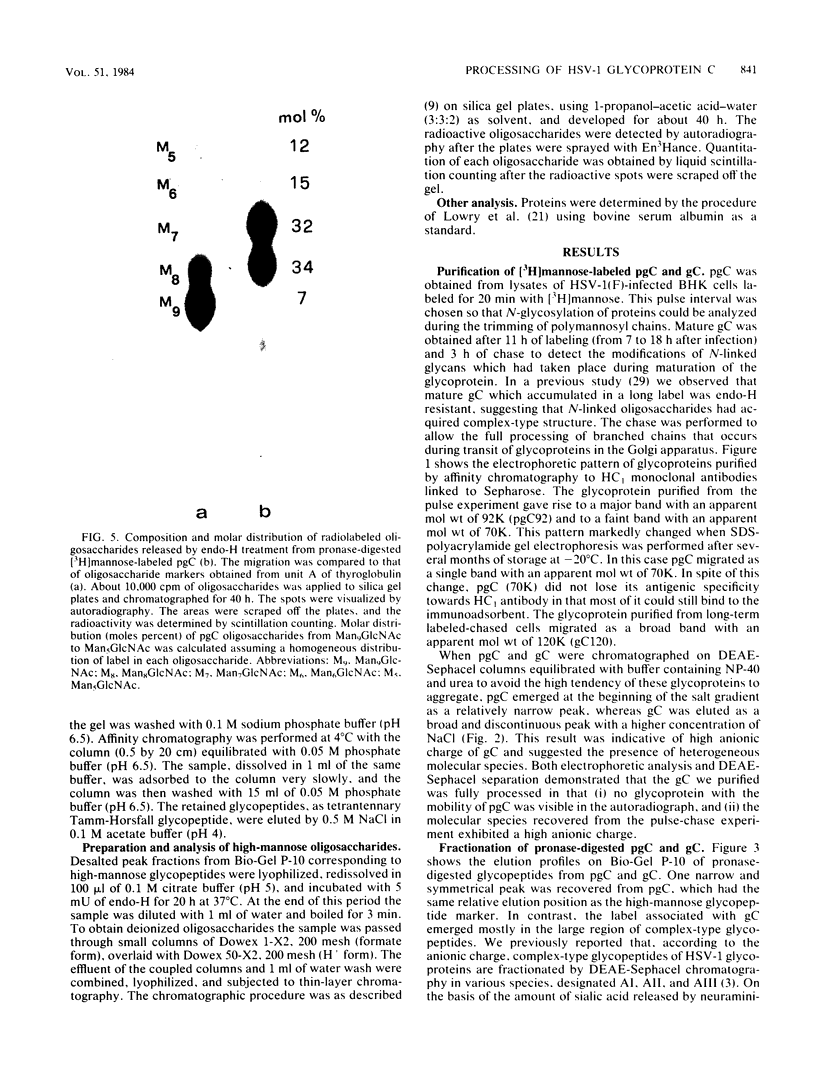
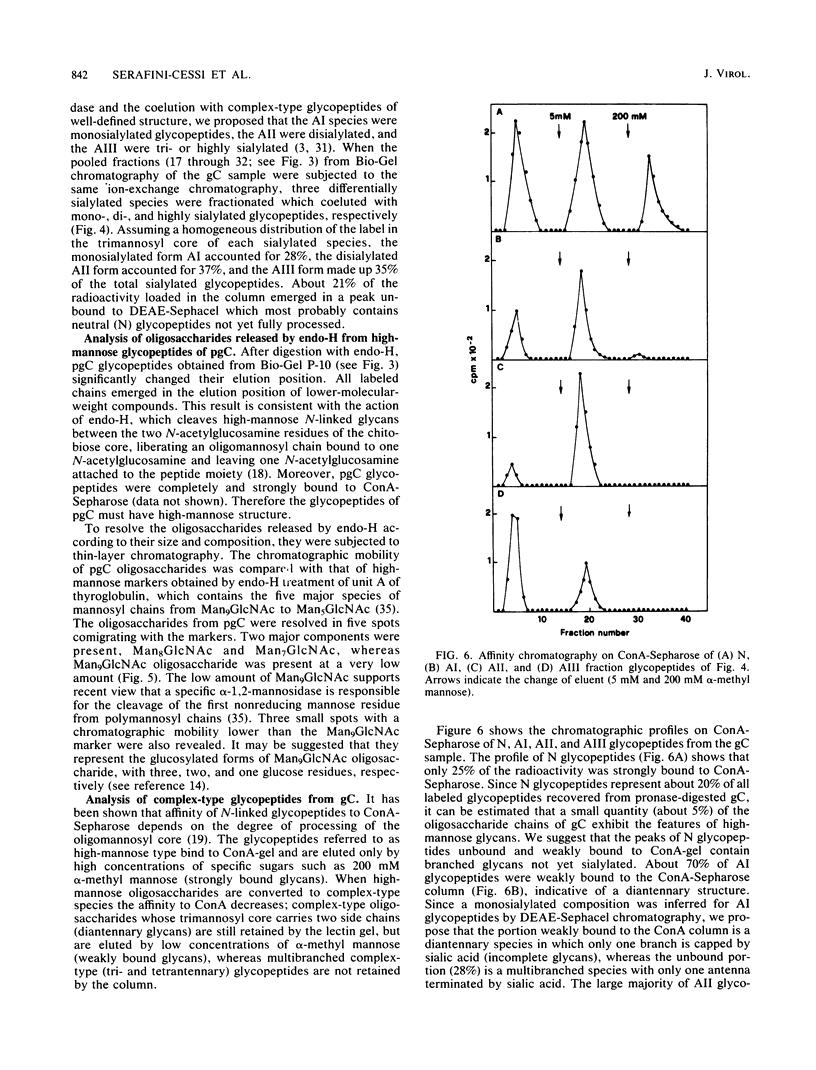
Immature and mature forms of glycoprotein gC were purified by immunoadsorbent from herpes simplex virus type 1-infected BHK cells labeled with [3H]mannose for a 20-min pulse or for 11 h followed by a 3-h chase. The nature of N-asparagine-linked oligosaccharides carried by the immature form, pgC (molecular weight = 92,000), and the mature gC (molecular weight = 120,000) has been investigated. All pronase-digested glycopeptides of pgC were susceptible to endo-beta-N-acetylglucosaminidase H treatment; thus they have a high-mannose structure. Using thin-layer chromatography to separate endo-beta-N-acetylglucosaminidase H-cleaved oligosaccharides, polymannosyl chains of different sizes, ranging from Man9GlcNAc to Man5GlcNAc, were separated. The major components were Man8GlcNAc and Man7GlcNAc, suggesting that pgC labeled in a 20-min pulse represents the form of glycoprotein already routed to the Golgi apparatus. Analysis of glycopeptides of mature gC showed that the majority (95%) of N-linked glycans were converted to complex-type glycans. Ion-exchange chromatography and affinity chromatography on concanavalin A-Sepharose and leucoagglutinin-agarose revealed that diantennary and triantennary glycans predominated, whereas tetrantennary chains were not present. Parts of the di- and triantennary chains were not fully sialylated. The high heterogeneity of complex-type chains found in mature gC may be related to the high number of N-glycosylation sites of the glycoprotein as predicted by DNA sequencing studies (Frink et al., J. Virol. 45:634-647, 1983).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke D., Keegstra K. Carbohydrate structure of Sindbis virus glycoprotein E2 from virus grown in hamster and chicken cells. J Virol. 1979 Feb;29(2):546–554. doi: 10.1128/jvi.29.2.546-554.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godelaine D., Spiro M. J., Spiro R. G. Processing of the carbohydrate units of thyroglobulin. J Biol Chem. 1981 Oct 10;256(19):10161–10168. [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Keil W., Niemann H., Geyer R., Schwarz R. T. The characterization of influenza A viruses by carbohydrate analysis. Curr Top Microbiol Immunol. 1983;104:247–257. doi: 10.1007/978-3-642-68949-9_15. [DOI] [PubMed] [Google Scholar]
- Kobata A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem. 1979 Nov 15;100(1):1–14. doi: 10.1016/0003-2697(79)90102-7. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Kumarasamy R., Blough H. A. Characterization of oligosaccharides of highly purified glycoprotein gC of herpes simplex virus type 1 (HSV-1). Biochem Biophys Res Commun. 1982 Dec 31;109(4):1108–1115. doi: 10.1016/0006-291x(82)91891-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsden H. S., Buckmaster A., Palfreyman J. W., Hope R. G., Minson A. C. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J Virol. 1984 May;50(2):547–554. doi: 10.1128/jvi.50.2.547-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Blomberg J., Lycke E. O-glycosidic carbohydrate-peptide linkages of Herpes simplex virus glycoproteins. Arch Virol. 1981;70(4):321–329. doi: 10.1007/BF01320247. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Norrild B., Andersen A. B., Pereira L., Jeansson S., Lycke E. Populations of herpes simplex virus glycoprotein gC with and without affinity for the N-acetyl-galactosamine specific lectin of Helix pomatia. Arch Virol. 1983;76(1):25–38. doi: 10.1007/BF01315701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfreyman J. W., Haarr L., Cross A., Hope R. G., Marsden H. S. Processing of herpes simplex virus type 1 glycoproteins: two-dimensional gel analysis using monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):873–886. doi: 10.1099/0022-1317-64-4-873. [DOI] [PubMed] [Google Scholar]
- Peake M. L., Nystrom P., Pizer L. I. Herpesvirus glycoprotein synthesis and insertion into plasma membranes. J Virol. 1982 May;42(2):678–690. doi: 10.1128/jvi.42.2.678-690.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush J. S., Turco S. J., Laine R. A. Erythroglycan biosynthesis in K-562 cells. Inhibition of synthesis by tunicamycin and lack of attachment to the G-protein of vesicular-stomatitis virus. Biochem J. 1981 Jan 1;193(1):361–365. doi: 10.1042/bj1930361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F. Guinea-pig kidney beta-N-acetylgalactosaminyltransferase towards Tamm-Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem J. 1983 Dec 1;215(3):483–489. doi: 10.1042/bj2150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Scannavini M., Campadelli-Fiume G. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology. 1983 Nov;131(1):59–70. doi: 10.1016/0042-6822(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Franceschi C., Sperti S. Specific interaction of human Tamm-Horsfall gylcoprotein with leucoagglutinin, a lectin from Phaseolus vulgaris (red kidney bean). Biochem J. 1979 Nov 1;183(2):381–388. doi: 10.1042/bj1830381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Studies on the synthesis and processing of the asparagine-linked carbohydrate units of glycoproteins. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1099):117–127. doi: 10.1098/rstb.1982.0160. [DOI] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]





