Abstract
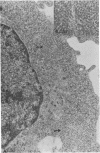
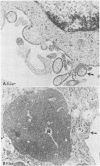
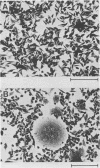
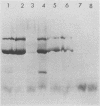
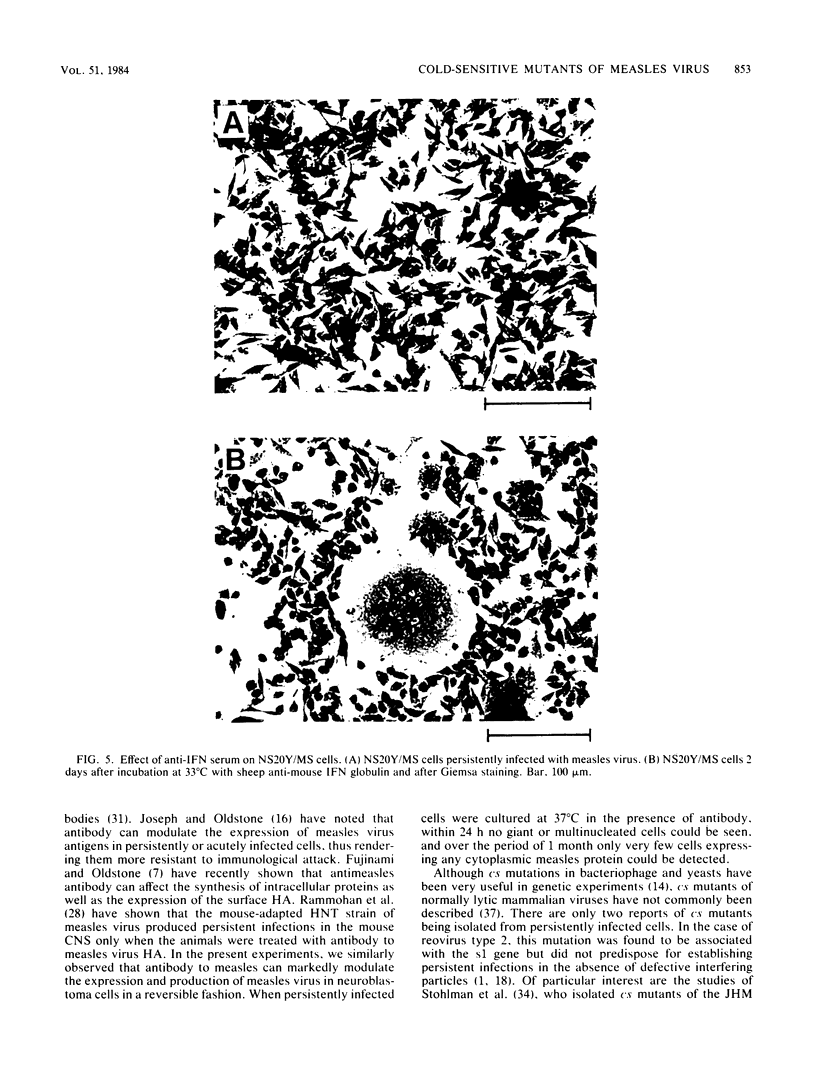
Clone NS20Y of the mouse neuroblastoma C1300 was infected with wild-type Edmonston measles virus, and, after a transition to a carrier culture, became persistently infected. Persistently infected clones were derived and characterized morphologically by the appearance of multinucleate giant cells and nucleocapsid matrices in cytoplasm and nucleus, but very few budding virus particles. Antimeasles antibodies markedly suppressed the expression of viral antigens and giant cells, and the effect was totally reversible. When the cells were cultured at 33 degrees C, the number of giant cells began to diminish and ultimately disappeared; in contrast, when cultured at 39 degrees C, the cultures invariably lysed. Yields at 33 degrees C were ca. 2 logs lower than those at 39 degrees C. Cells cultured at 33 degrees C produced relatively high levels of interferon, whereas those at 39 degrees C produced little or no interferon. When the persistently infected cultures were exposed to anti-interferon alpha/beta serum at a nonpermissive temperature, there was a marked increase in multinucleate cells, suggesting that maintenance of the persistence state and its regulation by temperature may be related to the production of interferon. Viral isolates from cells cultured at 39 degrees C were obtained, and 90% of viral clones were found to be cold sensitive. Complementation studies with different viral clones indicated that the cold-sensitive defect was probably associated with the same genetic function. Western blot analysis of the persistently infected cells indicated a significant diminution and expression of all measles-specific proteins at a nonpermissive temperature. Infection of NS20Y neuroblastoma cells with the cold-sensitive virus isolates resulted in the development of an immediate persistent infection, whereas infection of Vero or HeLa cells resulted in a characteristic lytic infection, suggesting that the cold-sensitive mutants may be selected or adapted for persistent infection in cells of neural origin.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Sheahan B. J. Semliki forest virus neurovirulence mutants have altered cytopathogenicity for central nervous system cells. Infect Immun. 1982 Apr;36(1):333–341. doi: 10.1128/iai.36.1.333-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholz C. M., Kiley M. P., Payne F. E. Isolation and characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Jul;16(1):192–202. doi: 10.1128/jvi.16.1.192-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrer M. J., Bloom B. R., Udem S. Characterization of measles polypeptides by monoclonal antibodies. Virology. 1981 Jan 30;108(2):381–390. doi: 10.1016/0042-6822(81)90446-3. [DOI] [PubMed] [Google Scholar]
- Fisher L. E., Rapp F. Role of virus variants and cells in maintenance of persistent infection by measles virus. J Virol. 1979 Apr;30(1):64–68. doi: 10.1128/jvi.30.1.64-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 Jul;125(1):78–85. [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Ventura P., Gibbs C. J., Jr, Tourtellotte W. W. Measles virus nucleotide sequences: detection by hybridization in situ. Science. 1981 May 8;212(4495):672–675. doi: 10.1126/science.7221554. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. A genetic method for determining the order of events in a biological pathway. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2046–2050. doi: 10.1073/pnas.70.7.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T. The possible viral etiology of multiple sclerosis. Adv Neurol. 1975;13:1–46. [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman R. S., Ahmed R., Fields B. N. Selection of a mutant S1 gene during reovirus persistent infection of L cells: role in maintenance of the persistent state. Virology. 1983 Nov;131(1):79–87. doi: 10.1016/0042-6822(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Kauffman R. S., Ahmed R., Fields B. N. Selection of a mutant S1 gene during reovirus persistent infection of L cells: role in maintenance of the persistent state. Virology. 1983 Nov;131(1):79–87. doi: 10.1016/0042-6822(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J. L., Breschkin A. M., Rapp F. Characterization of the Hallé SSPE measles virus isolate. J Gen Virol. 1982 Mar;59(Pt 1):57–64. doi: 10.1099/0022-1317-59-1-57. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Sakuma T., Kuwajima S., Yamamoto T. K., Iida H. Characterization of measles viruses in establishment of persistent infections in human lymphoid cell line. J Gen Virol. 1976 Dec;33(3):361–379. doi: 10.1099/0022-1317-33-3-361. [DOI] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Nowakowski M., Feldman J. D., Kano S., Bloom B. R. The production of vesicular stomatitis virus by antigen- or mitogen-stimulated lymphocytes and continuous lymphoblastoid lines. J Exp Med. 1973 Apr 1;137(4):1042–1059. doi: 10.1084/jem.137.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Rammohan K. W., McFarland H. F., McFarlin D. E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981 Apr 16;290(5807):588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Davidson W. B., Martin S. J. The role of defective interfering particles in persistent infection of Vero cells by measles virus. J Gen Virol. 1977 Apr;35(1):89–97. doi: 10.1099/0022-1317-35-1-89. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. I Interferon-inducing defective-interfering particles as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1978 Mar;85(1):175–186. doi: 10.1016/0042-6822(78)90422-1. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Bronson R. T., Fields B. N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983 Apr 29;220(4596):505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Sakaguchi A. Y., Weiner L. P. Characterization of the cold-sensitive murine hepatitis virus mutants rescued from latently infected cells by cell fusion. Virology. 1979 Oct 30;98(2):448–455. doi: 10.1016/0042-6822(79)90567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]
- Wright P. J., Cooper P. D. Isolation of cold-sensitive mutants of poliovirus. Intervirology. 1974;2(1):20–24. doi: 10.1159/000149400. [DOI] [PubMed] [Google Scholar]