Abstract
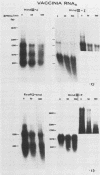
In this report we used Northern blot hybridization analysis to characterize the fate of several species of viral RNA transcribed from internal and terminal regions of vaccinia DNA in interferon-treated, infected mouse L cells grown in suspension. All species of viral RNAs were expressed but were reduced in amount. Larger-sized RNAs were reduced more than smaller-sized RNAs. This reduction appears to be related to the activation of the interferon-mediated double-stranded RNA-dependent 2-5A synthetase-endoribonuclease system, as the rRNA cleavage pattern characteristic of this system was observed early in infection and in cell extracts in response to exogenous 2-5A. Thus, in interferon-treated, vaccinia-infected mouse L cells in suspension, there is indiscriminate degradation of viral and cellular RNAs, and this RNA breakdown might play a role in the interferon-mediated inhibition of protein synthesis.
Full text
PDF
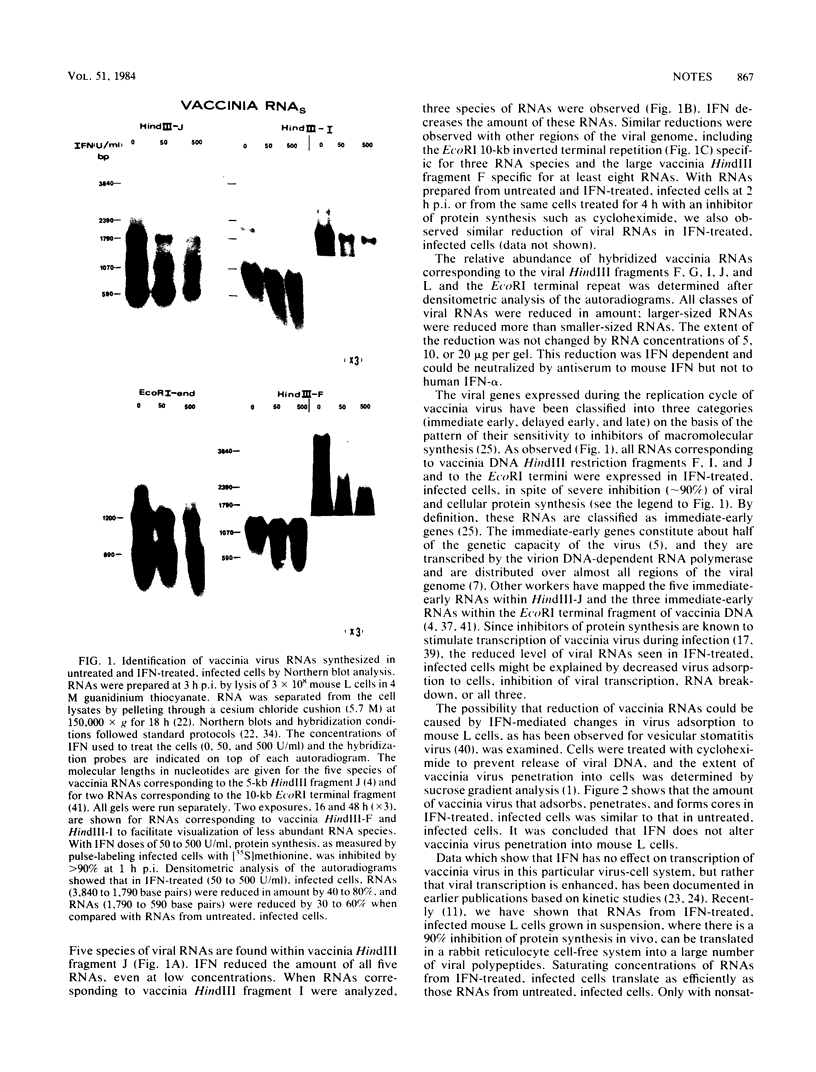



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. III. The effect of ultraviolet-irradiated virus on the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):1–12. doi: 10.1016/0042-6822(81)90606-1. [DOI] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R. F., Parr R. P., Moss B. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J Virol. 1979 Apr;30(1):365–374. doi: 10.1128/jvi.30.1.365-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Esteban M., McCarron R., McAllister W. T., Holowczak J. A. Vaccinia virus transcription: hybridization of mRNA to restriction fragments of vaccinia DNA. Virology. 1978 May 1;86(1):102–114. doi: 10.1016/0042-6822(78)90011-9. [DOI] [PubMed] [Google Scholar]
- Colby C., Duesberg P. H. Double-stranded RNA in vaccinia virus infected cells. Nature. 1969 Jun 7;222(5197):940–944. doi: 10.1038/222940a0. [DOI] [PubMed] [Google Scholar]
- Colby C., Jurale C., Kates J. R. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J Virol. 1971 Jan;7(1):71–76. doi: 10.1128/jvi.7.1.71-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Extension of the transcriptional and translational map of the left end of the vaccinia virus genome to 21 kilobase pairs. J Virol. 1981 Sep;39(3):733–745. doi: 10.1128/jvi.39.3.733-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Benavente J., Paez E. Effect of interferon on integrity of vaccinia virus and ribosomal RNA in infected cells. Virology. 1984 Apr 15;134(1):40–51. doi: 10.1016/0042-6822(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Control of expression of the vaccinia virus thymidine kinase gene. J Virol. 1981 Nov;40(2):456–464. doi: 10.1128/jvi.40.2.456-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Maki R. A., Miller D. B., Ball L. A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M., Danielescu G. The effect of interferon on the formation of virus polyribosomes in L cells infected with vaccinia virus. J Gen Virol. 1975 May;27(2):197–209. doi: 10.1099/0022-1317-27-2-197. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Maintenance of protein synthesis in spite of mRNA breakdown in interferon-treated HeLa cells infected with reovirus. Mol Cell Biol. 1983 Jan;3(1):64–69. doi: 10.1128/mcb.3.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Sen G. C., Brown G. E., Lebleu B., Kawakita M., Cabrer B., Slattery E., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Characteristics of an endonuclease activity. Eur J Biochem. 1977 Oct 3;79(2):565–577. doi: 10.1111/j.1432-1033.1977.tb11841.x. [DOI] [PubMed] [Google Scholar]
- Revel M., Kimchi A., Shulman L., Fradin A., Shuster R., Yakobson E., Chernajovsky Y., Schmidt A., Shure A., Bendori R. Role of interferon-induced enzymes in the antiviral and antimitogenic effects of interferon. Ann N Y Acad Sci. 1980;350:459–472. doi: 10.1111/j.1749-6632.1980.tb20649.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Silverman R. H., Cayley P. J., Knight M., Gilbert C. S., Kerr I. M. Control of the ppp(a2'p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982 May;124(1):131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Silverman R. H., Skehel J. J., James T. C., Wreschner D. H., Kerr I. M. rRNA cleavage as an index of ppp(A2'p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983 Jun;46(3):1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varich N. L., Sychova I. V., Kaverin N. V., Antonova T. P., Chernos V. I. Transcription of both DNA strands of vaccinia virus genome in vivo. Virology. 1979 Jul 30;96(2):412–430. doi: 10.1016/0042-6822(79)90099-0. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P. A., Wilcox D. K., Widnell C. C., Youngner J. S. Interferon-mediated inhibition of virus penetration. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1083–1086. doi: 10.1073/pnas.80.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Brown R. E., Gilbert C. S., Kerr I. M. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature. 1979 Dec 6;282(5739):582–586. doi: 10.1038/282582a0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wittek R., Moss B. Colinearity of RNAs with the vaccinia virus genome: anomalies with two complementary early and late RNAs result from a small deletion or rearrangement within the inverted terminal repetition. J Virol. 1982 May;42(2):447–455. doi: 10.1128/jvi.42.2.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson B. Vaccinia mRNA synthesis under conditions which prevent uncoating. Biochem Biophys Res Commun. 1967 Apr 20;27(2):169–175. doi: 10.1016/s0006-291x(67)80057-3. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]