Abstract
Functional imaging studies have begun to identify a set of brain regions whose brain activity is greater during ‘rest’ (e.g., fixation) states than during cognitive tasks. It has been posited that these regions constitute a network that supports the brain's default mode, which is temporarily suspended during specific goal-directed behaviors. Exogenous tasks that require cognitive effort are thought to command reallocation of resources away from the brain's default state. However, it remains unknown if brain activity during fixation periods between active task periods is influenced by previous task-related emotional content. We examined brain activity during periods of FIXATION (viewing and rating gray-scale images) interspersed among periods of viewing and rating complex images (‘PICTURE’) with positive, negative, and neutral affective content. We show that a select group of brain regions (PCC, precuneus, IPL, vACC) do exhibit activity that is greater during FIXATION (>PICTURE); these regions have previously been implicated in the “default brain network”. In addition, we report that activity within precuneus and IPL in the FIXATION period is attenuated by the precedent processing of images with positive and negative emotional content, relative to non-emotional content. These data suggest that the activity within regions implicated in the default network is modulated by the presence of environmental stimuli with motivational salience and, thus, adds to our understanding of the brain function during periods of low cognitive, emotional, or sensory demand.
Keywords: fMRI, rest, baseline, fixation, emotion, affect, parietal cortex, precuneus
Functional brain imaging studies have begun to identify a set of brain regions whose activity is consistently greater during “rest” periods (e.g., passive visual fixation or eyes closed resting) than during periods that involve a perceptual, cognitive, or emotional task. These regions include the posterior cingulate cortex (PCC), precuneus, inferior parietal lobule (IPL), anterior cingulate cortex (ACC), and ventral medial prefrontal cortex (vMPFC) [20, 32]. It has been shown that brain activity during “rest” and fixation states involves substantial energy consumption [27, 29], appears well-organized [4, 7, 12]. Given that this activity is sustained during periods during which subjects are not engaged in a specific task, but reduced when subjects engage in cognitively demanding tasks, one prevailing theory to explain these “rest” activations is that they represent the existence of a default mode of brain function [28, 30].
It has been suggested that task-related changes in cognitive and/or affective demands could alter the extent to which the brain’s default network returns to its “resting” state. There is evidence to suggest that prior cognitive state modulates functional connectivity of the default network [38], and that greater cognitive load [5, 9] and greater task-difficulty [21, 22] are associated with greater deactivations within ‘default’ regions during task engagement. Changes in an individual’s emotional state may also influence the brain’s default network. As such, Harrison and colleagues recently demonstrated that functional connectivity of default network regions is decreased when subjects recall sad, relative to neutral, life events [14]. Previously, another group had shown that attending one’s own affective reactions to emotionally evocative images [13] and having anticipatory anxiety prior to unpracticed task performance [34] and prior to potential aversive/painful shock [33] all attenuate task-related deactivations vACC/MPFC, PCC, and precuneus. Collectively, these studies suggest that task induced deactivations (TID) in default network regions can be interrupted or modulated by introspective emotional salience processing and/or evoked negative emotional experience.
Functional brain imaging studies of emotion have commonly employed box-car (e.g., epoch-related) designs consisting of alternating ‘ON’ and ‘OFF’ blocks during which subjects are instructed to engage in an active emotional processing task (viewing pictures or faces) and to emotionally disengage by viewing a blank or gray-scale images (FIXATION), respectively [25]. Such studies have broadly informed us about the functional neuroanatomy of emotion by demonstrating activations in certain cortico-limbic regions during the emotional state (>FIXATION) [23, 25, 26, 37]. However, little is known about brain ‘activation’ during the FIXATION periods, in contrast to emotional processing tasks, or if activity during FIXATION differ following emotional (vs. non-emotional) experience. In this study, we sought to address these questions by examining the pattern and extent of during FIXATION periods, which are interspersed between active task periods (viewing emotional and neutral pictures). Given prior findings of brain regions exhibiting activation during FIXATION (versus emotional task, or versus cognitive task) [13, 32], we had a priori prediction within the following regions of interest (ROI): PCC, precuneus, inferior parietal lobule (IPL), anterior cingulate cortex (ACC), and ventral medial prefrontal cortex (vMPFC).
Thirty-seven healthy, right-handed subjects (16 male, 21 female; aged 22–49 years, mean age ± SD: 32.8 ± 7.9) participated in this study in accordance with the Declaration of Helsinki after giving informed consent as indicated by the University of Chicago Institutional Review Board. No subject had a prior or current psychiatric disorder as verified by structured clinical interview [6, 24] or prior or current major medical or neurologic illness as confirmed by a physician evaluation.
The emotional pictures task consisted of twelve 20-second alternating experimental/active (viewing ‘PICTURE’) and control (viewing gray-scale images; ‘FIXATION’) epochs, in each of four functional runs. The PICTURE epochs consisted of pictorial stimuli (5 per block) selected from the International Affective Pictures System (IAPS) [18], and categorized as Positive, Negative, and Neutral based on normative ratings of valence. These emotional (Positive, Negative) images have been shown to evoke acute and transient changes in subjective affective experience and arousal as well as peripheral and central indexes of emotional reactivity [16, 19, 25]. The images within these categories were matched in color composition, general image complexity, and general content (presence of human faces). Positive, negative, and neutral picture blocks (2 blocks of each category per run) were counterbalanced within and across subjects, and no image was repeated. The FIXATION epochs (6 per run) consisted of blank, grey images (5 per block) with a centered fixation crosshair. Stimuli were presented through MR-compatible LCD goggles using PRESENTATION software (Neurobehavioral Systems, Albany, CA). In the PICTURE block, subjects were instructed to indicate by button press if the image made them feel positive, negative, or neutral (rate valence). In the FIXATION block, subjects were instructed to indicate by button press if the blank image was a dark, medium, or light shade of grey (rate shade), and to otherwise “rest, relax, and try not to think of anything.” The FIXATION epochs served as a simple visual-motor task to maintain the subjects’ attention but evoke little, if any, cognitive or emotional effort. After scanning participants viewed each of the 120 IAPS stimuli previously seen and subjectively rated each image on 9-point scales for valence (1=extremely unpleasant; 5=neutral; 9=extremely pleasant), and arousal (1=not at all arousing; 5=moderately arousing; 9=extremely arousing).
All scanning was performed with BOLD-sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric; Milwaukee, WI) at the University of Chicago Brain Research Imaging Center using a standard radiofrequency coil and associated software (LX 8.3, Neuro-optimized gradients). Whole brain functional scans were acquired using a T2*-weighted reverse spiral gradient-recall echo sequence (echo time=25ms, repetition time=2000ms, 64×64 matrix, flip angle=77deg, field of view=24cm, 30 contiguous 5mm axial slices per volume). A high-resolution T1 scan (3D-MPRAGE; repetition time=25ms; min echo time; field of view=24cm; slice thickness=1.5mm, 120 slices per volume) was also acquired for anatomical localization.
Data from all thirty-seven subjects met criteria for quality with minimal motion correction (<3mm displacement in any one direction) and were included in the data analyses. The first four volumes from each run were discarded to allow for T1 equilibration effects. Data were preprocessed and analyzed using statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). The scans were spatially realigned to correct for head motion, warped (non-linear) to an EPI template in Montreal Neurologic Institute (MNI) space, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to maximize signal and minimize residual differences in neuroanatomy. Statistical analyses were performed using the general linear model and Gaussian random fields theory as implemented in SPM2 [10, 11]. Individual statistical parametric maps were produced from linear contrasts of interest and subsequently analyzed as a group (n=37) in a second-level random effects model [15].
First, from a whole-brain voxel-wise search, we identified regions that show greater activity during FIXATION relative to the experimental task collapsed across valence categories from the linear contrast of FIXATION > PICTUREAll. Second, we examined brain activity during FIXATION blocks following each PICTURE type (Positive, Negative, and Neutral) from the following contrasts: FIXATIONPositive > PICTURE All; FIXATIONNegative > PICTURE All; FIXATIONNeutral > PICTURE All. We chose to contrast against the PICTUREAll condition for both steps in order to have an equal and matched comparison condition across different types of FIXATION periods. Third, these contrasts specific to each of the 3 types of FIXATION periods (following Positive, Negative, and Neutral pictures, respectively) were then entered into a repeated-measures ANOVA in order to examine activation among them in the context of the same comparison condition (PICTUREAll) and identify brain regions that show a main effect of the prior emotional content on activation during FIXATION. Then, significant main effects of emotion on the activations within a priori ROIs were followed-up with t-tests to identify which emotional content produced different degrees of activation during FIXATION with the following contrasts: FIXATIONNeutral > FIXATIONPositive; FIXATIONNeutral > FIXATIONNegative; FIXATIONNegative > FIXATIONPositive. Activated voxels were identified with a significance height threshold of P < 0.05, corrected for multiple comparisons using the False Discovery Rate [FDR] (Genovese et al., 2002) from a whole-brain search and having a minimum contiguous cluster size of 20 voxels. We identified the location of activations using an automated anatomical (AAL) template [39] based on the atlas of Tzourio-Mazoyer and colleagues [36].
All subjects performed the task as intended; however, behavioral data was not successfully recorded for one subject. During fMRI scanning on-line performance showed that subjects exhibited a difference in reaction time between the valence rating and the shade rating (mean ± SD, valence: 1444 ± 239 ms; shade: 784 ± 196 ms; P < 0.001). However, an ANOVA for main effect of condition showed no differences in reaction time for the shade rating with respect to the prior emotional/neutral conditions (Positive: 794 ± 198 ms; Negative: 781 ± 201 ms; Neutral: 801 ± 205 ms, P = 0.90). Post-scan ratings of IAPS stimuli confirmed the intended valence and level of arousal evoked by each of the emotional categories. Relative to Neutral images, Positive pictures were rated as more pleasant and Negative pictures were rated as more unpleasant (mean ± SD, Positive: 7.0±0.70 > Neutral: 4.8±0.85, P < 0.001; Negative: 2.09±0.81 < Neutral: 4.8± 0.85; P < 0.001). Of note, there was no difference in arousal ratings between Positive and Negative pictures (5.32 ± 1.32 and 5.82 ± 2.08, respectively, P > 0.05); however, Positive and Negative images were more arousing than Neutral images (Neutral: 2.32 ± 1.08, Ps < 0.001).
Following a whole-brain search, brain activation during FIXATION (> PICTURE) were observed in PCC, precuneus, medial and bilateral parietal cortex (including IPL), superior and inferior medial frontal regions (including vACC), bilateral insula, and bilateral temporal gyrus (Table 1). Repeated-measures ANOVA revealed significant differences in the magnitude of activation during the FIXATION period following the three emotional categories in precuneus and bilateral IPL but not other areas noted above (Table 2; Figure 1A). Follow-up t-tests revealed that the extent of activation during FIXATION within bilateral IPL and precuneus following both Positive and Negative pictures was less than that following the Neutral pictures (Table 2; Figure 1B–C). Of note, the magnitude of activation during FIXATION in the vACC and insula were not observed to be different following emotional and non-emotional states. No differences in activation in any of the regions identified in Table 1 were noted during the FIXATION period following Positive and Negative pictures (P > 0.05).
Table 1.
Brain areas exhibiting activation during FIXATION (> PICTURE)
| Brain Region | Side | MNI Coordinates |
Cluster Size |
Z Score |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| IPL | R | 58 | −46 | 50 | 3578 | 7.41 |
| L | −46 | −64 | 50 | 2195 | 5.72 | |
| L | −38 | −62 | 48 | 1 | 2.51 | |
| Precuneus | R | 12 | −64 | 40 | 830 | 6.68 |
| L | −16 | −56 | 34 | 545 | 4.77 | |
| L | −16 | −58 | 72 | 80 | 3.81 | |
| L | −22 | −48 | 10 | 11 | 4.85 | |
| R | 22 | −44 | 10 | 19 | 5.65 | |
| R | 18 | −42 | 8 | 2 | 2.83 | |
| R | 12 | −50 | 76 | 1 | 2.59 | |
| MTG | R | 66 | −28 | −12 | 875 | 5.67 |
| L | −60 | −56 | 22 | 51 | 4.32 | |
| L | −66 | −30 | −6 | 94 | 2.93 | |
| L | −46 | −20 | −4 | 6 | 3.59 | |
| L | −46 | −16 | −8 | 2 | 3.32 | |
| L | −64 | −24 | 8 | 2 | 2.58 | |
| STG | R | 62 | −52 | 22 | 1159 | 5.43 |
| L | −42 | −18 | −4 | 264 | 5.34 | |
| L | −56 | −32 | 18 | 573 | 4.68 | |
| R | 48 | −30 | −4 | 1 | 2.53 | |
| PCC | R | 14 | −38 | 12 | 107 | 5.3 |
| R/L | 0 | −34 | 28 | 93 | 4.56 | |
| L | −14 | −42 | 10 | 1 | 2.78 | |
| Insula | L | −40 | 18 | −4 | 389 | 4.93 |
| R | 44 | 10 | −12 | 263 | 3.96 | |
| R | 32 | 16 | 14 | 16 | 2.79 | |
| ACC | R | 16 | 40 | 4 | 495 | 4.61 |
| L | −10 | 38 | 8 | 257 | 4.45 | |
| R/L | 0 | 30 | −4 | 23 | 3.17 | |
Height threshold at P < 0.05, FDR-corrected. For each activation foci, laterality (left, right), peak Montreal Neurologic Institute (MNI) atlas coordinates, cluster size (number of contiguous voxels), and Z-score are provided. IPL, Inferior Parietal Lobule; MTG , Middle Temporal Gyrus; STG, Superior Temporal Gyrus; PCC, Posterior Cingulate Cortex; ACC, Anterior Cingulate Cortex; L, left; R, right.
Table 2.
Brain areas in which extent of activation during FIXATION is modulated by prior affective state
| ANOVA | FIXNeutral > FIXPositive | FIXNeutral > FIXNegative | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI | k | Z | MNI | k | Z | MNI | k | Z | |||||||
| Region | x | y | z | x | y | z | x | y | z | ||||||
| IPL | 54 | −58 | 26 | 560 | 5.55 | 56 | −66 | 26 | 156 | 4.44 | 54 | −60 | 26 | 1169 | 6.38 |
| −42 | −58 | 24 | 313 | 4.55 | −52 | −64 | 32 | 484 | 4.09 | −40 | −62 | 22 | 887 | 5.67 | |
| 44 | −64 | 50 | 134 | 3.97 | −42 | −40 | 50 | 26 | 3.06 | ||||||
| −46 | −30 | 32 | 31 | 3.95 | |||||||||||
| Precuneus | 4 | −56 | 36 | 257 | 4.27 | −14 | −70 | 56 | 26 | 3.30 | 4 | −58 | 36 | 738 | 4.89 |
| −2 | −48 | 36 | 35 | 3.19 | 2 | −62 | 36 | 1057 | 4.68 | ||||||
Brain regions that demonstrate an effect of prior emotional (Positive, Negative, Neutral) epoch on activation during FIXATION. Height threshold at P < 0.05, FDR-corrected. IPL, Inferior Parietal Lobule; FIX, FIXATION.
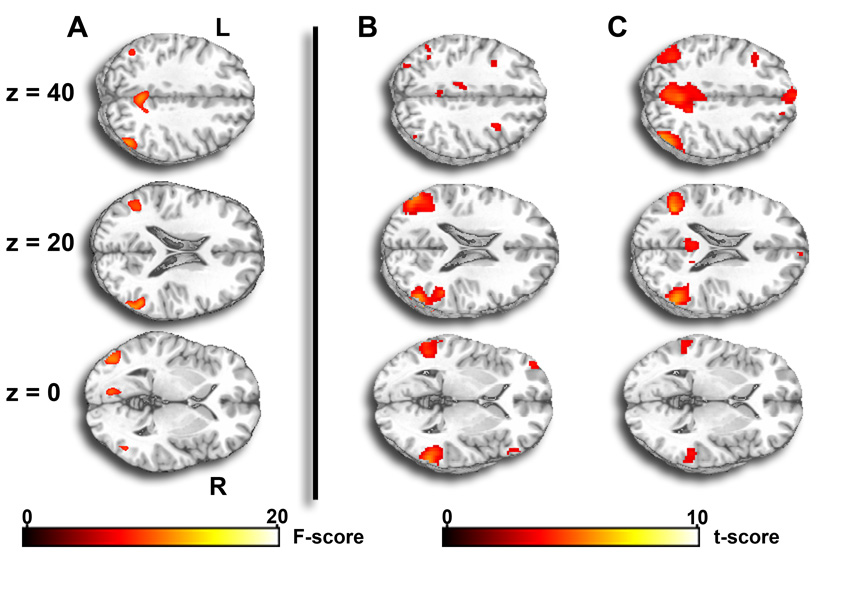
Fig 1. Statistical maps of effect of prior emotional state on activation during FIXATION.
A) F-map of brain areas exhibiting a main effect of emotional condition (positive, negative, neutral) on extent of activation during the FIXATION condition; B) t-map of brain areas exhibiting greater activation during FIXATION epochs that follow neutral pictures than FIXATION epochs that follow positive pictures; and C) t-map of brain areas exhibiting greater activation during FIXATION epochs that follow neutral pictures than FIXATION epochs that follow negative pictures. Map is superimposed on axial sections of a canonical brain image (at z-plane coordinates 40, 42, and 0 of the Montreal Neurologic Institute atlas). F-score and t-score scales are shown at the bottom. L, left; R, right. For details, see Table 2.
To our knowledge, this is the first study to demonstrate that affective experience influences brain activation during the FIXATION period. Here, we show that activity in certain brain regions (PCC, precuneus, medial and bilateral parietal cortex (including IPL), superior and inferior medial frontal regions [including vACC], bilateral insula, and bilateral temporal gyrus) is greater during the FIXATION period during which subjects engaged in a simple visual-motor task that requires little cognitive, sensory, or emotional demand, in comparison to PICTURE periods during which they processed emotionally salient and evocative pictures. From recent resting-state fMRI studies, this set of regions has been consistently implicated as the brain’s ‘default’ network [4, 7, 12, 28, 30]. We also demonstrate that the extent of activation of 2 specific regions (precuneus and IPL) during FIXATION is attenuated by the precedent processing of images with emotional relative to non-emotional content. Together, these data provide evidence that emotional experience can alter the activation of distinct set of brain regions during periods intended as “rest” and adds to our understanding of the brain’s activity and function during periods during which cognitive, sensory, and emotional demands are low.
The findings are broadly consistent with prior evidence of modulation of brain activity during FIXATION by prior tasks associated with high cognitive demand [20, 32]. The extent of activation during FIXATION has been shown to be modulated by the degree of cognitive effort required by the experimental/active task [38], such that as subjects engage in tasks that become more cognitively demanding they are less able to enter into task-unrelated mental states (i.e., stream of consciousness, free association, stimulus-independent thinking) [1, 8, 21, 22, 35]. It has been posited that regions within the brain’s default network, similar to those shown here to be more activated during FIXATION, also interact with and/or are modulated by additional demands, such as ongoing salience processing [31] and emotional states [13, 33, 34]. Recently, it has been demonstrated that recall of a sad event (relative to a neutral one) decreases the functional connectivity of default network regions including the PCC, precuneus, IPL, and mPFC [14]. Until then, the notion that induced affective states can alter the brain’s default state had not been directly examined.
Our results are consistent with such findings, and suggest that activity of IPL and precuneus during FIXATION are attenuated by the recent, preceding emotional state. Emotional states are believed to have evolved from reflexive reactions to stimuli that provided immediate survival functions, and can be characterized as motivationally tuned states of preparedness to decode stimulus salience in the external-internal environment and to adaptively guide an organism [2, 3, 17]. The present findings suggest that an emotionally evocative task may attenuate activity in certain regions previously implicated in the ‘default’ brain network, which is associated with “internally-generated” and/or “task-unrelated” thinking. This could serve to direct attention and allocation of resources towards processing the external milieu [31].
Our interpretations are constrained by some limitations. We did not collect self-report data on the subjective state of the participants during the FIXATION epochs, and cannot provide data on what subjects were feeling or thinking during these periods of “rest”; hence, we have no evidence that prior affective states affected the extent to which subjects engaged in task-unrelated thinking [21] or fully conclude that the subjects were at “rest.” Moreover, we did not collect on-line changes in emotional state and hence cannot examine the relationship between degree of emotionality and attenuation of activation during FIXATION periods. Last, the task of viewing emotional pictures is also associated with enhanced visual, semantic processing given that these valenced images are more complex and arousing than those that are neutral; therefore, these characteristics may have also increased cognitive and/or perceptual (in addition to emotional) demands, which confounds our interpretation.
In summary, we have provided evidence that evoked emotions can attenuate the extent of brain activation during FIXATION in discrete regions previously implicated as the brain’s default network. These findings add to accumulating data that task demands, emotional or cognitive, can disrupt activity during periods of “rest” and enhance our understanding of the functional significance of the brain’s activity during periods of low and/or absent cognitive, sensory, and emotional demands.
Acknowledgements
This work was made possible through grants from the National Institutes of Health (MH076198, MH063262, and MH069764), American Foundation for Suicide Prevention, and the Pritzker School of Medicine Summer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 2.Barrett LF, Mesquita KN, Ochsner JJ, Gross The experience of emotion. Annu Rev Psychol. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damasio AR. Descartes' Error. New York: Avon Books, Inc.; 1994. [Google Scholar]
- 4.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull. 2006;70:263–269. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 6.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 7.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- 11.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995:189–210. [Google Scholar]
- 12.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;volume 7:S754. [Google Scholar]
- 16.Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 17.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 18.Lang PJ, Bradley MM, Cuthbert BN. NIMH Center for the Study of Emotion and Attention. Gainesville, FL: University of Florida; 1997. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. [Google Scholar]
- 19.Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 20.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 21.McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the "stream of consciousness": an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 23.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 24.Pfohl B, Blum N, Zimmerman M, editors. Structured Clinical Interview for DSM-IV. Iowa City: University of Iowa College of Medicine; 1995. [Google Scholar]
- 25.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 26.Phillips ML, Drevets WC, Rauch SL, Lane R. The neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 27.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 30.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 31.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Peterson SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 33.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 36.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 37.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 38.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter B, Blecker C, Kirsch P, Sammer G, Schlienle A, Stark R, Vaitl D. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. 9th International Conference on Functional Mapping of the Human Brain. NeuroImage, New York, NY. 2003;Vol. 2 [Google Scholar]