Abstract
Voxel-based morphometry (VBM) was used to examine the relationship between gray matter (GM) volume and performance on two commonly used clinical neuropsychological measures of frontal lobe or executive function, the Trail Making Test part B (TrailsB) and the Controlled Oral Word Association Test (COWAT) in 221 cognitively healthy adults between the ages of 18 and 84. We hypothesized that these measures would be associated with GM volume in the dorsolateral frontal lobes. Voxel-based multiple regression was used to correlate cognitive function with modulated GM probability maps while controlling for age, education, gender, and total intracranial volume. A relationship with TrailsB was found in bilateral lateral inferior frontal gyri and left basal ganglia. A relationship with COWAT was found in the left lateral inferior and middle frontal gyri. Lesion studies have long implicated the importance of these regions for executive function. The present results confirm and extend those prior findings to healthy adults.
Keywords: VBM, Executive function, Frontal lobe, MRI
Introduction
Functional neuroimaging techniques have greatly advanced our understanding of normal cognitive function. However, the tasks typically used in brain activation paradigms rarely correspond directly to clinical neuropsychological tests. This is because neuropsychological tests are cognitively complex, and the timing and environmental constraints of the imaging setting prohibit administration in the same standardized fashion that they are given in the clinical setting. As a result of these limiting factors, few brain mapping studies make use of neuropsychological tests (Monchi et al. 2007; Moritz et al. 2004; Roth et al. 2006; Schlosser et al. 1998; Zakzanis et al. 2005).
One approach that may be helpful in understanding brain–behavior relationships with regard to neuropsychological function is voxel-based morphometry (VBM) developed by Ashburner and Friston (2000) (see also Good et al. 2001). VBM involves segmentation of T1-weighted MRI data into gray and white matter probability maps. These maps are then normalized to a standard atlas space and statistically analyzed voxel-by-voxel. This statistical parametric mapping technique allows the researcher to evaluate group differences in gray matter (GM) volume at a fine degree of spatial resolution. The advantage of whole-brain VBM over rater-based approaches, such as tracing anatomically defined regions of interest (ROI) analyses, is that VBM does not require a priori assumptions about the size, location or shape of the ROI. Manually defined ROIs are often defined along sulcal or tissue boundaries or other anatomical landmarks. The manual ROI approach implies that the neural substrate in question is confined to the ROI or set of ROIs defined by the researchers and that the neural substrate fills the entire ROI. This may not always be the case. A manually defined ROI may simultaneously exclude relevant brain areas and include less relevant areas, decreasing sensitivity to detect effects. With VBM the entire brain, or particular regions, can be considered at a much finer degree of spatial resolution.
The current study used VBM to examine the relationship between GM volume and performance on two neuropsychological tests of executive function, the Trail Making Test Part B (TrailsB; Reitan and Wolfson 1993) and Controlled Oral Word Association Test (COWAT; Benton et al. 1994) in healthy controls between the ages of 18 and 84 years. TrailsB is a visuomotor test that requires rapid mental sequencing and set shifting between numbers and letters. This paper-pencil timed test requires participants to search for and connect numbers and letters in alternating ascending sequence. COWAT is a letter-based phonemic verbal fluency task in which participants must orally produce as many words as they can in 1 min that begin with a specific letter (i.e. C, F, or L) presented to them (Lezak 1995; Spreen and Strauss 1998).
Functional MRI (fMRI) and lesion studies have investigated brain regions related to TrailsB, many indicating the importance of frontal lobe function for performance. For example, Stuss et al. (2001) examined the relationship between the TrailsB and specific regions within the frontal lobe and found that patients with dorsolateral frontal lesions were most impaired, but that damage to inferior medial regions of the frontal lobe was not significantly detrimental to TrailsB performance. Zakzanis et al. (2005) implemented the Trail Making Test with fMRI and found predominantly frontal and subcortical foci including superior frontal gyrus, dorsolateral frontal lobe, anterior insula and basal ganglia. Similar findings were observed by Moll et al. (2002).
COWAT has also shown to be indicative of frontal lobe function (Costafreda et al. 2006; Lezak 1995). Previous functional MRI of word generation and lesion studies with COWAT have consistently demonstrated the importance of the left lateral prefrontal cortex, particularly the inferior and middle frontal gyri, in verbal fluency tasks in intact and impaired brains (Costafreda et al. 2006; Royall et al. 2002; Schlosser et al. 1998; Stuss et al. 1998) though other language regions including the posterior temporal lobe are also involved (or coactive) in functional imaging studies (Schlosser et al. 1998).
Based on previous research, it was hypothesized that poorer performance on TrailsB would be associated with reduced GM volume in dorsolateral and subcortical regions. We hypothesized that poorer performance on COWAT would correlate with decreased GM volume mainly in the left inferior frontal gyrus (IFG) and adjacent prefrontal and premotor regions (Costafreda et al. 2006).
Materials and methods
Participants
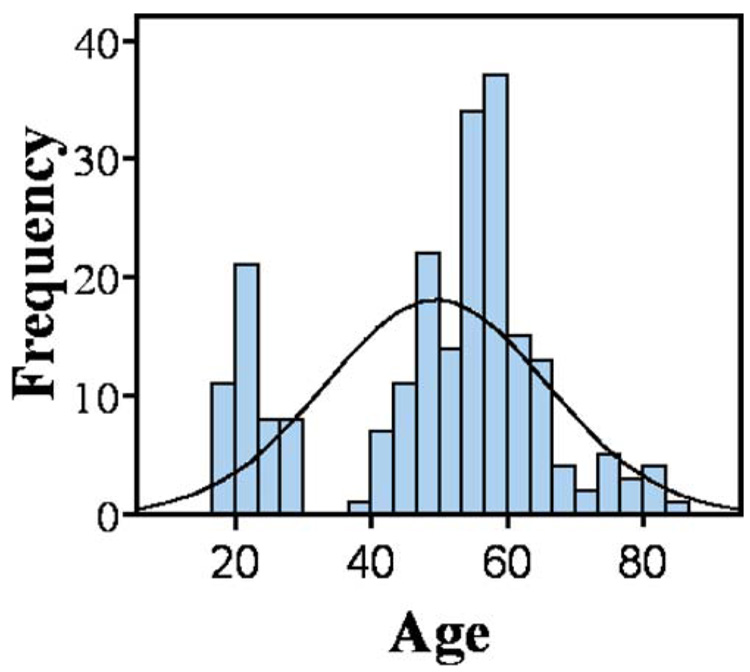
Participants consisted of 221 cognitively healthy individuals ranging in age from 18 to 84 who were recruited from the community by advertisements and direct mailings for several community outreach programs. They were recruited as controls for several ongoing studies of brain function and aging. The two tests that were analyzed (i.e., TrailsB and COWAT) overlapped across the protocols. Participants were screened for eligibility including general medical history and MRI scanner compatibility. Exclusion criteria included all of the following: a diagnosis of dementia, mild cognitive impairment, or other cognitive disorder; significant cognitive complaints; prior or current neurological disease (such as traumatic brain injury or epilepsy) or neurosurgery, current diagnosis of major Axis I psychiatric disorder, chronic major medical conditions (e.g., poorly controlled diabetes or hypertension, cardiac disease, cancer treated with radiation or chemotherapy within the past 5 years); and a modified Hachinski score (Rosen et al. 1980) greater than four. Health history was obtained through a comprehensive health questionnaire as well as screening interviews. Cognitive function was tested directly with a brief battery of neuropsychological tests administered by trained technicians. The cognitive data were scored independently by the testing technician and another technician. The double scored results and resolution of any non-clerical discrepancies were reviewed by a neuropsychologist. Participants who scored lower than two standard deviations on any test were excluded from the analysis. Additionally, participants who scored below 1.5 SD in any domain of cognitive ability across tests (e.g. memory, executive function, language) were also excluded. Current mood was assessed with the Center for Epidemiologic Study–Depression Scale (CES-D) and the State-Trait Anxiety Inventory (STAI). Participants showing evidence of significant affective dysfunction (CES-D >16; STAI trait scale in >95th percentile of age & gender-corrected normative data) were excluded from this study. The anatomical MRI data obtained from this study were required to be read as normal by a neuroradiologist for inclusion in statistical analyses. The demographic characteristics of the participants and descriptive statistics of the cognitive data are reported in Table 1. The distributions of participant ages are illustrated in Fig. 1. All provided written informed consent prior to participating.
Table 1.
Demographic and neuropsychological data
| TrailsB |
COWAT |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 49.56 | 16.36 | 50.02 | 16.17 |
| Education (years) | 16.00 | 2.50 | 15.97 | 2.49 |
| Gender: Female/Male | 136/82 | 130/81 | ||
| Sample Size (N) | 218 | 211 | ||
| COWAT (raw score) | – | – | 44.73 | 10.10 |
| Trail Making Test B (sec) | 60.83 | 21.30 | – | – |
COWAT Controlled Oral Word Association Test
Fig. 1.
Histogram of age distribution of the participants in the study. Each bin is 3.3 years. A hypothetical normal distribution is also shown
Neuropsychological tests
TrailsB and COWAT were administered in a standardized fashion. As per the standardized protocol, TrailsB was preceded by Trail Making Test part A. For COWAT, the total number of correct words given across three 60-s trials was used as the raw score in analyses. For TrailsB, the score used was the total time to completion. TrailsB scores were available for 218 individuals and COWAT scores were available for 211 individuals.
MRI scanning
MRI scans were obtained using a General Electric 3.0 Tesla scanner (Waukesha, WI). The T1-weighted volume was acquired with a 3D inversion recovery prepared fast gradient echo pulse sequence and provided high-resolution structural images with the following parameters: inversion time=600 ms, fast gradient echo read-out with TR/TE/flip= 9 ms/1.8 ms/20°; acquisition matrix=256×192×124 (interpolated to 256×256×124); field of view=240 mm; slice thickness=1.2 mm (124 slices); ±16 kHz receiver bandwidth. An experienced neuroradiologist reviewed all images prior to the analysis for clinical evidence of any neurovascular disease or structural abnormality that would exclude the subjects from the analysis.
Data analysis and voxel-based morphometry (VBM)
The VBM procedure as described by Good et al. (2001) was used to derive GM maps (while preserving volume) for each subject. This procedure utilized the VBM extension tools developed by Gaser (http://www.fil.ion.ucl.ac.uk/spm/ext/#VBMtools) and involved automated iterative skull stripping, segmentation of the images into GM, white matter (WM), and cerebrospinal fluid (CSF) probability images, and spatial normalization of the GM images to a customized GM template in standard MNI atlas space. The normalized brain images were then segmented and the resulting GM images were modulated using the Jacobian values obtained from the spatial normalization in order to preserve GM volume. Finally, the GM maps were smoothed with a 12 mm Gaussian kernel. The image processing and statistics were accomplished with Statistical Parametric Mapping (SPM2) statistical software.
The relationship between GM volume and neuropsychological test performance was examined using two identical multiple regression models selected from available models in SPM2. Performance on TrailsB and COWAT were the predictors in the two models. Age, education, gender, and total intracranial volume (TICV) were included as covariates of no interest. TICV was calculated using Sienax, an automated algorithm (Smith et al. 2002) in the FMRIB Software Library (FSL.3.3) that is completely rater free and reproducible. The statistical model used an alpha level set at p<0.001 (uncorrected) and an extent threshold of 20 2×2×2 voxels. This procedure yielded an estimate of the relationship between performance on TrailsB and COWAT and GM volume after adjusting for the covariates. The Wake Forest University Pick Atlas GUI (Maldjian et al. 2003) was used to restrict the analyses to GM and the cerebellum using a dilation factor of 1 to be more inclusive of the cortex. In addition to the voxel-based analyses, we also determined the simple correlations between age and neuropsychological performance after adjusting for education using SPSS v12.0.
Results
The demographic data are presented in Table 1. The mean age was approximately 50. The mean education level was 16 years. The means and standard deviations for the cognitive tests were in the average to high average range compared to normative groups (Spreen and Strauss 1998). There was a significant correlation between age and TrailsB (r=0.40, p<0.001), but not between age and COWAT (r=0.02), perhaps due to the high levels of education across the age range.
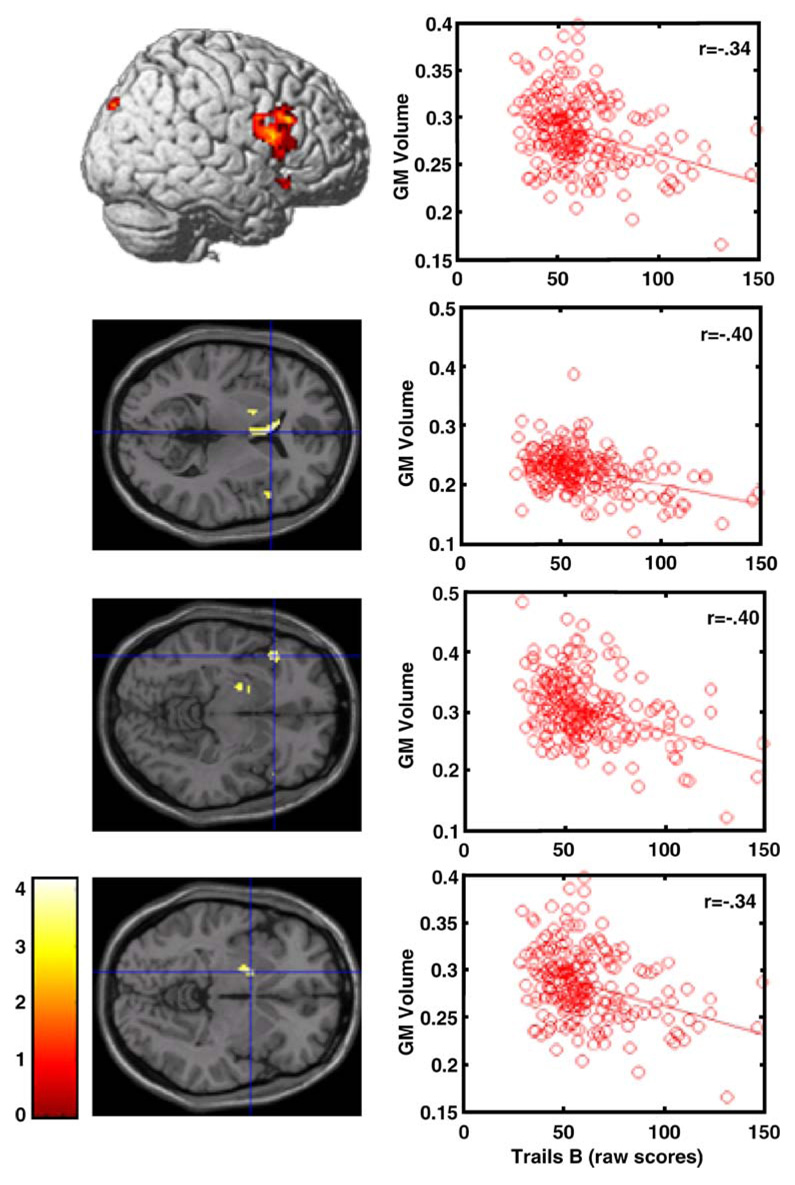
The inverse relationship between TrailsB time to completion and GM volume was significant in the superior portion of the right inferior frontal gyrus, and also in the left inferior frontal gyrus, caudate, globus pallidus and right posterior parietal lobe (Fig. 2, Table 2). Figure 2 shows the location of four of these findings in a lateral surface rendering and slice views. The plots in Fig. 2 show the relationship between TrailsB performance and GM volume (spatially summarized for each individual by the first eigenvariate across all voxels within the cluster) for the four respective locations.
Fig. 2.
TrailsB performance and GM volume relationships. Locations and plots of significant clusters where a relation-ship exists between TrailsB performance and GM volume at a threshold of punc<0.001, while controlling for age, education, gender, and TICV. The respective plots represent the linear relationship between performance on TrailsB and the first eigenvariate of the plotted cluster
Table 2.
MNI coordinates, t-values and cluster sizes for the VBM analyses
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| x | y | z | Peak t value | k | |
| TrailsB/GM Volume correlation | |||||
| R Inferior Frontal Gyrus (pars oper.) | 54 | 8 | 18 | 4.22 | 447 |
| Left Caudate | −4 | 18 | 6 | 4.00 | 295 |
| Left Inferior Frontal Gyrus (pars orb.) | −46 | 22 | −8 | 3.87 | 57 |
| Left Globus Pallidus | −20 | 2 | −4 | 3.66 | 99 |
| Left Uncus | −22 | 2 | −22 | 3.68 | 56 |
| Right Superior Occipital Lobe | 16 | −86 | 36 | 3.46 | 89 |
| COWAT/GM Volume correlation | |||||
| L Middle Frontal Gyrus | −36 | 38 | 24 | 3.65 | 29 |
| L Inferior Frontal Gyrus (pars orb.) | −56 | 22 | 28 | 3.46 | 30 |
| L Inferior Frontal Gyrus (pars orb.) | −56 | 22 | 0 | 3.40 | 31 |
All t-statistics are significant at p<0.001 uncorrected, corresponding to critical t-value of 3.13.
There was one small region (24 voxels) where a significant positive correlation was found (0, −22, 82; t=3.45, p<0.001). This was within the longitudinal fissure in the midline near the top of the cranium and may reflect thickened or calcified falx rather than GM.
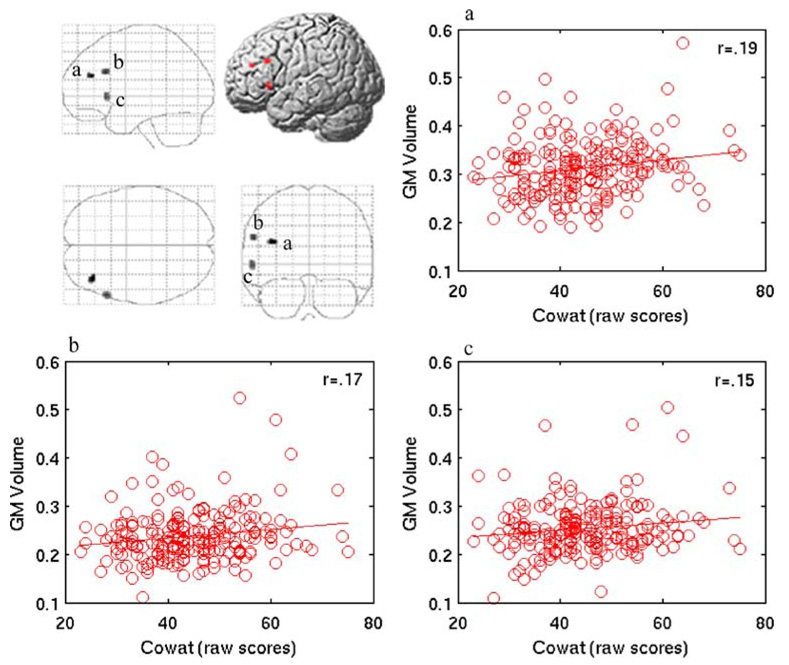
The analysis of the relationship between COWAT performance and GM volume revealed three significant clusters in the left inferior and middle frontal gyri (Fig. 3, Table 2). In Fig. 3, the linear relationship between COWAT performance and GM volume is shown as well as a rendered surface and maximum intensity projection depicting all significant locations in the brain. The plots in Fig. 3 represent the significant clusters. GM volume in each cluster is represented by the first eigenvariate across all adjusted voxels in the cluster for each participant and plotted against COWAT performance. No regions exhibited a negative correlation.
Fig. 3.
This glass brain maximum intensity projection and left lateral rendering of the cortex illustrate left lateral prefrontal regions in which COWAT performance was positively correlated with GM volume at a threshold of punc<0.001, while controlling for age, gender, education, and TICV. The linear relationships between the three significant GM clusters and COWAT performance are plotted. The plots are labeled a, b, and c, corresponding to its labeled cluster
Discussion
In this report we have presented correlational maps of GM and two common neuropsychological tests in a large cognitively normal sample of adults. When examining the relationship between timed performance on TrailsB and GM volume, results indicated that the superior and posterior aspect of the right inferior frontal gyrus, right posterior parietal lobe, and left basal ganglia (caudate and globus pallidus) were regions of significance. This finding is consistent with previous functional MRI and lesion studies indicating the importance of frontal and subcortical systems for this particular test (Lezak 1995; Reitan 1971; Stuss et al. 2001; Zakzanis et al. 2005), and more specifically, the importance of the right lateral frontal lobe for tasks that require sustained attention and set shifting (Pardo et al. 1991; Royall et al. 2002), two major components of TrailsB. Conversely, some have not found a significant relationship between frontal lobe volume and the Trails Making Test PartB–PartA difference (Anderson et al. 1995; Reitan and Wolfson 1995), although those studies are not directly comparable since they were of brain-impaired patients and used ROI-based methodologies. The frontal and subcortical findings in the caudate and globus pallidus are in agreement with prior neuroanatomical (Tekin and Cummings 2002) and functional imaging literature on executive functions, including TrailsB (Moll et al. 2002; Zakzanis et al. 2005) as well as others involving set shifting (Monchi et al. 2007; Monchi et al. 2001) and planning (Baker et al. 1996; Dagher et al. 1999; Owen et al. 1996).
The COWAT measure exhibited a relationship with GM in the left inferior and middle frontal gyri. This is not unexpected for a test of higher language and divergent reasoning. What is interesting is that this finding was present in intact cognitively normal brains using a static measure of gray matter volume rather than a functional or metabolic scan. Although the location of the result is plausible, the correlation itself is somewhat low but statistically significant due to the number of subjects in the study. In accordance with these results, others have strongly implicated the importance of the frontal lobe for this verbal fluency measure (Lezak 1995; Royall et al. 2002), and many have demonstrated the importance of the left inferior frontal gyrus and dorsolateral frontal lobe for verbal fluency tasks in intact and impaired brains (Costafreda et al. 2006; Friston et al. 1991; Schlosser et al. 1998; Stuss et al. 1998).
These findings were above and beyond any linear covariance that could be explained by age, education, gender, or cranial volume. These covariates were important elements of the model because previous research has demonstrated that age, education and gender are related to aspects of cognition (Buckner 2004; Coffey et al. 2001; Salthouse 1996), and age is also related to gray matter volume (Blatter et al. 1995; Good et al. 2001; Lemaitre et al. 2005; Murphy et al. 1992; Pfefferbaum et al. 1990; Raz et al. 1997; Salat et al. 1999; Thompson et al. 2003). That the findings observed here were predominantly frontal does not imply that other areas are not involved. Indeed functional imaging shows this to be the case (Costafreda et al. 2006; Roth et al. 2006; Shafritz et al. 2005). Our results simply suggest that in normal adults, the relationship between these neuropsychological tests and gray matter volume is primarily frontal.
Despite the fine resolution of these neuroanatomical analyses, results of the current study are correlational in nature and therefore do not confer causality, nor do they necessarily infer a neural substrate for any of the measures. Studying normal adults restricts the range of variance in the sample, for both neuropsychological function and gray matter, and limits the generalizability to other populations. Future studies could examine additional groups of participants, such as cognitively impaired patients, that would not only expand the generalizability of the current findings, but also provide more variance of neuropsychological performance and a broader range of GM differences among participants. Some moderating variables including age, education, gender, and TICV were taken into account, but there are certainly others that we have not modeled (such as IQ), or that are not ascertainable at this time (as in the case of participants who may be in a preclinical phase of a future neurodegenerative disease). In this study we relied on self-report of participants to rule out Axis I psychiatric disorders and medical disorders. Lack of a formal structured clinical interview to complement the self-report health history questionnaire is a limitation.
Further, the results are limited by the type of data used in these analyses. The neuropsychological tests are complex and impure from a cognitive neuroscience perspective, involving multiple cognitive processes. The MRI data in these analyses were static structural gray matter images based on probabilistic tissue classification from T1 weighted scans. While T1 imaging is excellent for tissue contrast, it is relatively insensitive to other clinically relevant markers such as ischemic change. Structural anatomic tissue contrast may not necessarily reflect the metabolic and physiological integrity of the tissue itself. Furthermore, the voxel-based approach requires spatial transformation of the GM map to a standard atlas space. It has been demonstrated that such transforms may be less accurate for atrophic brains (Dickerson et al. 2005; Sandstrom et al. 2006). Although we used a subject specific template to help address this issue, differences in spatial transformation may have affected our data. This concern is relevant to these data as well as any standard-space voxel-wise analysis across age or disease. In this paper we have not explicitly tested the relationship between aging and GM morphometry or cognition. We assumed the relationship was linear and used covariance to remove the effect of age, but nonlinear age-effects may not have been modeled with our approach. Future work in our laboratory will address the effects of age and other risk factors for dementia (such as genetic, cardiovascular, environmental and demographic factors) on brain morphometry. Despite the correlational nature of these data, the results may provide some approximation of the broad neural correlates of these particular commonly used tests in a wide range of cognitively normal adults.
Acknowledgements
This study was supported by NIH grants AG021155, MH65723 and a Merit Review grant from the Department of Veterans Affairs. The assistance of Shelly Fitzgerald, Britta Jabbar, Taylor Schmitz, Allie Wichmann, Michael Ward, Gemma Gliori, and Howard Rowley is greatly appreciated. We especially thank the participants of this study.
Contributor Information
Lisa M. Newman, Department of Veterans Affairs, Madison VA Hospital, Madison, WI 53705, USA Department of Medicine, University of Wisconsin, Madison, WI 53705, USA.
Mehul A. Trivedi, Department of Veterans Affairs, Madison VA Hospital, Madison, WI 53705, USA Department of Medicine, University of Wisconsin, Madison, WI 53705, USA.
Barbara B. Bendlin, Department of Veterans Affairs, Madison VA Hospital, Madison, WI 53705, USA Department of Medicine, University of Wisconsin, Madison, WI 53705, USA.
Michele L. Ries, Department of Veterans Affairs, Madison VA Hospital, Madison, WI 53705, USA Department of Medicine, University of Wisconsin, Madison, WI 53705, USA.
Sterling C. Johnson, Department of Veterans Affairs, Madison VA Hospital, Madison, WI 53705, USA Department of Medicine, University of Wisconsin, Madison, WI 53705, USA.
References
- Anderson CV, Bigler ED, Blatter DD. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. Journal of Clinical and Experimental Neuropsychology. 1995;17:900–908. doi: 10.1080/01688639508402438. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, et al. Neural systems engaged by planning: A PET study of the tower of London task. Neuropsychologia. 1996;34(6):515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Benton LA, Hamsher K, Sivan AB. Controlled oral word association test. Multilingual aphasia examination. 3rd ed. Iowa City: AJA; 1994. [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson C, Burnett BM, et al. Quantitative volumetric analysis of brain MR: Normative database spanning 5 decades of life. American Journal of Neuroradiology. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. Cognitive correlates of human brain aging: A quantitative magnetic resonance imaging investigation. Journal Neuropsychiatry Clinical Neuroscience. 2001;13(4):471–485. doi: 10.1176/jnp.13.4.471. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: A correlational PET activation study with the tower of London task. Brain. 1999;122(Pt 10):1973–1987. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Investigating a network model of word generation with positron emission tomography. Proceedings of the Biological Society. 1991;244(1310):101–106. doi: 10.1098/rspb.1991.0057. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26(3):900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting: An fMRI study of the trail making test. Arquivos de Neuro-Psiquiatria. 2002;60(4):900–905. doi: 10.1590/s0004-282x2002000600002. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130(Pt 1):233–244. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin card sorting revisited: Distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2001;21(19):7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CH, Johnson SC, McMillan KM, Haughton VM, Meyerand ME. Functional MRI neuroanatomic correlates of the hooper visual organization test. Journal of the International Neuropsychological Society. 2004;10(7):939–947. doi: 10.1017/s1355617704107042. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Archives of Neurology. 1992;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory: A positron emission tomography study in humans. European Journal of Neuroscience. 1996;8(2):353–364. doi: 10.1111/j.1460-9568.1996.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Jernigan TL, Zipursky RB, Rosenbloom MJ, Yesavage JA, et al. A quantitative analysis of CT and cognitive measures in normal aging and Alzheimer’s disease. Psychiatry Research. 1990;35(2):115–136. doi: 10.1016/0925-4927(90)90015-x. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reitan R. Trail making test results for normal and brain-damaged children. Perceptual and Motor Skills. 1971;33(2):575–581. doi: 10.2466/pms.1971.33.2.575. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead–Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd ed. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Reitan R, Wolfson D. Category test and trail making test as measures of frontal lobe functions. Clinical Neuropsychologist. 1995;9:50–56. [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Annals of Neurology. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Roth R, Randolph J, Koven N, Isquith P. Neural substrates of executive functions: Insights from functional neuroimaging. In: Dupri J, editor. Focus on neuropsychological research. New York: Nova; 2006. [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: A review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. Journal Neuropsychiatry Clinical Neuroscience. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Archives of Neurology. 1999;56(3):338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sandstrom CK, Krishnan S, Slavin MJ, Tran TT, Doraiswamy PM, Petrella JR. Hippocampal atrophy confounds template-based functional MR imaging measures of hippocampal activation in patients with mild cognitive impairment. AJNR American Journal of Neuroradiology. 2006;27(8):1622–1627. [PMC free article] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(4):492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25(2):600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4(3):265–278. [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The trail making test: A study in focal lesion patients. Psychological Assessment. 2001;13(2):230–239. [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. Journal of Neuroscience. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the trail making test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]