Abstract
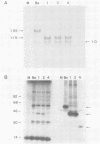
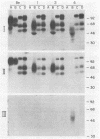
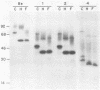
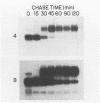
We have constructed two pseudorabies virus (PRV) mutants, each with a unique EcoRI restriction site in the nonessential gIII envelope glycoprotein gene. Since no natural PRV isolate has been reported to contain EcoRI sites, the isolation and single-step growth curve analysis of these mutants established that PRV can carry such a site with little ill effect in tissue culture. Virus carrying these defined mutations produced novel gIII proteins that enabled us to begin functional assignment of protein localization information within the gIII gene. Specifically, one viral mutant contained an in-frame synthetic EcoRI linker sequence that was flanked on one side by the first one-third of the gIII gene and on the other side by the last one-third of the gene. The resulting protein lacked the middle one-third of the parental species, including five of eight putative N-linked glycosylation signals, but was still glycosylated and found in enveloped virions; it was not secreted into the medium. A second viral mutant contained an in-frame synthetic EcoRI linker sequence that additionally specified a nonsense codon at position 158, producing a gIII protein that was glycosylated and secreted into the medium; the fragment was not found in enveloped virions. By endoglycosidase and pulse-chase analyses, we established a precursor-product relationship between the various forms of gIII expressed in the parental and mutant strains, and perhaps determined certain features of the gIII protein that are required for its efficient export within the cell.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Demarchi J. M., Kaplan A. S. Characterization of defective interfering viral particles present in a population of pseudorabies virions. Virology. 1974 Sep;61(1):29–37. doi: 10.1016/0042-6822(74)90239-6. [DOI] [PubMed] [Google Scholar]
- Bennett L. M., Timmins J. G., Thomsen D. R., Post L. E. The processing of pseudorabies virus glycoprotein gX in infected cells and in an uninfected cell line. Virology. 1986 Dec;155(2):707–715. doi: 10.1016/0042-6822(86)90230-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. XI. Sulfated, structural glycoproteins. Virology. 1976 Apr;70(2):561–563. doi: 10.1016/0042-6822(76)90300-7. [DOI] [PubMed] [Google Scholar]
- Keeler C. L., Jr, Whealy M. E., Enquist L. W. Construction of an infectious pseudorabies virus recombinant expressing a glycoprotein gIII-beta-galactosidase fusion protein. Gene. 1986;50(1-3):215–224. doi: 10.1016/0378-1119(86)90326-4. [DOI] [PubMed] [Google Scholar]
- Kerr C. L., Pennington T. H. The effect of monensin on virion production and protein secretion in pseudorabies virus-infected cells. J Gen Virol. 1984 Jun;65(Pt 6):1033–1041. doi: 10.1099/0022-1317-65-6-1033. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Thiel H. J., Schreurs C., Rziha H. J. Location of the structural gene of pseudorabies virus glycoprotein complex gII. Virology. 1986 Jul 15;152(1):66–75. doi: 10.1016/0042-6822(86)90372-7. [DOI] [PubMed] [Google Scholar]
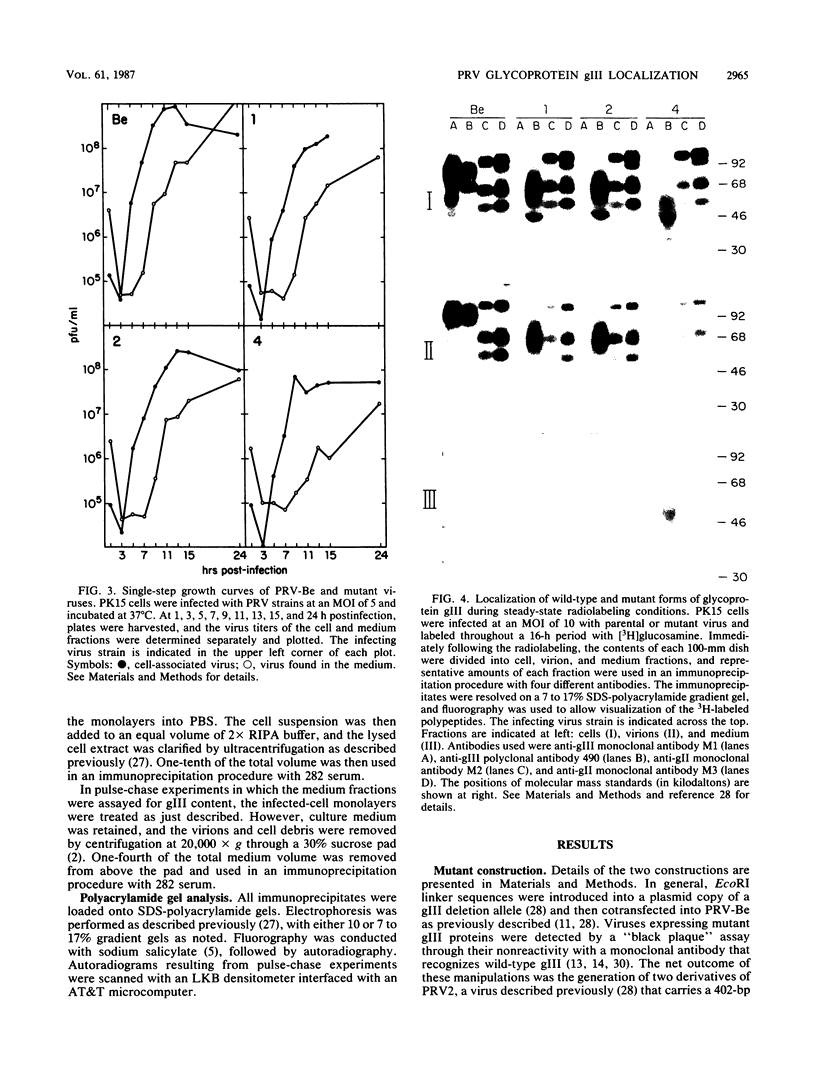
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Weis J. H., Enquist L. W., Watson R. J. Construction of E. coli expression plasmid libraries: localization of a pseudorabies virus glycoprotein gene. J Mol Appl Genet. 1984;2(5):485–496. [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Kennell W. L., Lamm D. L. Visualization of minute centers of viral infection in unfixed cell cultures by an enzyme-linked antibody assay. J Immunol Methods. 1981;40(3):297–305. doi: 10.1016/0022-1759(81)90361-6. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wenske E. A., Courtney R. J. Glycosylation of herpes simplex virus type 1 gC in the presence of tunicamycin. J Virol. 1983 Apr;46(1):297–301. doi: 10.1128/jvi.46.1.297-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]